Despite the 40 years history, the comparable survival of Hemodialysis and Peritoneal Dialysis (PD), and the improved PD technique survival, the percentage of patients performing PD is low. After a short history review and data description, we analyze the many non-medical factors (“the vicious circle”) that contribute to the underutilization of PD: inadequate medical training, lack of infrastructures, small PD units, inadequate patient education for choice of dialysis modality, lack of multidisciplinary end-stage renal disease units, the proliferation of hemodialysis centers, or the trends in government reimbursement. Several of these factors are modifiable, and we propose future strategies to increase the use of PD.
Pese a sus ya casi cuarenta años de historia y la mejora de la supervivencia tanto de la técnica como de los pacientes, la modalidad de diálisis peritoneal (DP) sigue siendo la menos utilizada. Tras un repaso histórico y un análisis de los datos actuales, analizamos los factores no médicos («el círculo vicioso») que contribuyen a la infrautilización de la DP: la formación deficiente de los especialistas, la falta de infraestructuras, las pequeñas unidades de DP, la falta de información a los pacientes, la proliferación de unidades de hemodiálisis, la escasez de consultas de enfermedad renal crónica avanzada o la forma de financiación de la diálisis. Y proponemos estrategias de futuro para mejorar y promocionar el uso y desarrollo de la DP.
On the brink of forty years of continuous ambulatory peritoneal dialysis (CAPD), the development of this technique has not followed a "natural" evolution, and far from being an "adult" and recognised technique, at least in Spain, it has not reached its 'independence', it is not taken into account, and sometimes gives the impression that it is declining while still in its youth.
But this is not a local issue, other countries are facing the same problem. International and domestic economic environments are driving political, structural and functional changes that affect health, and therefore also peritoneal dialysis (PD). However, the different incidence and prevalence of PD across countries suggests that the current situation of PD itself cannot be defined as a "disease" of the technique, since there must be other factors to explain these differences in use and development.
If we want the "young but sufficiently prepared" PD technique to have a future and not wither away, we will have to review and analyse what has happened, what is happening, and what we do not want to happen. We must learn from the past and present to break down barriers that impede its progress and implement future strategies.
PAST
The first clinical use of the peritoneum was in 1743 when Warrick decided to treat a patient with repeat ascites by burning abdominal lymphatic nodes to which he attributed the origin of ascites, with a solution of Burgundy wine and Bristol water in equal parts. For this, after draining the ascites he infused said solution and although clinically the patient tolerated this badly, after recovering ascites took longer to recur. Encouraged by this fact, in the next attempt he infused more concentrated wine. Moreover, when the woman miraculously recovered, she did not suffer repeat ascites1 again.
In the following century, with the increase in abdominal surgery, peritoneal functional studies were initiated, demonstrating that the peritoneum acted as a semipermeable membrane: F. von Recklingshausen published a very detailed description of the anatomy, histology and physiology of the peritoneum2, G. Wegner showed that adding an osmotic agent it is possible to achieve ultrafiltration3, EH Starling showed that the transport of water and solutes across the peritoneum and blood is bidirectional4 and J. Putman after repeated animal experiments published "The living peritoneum as a dialysing membrane'5
And in the 20th century, Ganter, in 1923, performed the first peritoneal lavages for dialysis in humans, describing the technique in detail with many instructions and recommendations6.
In 1927 H. Heusser and H. Werder were the first to treat 3 patients suffering from acute renal failure (ARF) due to mercury poisoning with continuous flow PD (using two catheters)7. They were biochemically successful, as they were able to demonstrate a decrease in the levels of blood urea, but not clinically, because the patients died. Rosenmark in 1934 tried to apply this technique again, using glucose as an osmotic agent for the first time. He did not achieve clinical success, but was again able to show a decrease in urea levels8.
It was not until after the II WW that, in 1946, J. Fine, A. Seligman and H. Frank, in Boston, and R. Reid, in England, reported the first cases of ARF treated and resolved with continuous PD9,10.
Thus we come to the 1950s, with PD used as a last resort to treat ARF due to its many significant complications and because key aspects of the technique had not yet been defined, such as type of access, dialysate, flow, etc. It was unclear whether peritoneal lavage should be by continuous flow (two catheters) or intermittent (the same catheter to infuse and drain), but ultimately the lowest rate of leakage and peritonitis tipped the balance towards the second option, and after Arthur Grollman’s studies11 the solution was allowed to remain a certain time in the peritoneal cavity.
