To the Editor,
With the development of synthetic membranes and the fact that ethylene oxide is no longer used as a sterilizing agent, hypersensitivity reactions in haemodialysis have decreased considerably. However, they still exist. We describe a particular case from which we learnt some interesting things.
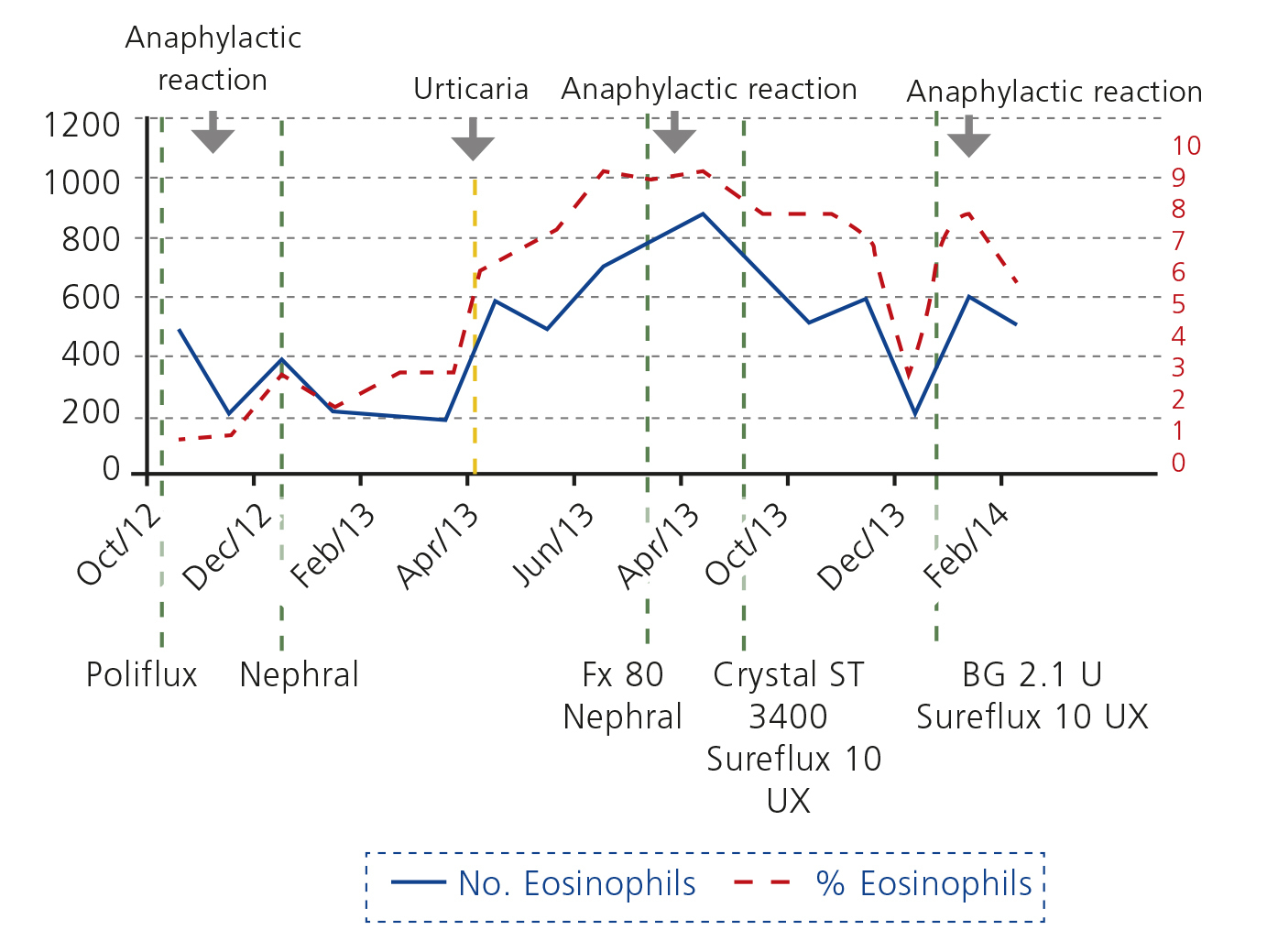
Ours was an 86-year-old male patient, smoker, on haemodialysis due to chronic interstitial nephropathy, with hypertension, ischaemic heart disease, type 2 diabetes mellitus, chronic obstructive pulmonary disease (COPD) and dyslipidaemia. He was on treatment with amlodipine, doxazosin, omeprazole, saxagliptin, pravastatin, paricalcitol, and beta 2 combined with inhaled steroids and during dialysis intravenous iron sucrose (Feriv®) and alpha erythropoietin (Eprex®). With no known history of allergies, urticaria or eosinophilia. He began haemodialysis during an episode of overall cardiorespiratory failure due to COPD decompensation with haemodynamic instability, volume overload and hypotension. He required treatment with steroids. He was dialysed with ultrapure water through a Poliflux21H® dialyser (polyamide polymer combination, polyarylethersulfone and high permeability polyvinylpyrrolidone, heat sterilised) and for one month suffered hypotension and clinical angina during dialysis. Once he had overcome these symptoms, steroid treatment was suspended and a week later, five minutes after connection, he suffered severe bronchospasm and hypotension with a generalised burning sensation, which responded to the administration of expanders, high flow oxygen, inhaled bronchodilators, antihistamines and intravenous steroids; it was not necessary to interrupt dialysis. During the next three dialysis sessions he suffered a repetition of these symptoms. No eosinophilia was seen. Antiheparin antibodies were negative. Dialysis fluid culture and endotoxins were negative. IgE was normal. No contact with ethylene oxide in any material. No thrombocytopaenia. In the following session, he was pre-treated with dexchlorpheniramine and methylprednisolone and the dialyser was replaced by NEPHRAL® (polyacrylonitrile AN 69 sterilised by gamma rays). The symptoms did not reappear, so prophylaxis was stopped after a month. During the next month, after six months on the programme, he developed generalised urticaria, and peripheral eosinophilia. At different stages his medication was changed without any clinical or analytical improvement. The dialyser was changed to a FX80M® (heat sterilised helixone) and hypersensitivity reappeared in the first session. Since then, again with NEPHRAL®, which was well tolerated, but urticaria and eosinophilia became constant. At 12 