The aim of this article is to update the 2010 recommendations on the evaluation and management of renal disease in HIV-infected patients. Renal function should be monitored in all HIV-infected patients. The basic renal work-up should include measurements of serum creatinine, estimated glomerular filtration rate by CKD-EPI, urine protein-to-creatinine ratio, and urinary sediment. Tubular function tests should include determination of serum phosphate levels and urine dipstick for glucosuria. In the absence of abnormal values, renal screening should be performed annually. In patients treated with tenofovir or with risk factors for chronic kidney disease (CKD), more frequent renal screening is recommended. In order to prevent disease progression, potentially nephrotoxic antiretroviral drugs are not recommended in patients with CKD or risk factors for CKD. The document provides indications for renal biopsy and advises on the optimal time for referral of a patient to the nephrologist. The indications for and evaluation and management of dialysis and renal transplantation are also addressed.
El objetivo de este documento es actualizar las recomendaciones sobre la evaluación y el manejo de la afectación renal en pacientes con infección por el VIH del año 2010. La función renal debe monitorizarse en todos los pacientes e incluir la medida de la concentración sérica de creatinina, la estimación del filtrado glomerular (ecuación CKD-EPI), la medida del cociente proteína/creatinina en orina y un sedimento urinario. El estudio básico de la función tubular ha de incluir la concentración sérica de fosfato y la tira reactiva de orina (glucosuria). En ausencia de alteraciones, el cribado será anual. En pacientes tratados con tenofovir o con factores de riesgo para el desarrollo de enfermedad renal crónica (ERC), se recomienda una evaluación más frecuente. Se debe evitar el uso de antirretrovirales potencialmente nefrotóxicos en pacientes con ERC o factores de riesgo para evitar su progresión. También se revisan las indicaciones de la biopsia renal, cuándo enviar el paciente al nefrólogo y las indicaciones, evaluación y manejo del paciente en diálisis o del trasplante renal.
1. INTRODUCTION
With the progressive change in the natural history of infection by the human immunodeficiency virus (HIV), the sustained decrease in the incidence of the acquired immunodeficiency syndrome (AIDS) and related mortality most HIV-infected patients have an estimated mortality that is similar to that of the general population. Furthermore, with greater longevity there has been an increase in comorbidity related to chronic conditions and the importance of renal diseases as a cause of morbidity and mortality in HIV-infected patients has been pointed out. Many of the causes of acute and chronic kidney disease in HIV-infected patients are similar to those of the general population, although some of them are specific and/or more frequent in these patients, such as HIV-associated nephropathy, glomerulonephritis mediated by immune complexes, thrombotic microangiopathies and antiretroviral and non-antiretroviral drug-induced nephrotoxicity.
The objective of this document is to provide recommendations, based on scientific evidence, (GRADE system [Grading of Recommendations of Assessment Development and Evaluations]1,2), on the prevention, diagnosis and management of renal disease in HIV-infected patients carried out by the Panel of experts from the AIDS Working Group (GESIDA) of the Spanish Society of Nephrology (S.E.N.) and the Spanish Society of Clinical Biochemistry and Molecular Pathology (SEQC) published as a monography1 (http://www.revistanefrologia.com/modules.php?name=articulos&idarticulo=12674&idlangart=EN). This manuscript updates a previous consensus document published in 2010 by the GESIDA and the Secretariat of the Spanish National AIDS Plan3.
2. RENAL EVALUATION OF HIV-INFECTED PATIENTS AND regularity of the assessments
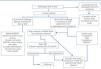
Regular evaluation of risk factors for chronic kidney disease (CKD), of renal function and of the presence of renal damage markers are early detection of renal disease, its aetiological diagnosis and its follow-up, as well as the adjustment of doses of nephrotoxic drugs or those that are eliminated through the kidneys. A basic renal study or screening is recommended in all HIV-infected patients and a comprehensive renal study is recommended only in selected patients whose basic renal study presents disorders (Table 1).
nRecommendations on renal evaluation
The evaluation of renal damage in HIV-infected patients will include:
1. Measuring serum creatinine concentration and estimating glomerular filtration rate (GFR), mainly by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation or, where applicable, the MDRD (Modification of Diet in Renal Disease) equation. Quality of the evidence: High. Degree of recommendation: Strong.
2.Measuring urine protein/creatinine ratio (uPCR), preferably first-morning-void urine sample or, when unavailable, a random urine sample will be acceptable. In patients with diabetes mellitus and/or high blood pressure (HBP), the ACR will also be determined. Recommendation based on consensus.
3. Basic evaluation of tubular function by the above mentioned analyses (points 1 and 2), serum phosphate concentration and glucosuria detection by urine test strip, preferably in the first-morning-void urine sample. Recommendation based on consensus.