Different types of peritoneal catheters were tried, because kinking, obstruction and entrapment problems were frequent. Maxwell and his team designed a nylon catheter, flexible and non-irritating, placed by trocar through the midline, which made it easier to apply this method. It was also Maxwell who, in 1959, introduced the hanging bottle system, operated manually, which, once its contents had been infused into the peritoneal cavity, when lowered, served to collect the drainage. He convinced Baxter to market a dialysis solution and the technique was standardised12.
In late December 1959, a woman entered San Francisco after clinical and laboratory uraemia had been detected during her first postpartum check-up. Dr. R. F. Ruben implanted a peritoneal catheter and began treatment with peritoneal lavages. After a few days the patient had recovered, but further study showed that she was suffering from chronic renal failure (CRF). However, the catheter was left in place and peritoneal lavages were repeated when there were clinical signs of uraemia…and the patient and her laboratory indices improved once more. And again, and again ... peritoneal washings were initiated when a creatinine value of 20 mg/dl was reached and were suspended when the value had dropped to 13 mg/dl. In this manner, the patient was treated during additional six months13. Although this was the first case of CRF treated and maintained with regular PD, it was never published because the authors thought it was of no interest, as it was only one case and the patient had a short survival.
Thus we continue into the sixties, with several centres treating patients with chronic uraemia by means of regular PD. At first, plastic cannulas were left at the point of entry of the peritoneal catheter which were stoppered during rest periods and served as an "entry point" for the catheter used in each PD session14. Frequent infectious complications led the Tenckhoff, Shilipetar and Boenmade team to implant a catheter in each dialysis session. This team were able to achieve a fairly large regular home PD program (physicians made home visits to implant the catheter)15,16. But the limitations posed both by repeated catheter implantation and the large volumes of solutions required, as also the high infection rate caused by so many connections and disconnections, made regular PD a technique that was only used as a prelude to haemodialysis (HD) or used in special clinical situations, and it was not considered to have a successful future.
Several innovations have changed this scenario:
On the one hand, the simple modification implemented by West and Roberts17, introducing a stylet into the catheter reducing the need for sutures and the rate of dialysate leakage and simplifying implantation.
On the other, Tenckhoff’s design in 1968 of a straight silicone catheter with one or two Dacron felt cuffs18 that made it possible to keep it implanted for a long time (today it is still a reference model).
Lastly, the first cycling machines that instilled and drained the solution automatically without having to make multiple disconnections19 made their appearance.
Different models of catheters and cycling machines were designed based on the original models, making it possible to increase the number of patients that were treated by the technique known as regular PD: continuous peritoneal lavages for 48-72 hours once or twice a week. But large amounts of dialysis solution were still necessary, contained in large glass carafes, whose sterility was difficult to guarantee.
Until, in 1975, J. Moncrief and R. Popovich suggest that the same efficacy could be achieved using less volume of fluid, with longer dwell time and treatments applied every day. Although they wanted to publish this experience, the paper was not accepted, although it appears in the book of abstracts of the meeting of the American Society for Artificial Internal Organs of 197620. The idea, given the name of "CAPD" soon caused excitement in many countries. Basically it imitated the hanging bottles system, but far fewer exchanges were performed, allowing daytime dwell times of 4-6 hours and night-time ones of 8 hours.
In 1977 the first multi-centre study was performed, although with few patients, and it showed that CAPD was more efficient than periodic PD21. In Canada, Dr. Oreopoulus replaced the crystal bottle with a plastic bag that, once its contents were infused, was rolled up and kept beneath outer clothing to be used later as a drainage bag22.
Peritonitis remained a significant problem until, thanks to the addition of a titanium connector between the catheter and the transfer line (Nolph, 1979) and the design of a double bag system that allows "purging before infusion" (Buoncritiani group, 1980), this problem also appears to be on the way to being solved23,24.
During the eighties new types of catheters were designed with the aim of reducing access complications, integrated double bags and luer-locks to reduce the risks of contamination, and the first prototypes of home cyclers appear.
PD technique progresses and expands rapidly as from the nineties: connections improve, new dialysis solutions appear such as Nutrineal® (1995) or Extraneal® (1996), container bag material becomes more biocompatible. With information technology in the late twentieth century, cycling machines that register treatments appear and even telemedicine becomes possible; and more biocompatible solutions are used substituting lactate by bicarbonate (2001-2003).