months, in a single session, a plate dialyser Crystal ST 3400® (polyacrylonitrile AN 69 without polyvinylpyrrolidone [PVP] as amalgamating agent) was used, and isolated severe hypotension reappeared without respiratory symptoms, explained by the high surface area of the dialyser, so in the next session we used Sureflux 19 UX® (cellulose triacetate sterilised by gamma rays), and we continued with this dialyser for five months. Pruritus partially disappeared and eosinophilia decreased (Figure 1). After 15 months, we used a 2.1 U® (polymethylmethacrylate [PMMA] sterilised by gamma rays) dialyser, resulting in new hypersensitivity reaction within minutes of connection. Since then, this patient has been dialysed with Sureflux 19® and has suffered no new episodes of hypersensitivity.
The patient had a type A1 anaphylactoid reaction related to polyamide, helixone and PMMA membranes. Urticaria and eosinophilia with polyacrylonitrile. Haemodynamic instability with polyacrylonitrile on plates without PVP and good tolerance to cellulose triacetate membranes.
In the literature, there are examples of anaphylactic and anaphylactoid reactions or tolerance to different types of synthetic membranes in the same patient2-7, which are attributed to differences in the manufacturing of these membranes and the amount of PVP used to hydrophilise the membranes, but in none of these cases a change of dialyser lead to the disappearance of symptoms and the development of chronic urticaria with eosinophilia. In this regard, the absence of anaphylactoid reaction when using a polysulphone dialyser with plates with no PVP as amalgamating agent suggests a significant role of this product as the cause of the patient’s disorders. Other causes that should be assessed are endotoxin backfiltration in highly permeable membranes (absent in low permeability membranes), hypersensitivity to intravenous iron or the use of angiotensin-converting enzyme (ACE) inhibitors. We do not believe this to be the case, since there were no reactions with high-flux dialysers that did not have PVP as an amalgamating agent. Cultures ruled out the presence of endotoxins, no ACE inhibitors were used and the modification or suspension of intravenous iron did not cause clinical changes. It is necessary to take into account the severity of these reactions, which did not always occur at the beginning of the session, thus decreasing clinical suspicion. This case illustrates a gradation in clinical manifestations, a variation on previously published cases, and provides new information on the effectiveness of cellulose triacetate membranes in intolerance to other synthetic membranes.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Analytical evolution