4. When an abnormality in the basic study has been detected, more specific studies are advised. In the case of potential tubular involvement secondary to antiretroviral toxicity, the serum and urinary concentrations of phosphate and urate will be determined, as well as the respective fractional excretions calculation, the acid-base balance in blood study and the measurement of concentration of urinary and serum potassium. In the case of suspected glomerular disease, specific studies will be carried out in accordance with whether the glomerulopathy is suspected to be primary or secondary. Furthermore, imaging tests or consultation of the Nephrology department will be considered in accordance with the referral criteria, which are described in this document. Recommendation based on consensus.
5. The urine test strip may be useful for detecting the presence of urinary infection (esterase and nitrites), tubular abnormalities (non-hyperglycemic glucosuria) or urinary sediment abnormalities (hematuria), but must not be used for assessing proteinuria. Recommendation based on consensus.
6. The specimen of choice is random urine, preferably the first urine of the morning, since it has shown a good correlation and concordance with the values obtained in 24-hour urine, with the exception of nephrotic range proteinuria (>3g/day), in which the recommended specimen is 24-hour urine. Recommendation based on consensus.
•Recommendations on the regularity of the assessment (Table 2 and Figure 1)
1. In all HIV-infected patients, the basic renal study should be carried out to detect renal disease at diagnosis of the HIV infection, and systematically in its subsequent follow-up. Quality of the evidence: High. Degree of recommendation: Strong.
2. This study should be carried out in patients who do not receive antiretroviral treatment (ART): 1) when HIV-infection is diagnosed; 2) once per year if there are no risk factors for the development of nephropathy; 3) every six months when one or more of these factors are present; and 4) before beginning ART. Quality of the evidence: Low. Recommendation based on consensus.
3. In individuals treated with TDF, the frequency of tests must be increased. In patients without CKD risk factors, it is recommended to perform tests coinciding with those carried out for the efficacy of ART (1-3 months after the start of the treatment and subsequently every 6 months). In patients with CKD or CKD risk factors, it is recommended to perform the assessment within the month in which the drug is introduced. Each test will include the determination of serum phosphate and glucose and the urine test strip, preferably in the first urine of the morning (non-hyperglycemic glucosuria). Quality of the evidence: Low. Recommendation based on consensus.
4. In patients treated with the coformulation of tenofovir, emtricitabine, cobicistat and elvitegravir (TDF/FTC/COBI/EVG, Stribild®), the summary of characteristics of the European Medicines Agency recommends monthly tests during the first year and subsequently every 3 months. Quality of the evidence: High. Degree of recommendation: Strong.
5. In the absence of abnormalities in the basic study, an annual follow-up is recommended that includes: measurement of serum creatinine concentration, estimation of the glomerular filtration rate (GFR), preferably using the CKD-EPI equation, determination of the uPCR in the first urine of the morning and the urinary sediment study. Quality of the evidence: Low. Recommendation based on consensus.
3. REFERRAL TO NEPHROLOGY AND INDICATIONS FOR RENAL BIOPSY
Consultation of the Nephrology department must be considered as collaboration for adequate interpretation and approach to renal problems, particularly when they are complex or require diagnostic or therapeutic approaches. In addition, renal biopsy indications are the same as for patients without HIV infection and there is no evidence that HIV-infected patients have more complications related to renal biopsy than non-infected patients.
• Recommendations for referring patients to Nephrology
It is recommended to refer to Nephrology those patients presenting of the following abnormalities:
- uPCR >0.5g/g (>50mg/mmol), urinary albumin-creatinine ratio (uACR) >300mg/g (0.3g/g or 30mg/mmol) of uncertain aetiology.
- GFR < 45ml/min/1.73 m2
-Non-urological hematuria (>25-30 red blood cells per field) of uncertain aetiology.
- Acute renal function deterioration or progressive deterioration of uncertain aetiology.
-CKD and HBP refractory to treatment.
-Abnormal potassium values (>5.5mEq/l or <3.5mEq/l) of uncertain aetiology.
-Anemia of renal origin.
Quality of the evidence: Low. Recommendation based on consensus.
• Recommendations for indicating a renal biopsy
1. The indication of a renal biopsy will always be individualised in accordance with the balance between the risk of the biopsy and the benefits that it may bring. Quality of the evidence: Low. Recommendation based on consensus.
2. Renal biopsy indications in VIH-infected patients are the same as for patients without HIV infection Quality of the evidence: Low. Recommendation based on consensus.
3. Renal biopsy indications are:
- Nephrotic syndrome (uPCR >3g/g).
-Nephritic syndrome.