With all these advances, the proportion of patients on CAPD increases rapidly until, in the first decade of the century it reached a point where this trend is stagnant or even reversed.
TODAY
A. Data
We have left behind a decade in which there has been a slow but steady decline in the number of patients on renal replacement therapy (RRT), although there are still significant differences in the use of various forms of therapy. According to the registry maintained by the Spanish Society of Nephrology (S.E.N.), there is a national average incidence of 120 pmp, with curious discrepancies between Autonomous Communities (CCAA). Consequently, higher incidence figures double the lowest incidence figures. When analysed by age groups, the greatest incidence is in patients over 75 years of age25.
If we analyse the position of Spain relative to the rest of Europe, we see that we are within an acceptable average26. Not as acceptable if we break down the use of different forms of treatment. Consequently, in Spain, use of PD is not among the highest: most developed countries have higher figures, and even some of those not considered as such. We once more see significant differences among the different Autonomous Communities, although in some, such as Cantabria, for example, the lower use of PD is due to a greater number of early transplants.
But it is also important to know how many patients remain in kidney replacement therapy. Prevalence data are progressively increasing, and we have already passed and remain above 1000 pmp. Once more, there is no difference between the data of different modalities of therapy, because renal transplant is increasing. The differences between various Autonomous Communities persist, but are not as striking. And following the death of older patients, the largest age group is now that of 50-75 years of age. Compared with other countries in the European registry, we see that this time our prevalence data puts us near the top positions; we could say that we are doing quite well and have good survival rates. Differences in prevalence of various forms of therapy are more dependent on the group of transplanted patients, but are quite similar both between the different Autonomous Communities and compared with the rest of Europe.
In conclusion, progressive decrease in patients entering kidney replacement therapy and increased prevalence are confirmed. But PD continues to be the least frequently used mode of dialysis.
And why is that? Is it because PD is a technique that is inferior to HD? Numerous studies have shown that clinical outcomes of PD are similar, if not better, than those of HD. And that survival rates are similar for both techniques27-33. In Spain mortality is greater with HD independent of age group25.
B. Context
It is well known to all and repeatedly and widely cited in the media: that there is an economic crisis. Moreover, due to the crisis, cuts have been applied. One of the areas most under the spotlight and where most cuts can be applied is the health sector, which from the beginning of the 21st century has progressively increased its spending which has been restrained only in recent years precisely because of the crisis, but the sum spent on the health sector is not exactly the highest in Spain34,35(Figure 1).
The highest sum assigned within the health sector is that assigned to specialised care36. And that includes us, as specialists. Renal patients are a very small sector of the sick population but, however, account for a significant part of health resources37. Therefore it has become necessary to study how to streamline spending. And this has been done both nationally and internationally37-44.
If there is a country where you need money to be healthy, it is the USA. The American system showed that treating kidney patients represented a major expense; which increased if these were treated with HD instead of PD. Yet most of their patients were treated with HD. Based on these data they carried out a five-year cost estimate based on several assumptions: how much it would cost to maintain the current situation and the changes that would have to take place in the rate of use of PD to achieve savings of a billion dollars. They found that in all cases in which the use of PD was increased, savings were achieved and that when this ratio was reduced, not only were no savings achieved, but even the initial calculated expense was exceeded45,46.
A similar study has been carried out in Spain37. After analysing the cost of each form of dialysis, taking into account the forecasted increase in population using this technique and considering that more than half of patients start unscheduled dialysis, a 15-year cost estimate was made according to several scenarios: first, maintaining the current situation; second, increasing the rate of kidney replacement therapy; third, increasing PD; and fourth scenario, combining the second and third ones. The conclusion was that all scenarios meant a lower cost per patient/year that maintaining the status quo and that significant savings were achieved in scenarios where the use of PD was increased. And this without calculating collateral costs such as additional tests or indirect costs due to morbidity47,48.
However, if no one dialysis technique is clinically superior to another49 and if it is possible to use cheaper dialysis techniques50, why is PD the least used?51-53, what other factors influence this situation?
Numerous factors have been described, in addition to those directly dependent on technique, related to both patients and the centres, governments, health systems or even professionals51,54-59. Many of them both in and outside Spain.
THE FUTURE
A. identifying barriers
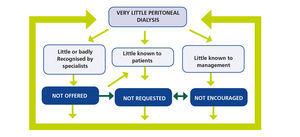
And this is the direction we need to take: to break this vicious circle, which results in less use of PD (Figure 2). A circle maintained by three main axes.
1. Peritoneal dialysis is poorly understood and/or poorly recognised by professionals
Therefore, not offered to patients. There are several reasons for this lack of knowledge or disrepute of PD.
1. Specialists training plans: The national training program requires Nephrology residents teaching units to have a HD unit, but considers PD optional. In addition, the estimated rotation time in PD is markedly lower60. As a result, residents do not receive adequate training. Consequently, in a recent survey of residents in the specialty, more than half acknowledged being poorly trained in PD61. And this is not exclusively a Spanish problem, a similar survey conducted in the United States showed American residents complained about the same lack58.
2. Lack of Resources (material and human) Even though, as stated in national PD62 guidelines published almost ten years ago to have a PD unit only three small rooms and very few staff are needed. Although initial rates of patient/physician-nurse have been adjusted for patient complexity, few centres have specialised staff dedicated solely to PD63,64. This, coupled with lack of experience means DP patients are considered "extra work"65. With regards to physicians, combining patient care in HD (which is three times a week) and PD (patients which one must "remember" as they are not present) means that the care of these "not so visible" patients is not calculated. In the case of nurses, nurses dedicated to PD may feel discriminated against compared to their peers in HD due to the greater proportion of patients treated and greater personal, dedication required, since these are not "routine" patients. In addition, her coworker in HD, unaccustomed to the PD technique may feel burdened by the extra work caused by PD patients during afternoon shifts or in emergency situations. Again, this is not only a problem in Spain, but has also been described in other countries65,66.
3. “Centre Effect” This is another factor. Several studies have shown that the greater the size of the PD unit and the greater the cumulative number of patients treated, the better the results. The minimum size or "key number" of treated patients should be greater than 20 to 25 to ensure good results and less problems51,66-70. However, in Spain, about half of the PD units do not reach these numbers64.
4. Proliferation of HD Units: It is difficult to promote the use of PD when HD units continue to open at a rate that exceeds the number of patients and at a cost that has to be covered. To cover the costs of these units, pressure is put on patients, and patients who are eligible for PD are diverted to HD. Moreover, once HD units are full, and given the number of new patients, it is justified to continue creating HD units, which are provided with personnel and material even before they open.
Patients are not put on PD, precisely to amortise HD units, which means that the few patients who are still using this technique are proportionately more expensive, because they still require personnel and said personnel could handle a larger number of patients50,63-65. It has been seen, furthermore, that hospital centres that have an HD unit outside the hospital send less patients to PD50,64. And this situation continues in spite of the fact that the cost of PD is less than that of HD in attached centres37,50.
Some authors have even dared to mention other ulterior motives in this proliferation of HD units, such as physician participation in the profits of these units, or sponsorship or funding by pharmaceutical companies since more of their products are used in HD54,58,64,66.
5. The burden of care: and last but not least, a factor that limits PD is the fact that PD patients give rise to more work. Because problems have to be solved remotely and/or instantly, so that patients feel they are under constant supervision71. Because the clinical management of PD patients is different to that of HD patients: with regards to control of blood volume, efforts to maintain residual renal function, nutrition, etc. Because PD patients give rise to more work, professionals not used to habitually dealing with them do not become involved in their control and when the head physician is absent, and problems crop up, they choose the "safer” option of transferring these patients to HD. There are many factors that require control at a distance in a patient not seen three times a week. Self-care that must be maintained and encouraged to prevent patients’ psychological distress, teleconsultation due to patients’ remoteness from centres, the need for rapid resolution of technique-related problems, which in some cases may require the help of surgical or interventional specialists71. For these reasons, or if one is tired of problems, difficulties, or in cases of sheer boredom, there is always the easy alternative of sending the patient to HD.
2. Little known to Patients
Therefore, not requested, the reasons for this can be summarised as:
1. Pre-dialysis information: Initial information which patients have on PD is always less than on HD, and is much less the older the patient. We dedicate little time to patient information and much less in fact, to those who are least informed. It was shown in a recent study that we dedicate less than one hour to inform almost half of our patients. So it is only to be expected that, when interrogated, they consider they were poorly informed72.
Several studies have shown that more and better information to patients on RRT techniques increases the percentage of those choosing PD73,74. In Spain, the Support Group for the Development of Peritoneal Dialysis (GADDPE) confirmed that the use of educational and information tools for patients decision making patient increases the rate of PD chosen over HD. The European Renal Best Practices Advisory Council has also stated the convenience and need to inform patients49.
It is not only necessary to inform patients to improve the rate of PD choices but in our country the law requires us to do so. Law 41/2002 clearly indicates that the patient has the right to know all the treatment options for their disease, that the professionals who will care for them must give them that information and that this must be detailed in an informed consent form75.
2. Chronic Advanced Kidney Disease Consultations: Informing a patient takes time and, in Spain, about 50% of patients start dialysis in a non-programmed manner, and, therefore, HD through a vascular catheter76. Aware of this problem, the Spanish Nephrology Association published guides for CAKD consultations77 one of the goals of these is to precisely inform patients and control them closely until they are included in a dialysis program. However, not all centres have such consultations and people with advanced kidney disease are still seen in general practice until they are included in dialysis programs. The results obtained by CAKD consultations are spectacular, as long as patients do not arrive with such reduced renal function that these consultations become mere entryways to dialysis78-81.
3. Little Known to Management
Therefore, not promoted. But in light of the statements of our rulers, lack of knowledge of PD does not seem to be the problem82,83.
There are several ways in which Management may intervene:
1. Preventive Nephrology: In health, management usually sees only the tip of the iceberg of disease, forgetting that the largest and most dangerous portion is not visible (Figure 3).
Management focuses on the therapeutic aspect without considering the important role of prevention. Many preventive processes, both in our field and in medicine in general, have proven clinical efficacy and cost-effectiveness.
The classical scheme of preventive medicine can be applied perfectly to nephrology specialists but what concerns us more closely is secondary preventive nephrology. Many risk factors have to be prevented and controlled, activities that, interestingly, coincide to a large extent with the aims of CAKD consultations77,84,85 (Figure 4).
And if health gains are not sufficient, different studies have shown that preventive activities represent a cost-effective investment for medium and long term savings86-89.
2. Type of Health Financing: Countries that possess health services that are mostly public have greater access to PD, and, therefore, a greater number of patients on PD, in comparison with countries with a greater number of private health schemes51,63.
In Spain almost half of HD centres are located outside hospitals and most are owned by large companies. The price per HD session is more expensive in a hospital than in centre which is not a hospital, but only centres outside hospitals offer regular chronic HD services; they do not attend emergencies, nor do they attend 24x7, and costs of medication, transport, additional tests, etc. impact on the referring hospital90.
PD, nationally, is agreed with private companies. The price paid for a day's therapy is relatively low but is fixed regardless of the number of exchanges performed by the patient, and once again, does not include medication, transport, etc.
Various economic studies on RRT performed in Spain have consistently shown that actual costs are not related to those reported officially91,92.
The method of financing dialysis techniques has a significant influence on their distribution. We can see an example of this in the United States. Until 2011, each of the different types of dialysis was paid differently, both as to fixed costs (property infrastructure, staff, electricity, equipment, etc.) as to drug costs, the coverage is lower for PD. From 2011 the US healthcare system pays centres the same amount per patient regardless of the type of dialysis. Since the costs of the assignments are lower for PD, to obtain greater benefits centres will increase the use of PD. Changes in reimbursements for dialysis is encouraging the development of PD programs93-95.
3. Incentives for Professionals: According to managers of large hospitals, lack of support by specialists has been one of the causes of lack of promotion of PD96. And aware that the number of professionals in public centres is not affected, contrary to what happens in centres outside hospitals, by the number of patients treated and the type of dialysis technique offered66, most managers would agree that it is necessary to encourage specialists96.
In Spain we have a model example of extensive public-health centres coordination, with resources, which due to its achievements, has been copied by other countries: the National Transplant Program, in which incentives for professionals is one of the reasons for its success. This model has proven to be beneficial to the patient, professionals and the system, and it would be readily applicable to PD.
B. Future Strategies
Knowing the barriers that impede PD growth, it is easy to plan future strategies97-100.
1. For peritoneal dialysis to be regarded more positively it is necessary to:
- Modify specialists’ training plan, with longer and mandatory rotation in PD. With the objective that training in both dialysis techniques should be independent and of similar duration. This is not practical or reasonable in centres with small units with few PD patients, where usually only one physician is responsible for all dialysis procedures.
- Avoid the proliferation of multiple small units and in areas with several hospitals assess the benefit of creating referral PD units. This would improve their experience, and therefore clinical outcomes, and also the training of residents.
- Reconsider accreditation for MIR teaching in those hospitals that do not offer PD, or, where applicable, mandatory external rotation of residents in a centre with experience in PD.
- Encourage, facilitate and foster commitment to PD within nephrology teams and increase the training and rotation of physicians and nursing staff in PD facilities. While full dedication promotes growth and success of PD units, it seems unreasonable to leave the care of these patients to a single specialist and/or nurse. A “rotating” second party system working with the "head specialist" would ensure continuity in patient care, and staff training and rotation.
- Demand adequate provision of dedicated human and material resources for PD. In the same way that the patient/nurse ratio for HD is set, this ratio should be considered and reviewed for PD. The increasing complexity of the condition of patients who start dialysis and the greater personal dedication required by PD patients (while teaching PD technique, how to resolve doubts and problems, catheter monitoring and control, recycling, conducting additional tests, complementary techniques, etc.) makes it advisable to reduce the number of patients initially estimated77. Perhaps 25 patients per nurse would be more realistic and effective. And even this ratio should be reduced in the case that the same nursing staff works with PD and HD. In cases where the ordinary nursing care for PD is only offered specifically and exclusively on the morning shift, compensation for the loss of benefits should be sought (economic or workday) by shift work, so this post is attractive and therefore not rejected.
- Stop increasing the creation of HD units and adapt them to the real increase of RRT patients.
- Create fast track routes for surgery or interventional techniques related to PD. In the same way that fistulas or vascular catheter problems are considered urgent and usually have established routes for prompt care, problems related to peritoneal catheters or the abdominal cavity should also be considered priorities and they should be resolved as soon as possible to prevent or delay patients going to HD, even if only temporarily.
- Enhance aspects such as telemedicine: a good many of the problems or concerns of patients can be solved by telephone both by specialists and nursing staff. Such consultations shall be considered and counted as care work, as if they were face to face, and should be facilitated with evening shifts, night shifts and holiday shifts, for example, by staff localization. Making use of computer technology that enables data transmission or remote viewing, facilitates patient management, decreasing hospital visits.
2. So that patients request PD it is necessary to:
- Inform all patients on all RRT techniques, regardless of the way in which they began dialysis. This information should be detailed, over several planned sessions for better assimilation, preferably offered by different sources (personally or by means of visual or audiovisual media) and understandable to the patient. Ultimately, this information must be validated by the patient by means of an informed consent form.
- Create, develop and enhance ACKD consultations so that more people are informed when they begin their programmed dialysis. The criteria and requirements for these units are already defined and patients should be referred early to these units and not only when they are candidates for dialysis.
- Promote and disseminate knowledge of PD. In Spain, the creation and actions of GADDPE are an example of the effectiveness of this point.
3. For Management to encourage PD it is necessary to:
- Promote and encourage preventive care of CKF collaborating with primary care and other specialties to develop preventive nephrology. Schedule teaching sessions or joint protocols and facilitate contact and consultation with the Department of Nephrology (online consultation, rapid referral pathways, etc.) Reduce the number of patients with undetected ACKD.
- Continue to push for the creation and increase of ACKD consultations.
- Request specific funding for dialysis programs encompassing all items, materials and drugs, hospital admissions, transport, staff, etc. This can be positive for the management of health centres and stimulate the support and development of the PD.
- Involve specialists in the promotion of PD. for example, with an incentives system similar to that used for transplants.
- The barriers to the development of PD are like the skins of an onion: when you remove one, other difficulties appear. Overcoming each one requires enthusiasm, dedication and innovation by applying new formulas that should be updated as populations age and morbidity increase.
Conflicts of interest
The author declares that she has no conflicts of interest related to the contents of this article.
KEY CONCEPTS
Figure 1. Evolution of health expenditure
Figure 2. The vicious circle that maintains low utilization of peritoneal dialysis
Figure 3. The iceberg of kidney disease
Figure 4. Nephrology and Preventive Medicine