- Persistent urinary abnormalities (uPCR >1g/g that does not respond to treatment with renin-angiotensin system blockers or if there is persistent microhematuria >25-50 red blood cells/field or outbreaks of hematuria).
- Acute renal failure suspected to be of glomerular, immunoallergic or unknown origin.
- TMA and malignant hypertension.
4. DIAGNOSTIC APPROACH TO RENAL FUNCTION DETERIORATION
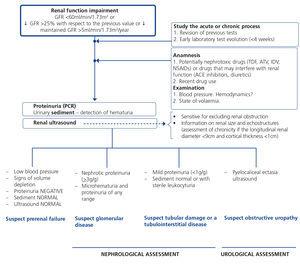
Renal function deterioration may occur in its acute form (acute renal failure) or gradually, in which case we speak in terms of progression. To evaluate progression, both GFR and proteinuria (or albuminuria) values must be considered, since both markers are related to progression to more advanced CKD stages. Following renal failure diagnosis, the aetiological diagnosis must be carried out, assessing whether the cause is prerenal, parenchymal or obstructive by an adequate anamnesis and physical examination, determination of uPCR, a study of the urinary sediment (detection of hematuria, leukocyturia, cylindruria) and a renal ultrasound study. Figure 2 displays an algorithm to be followed when there is renal function deterioration.
• Recommendations
1. When there is any renal function deterioration, it is necessary to study whether the process is acute or chronic, observing the changes in previous results (mainly of serum creatinine concentration and eGFR). Quality of the evidence: Low. Degree of recommendation: Strong.
2. The following are considered to be progression criteria: a) the change to a more severe CKD stage accompanied by a decrease in the eGFR of >25% with respect to the baseline value; b) a sustained decrease in the GFR >5ml/min/1.73m2/year.
5. DIAGNOSTIC APPROACH TO THE PRESENCE OF HemaTURIA
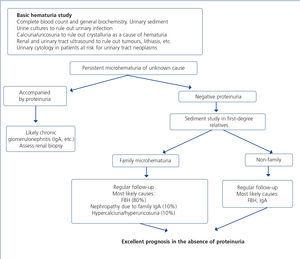
When hematuria is present, a specific clinical history, a complete physical examination, an analysis (including complete blood count, serum creatinine concentration and GFR estimation) a urinary sediment study, a urine culture, uPCR and a renal ultrasound should be carried out with the aim of distinguishing between urological and glomerular causes. Figure 3 displays an algorithm to be followed in the presence of persistent macro- or microscopic hematuria.
• Recommendations
1. In patients with hematuria, a differential diagnosis should be carried out between urological and glomerular origins. Quality of the evidence: Low. Recommendation based on consensus.
6. MANAGEMENT OF CHRONIC KIDNEY DISEASE PROGRESSION FACTORS, CARDIOVASCULAR RISK FACTORS AND OTHER RENAL ABNORMALITIES PRESENT IN HIV-INFECTED PATIENTS WITH CHRONIC KIDNEY DISEASE
Several variables, including higher glomerular filtration rate at the time of renal biopsy, a higher CD4 lymphocyte count, an undetectable HIV plasma viral load, the absence of hepatitis C virus (HCV) coinfection and/or HCV viremia, the use of ACE inhibitors or ARBs and ART, are factors that have been associated with better renal prognosis and/or higher survival rates in HIV-infected patients diagnosed with CKD by biopsy. We must highlight the importance of controlling proteinuria and HBP and insist on the prevention and early treatment of episodes of acute renal function deterioration.
6.A. Cardiovascular risk
CVD is one of the main causes of death not related to HIV infection. In these patients, there is an increase in CVR associated with factors such as the chronic inflammatory response to HIV infection and with secondary metabolic effects of antiretroviral medication. Furthermore, HIV-infected patients have shown an increased life expectancy, which means that they have a higher prevalence of CVR factors such as HBP and diabetes mellitus. The early diagnosis, treatment and prevention of CVD have become one of the priorities in the care of HIV-infected individuals. CKD patients have very high CVR. As such, the targets that must be achieved in some risk factors are stricter than in the general population.
• Recommendations
1. CKD patients are considered to be very high CVR patients. It is recommended to carry out a CVR evaluation in the baseline visit and at least once a year. Recommendation based on consensus.
2. All cardiovascular risk factors will be treated. Recommendation based on consensus.
3. In diabetic patients, albuminuria should be monitored as an early marker of diabetic nephropathy and HBP. Quality of the evidence: High. Degree of recommendation: Strong.
6.B. Proteinuria management
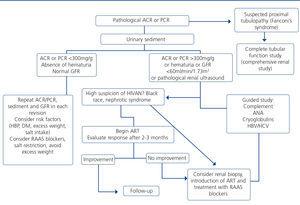
We will define moderate asymptomatic proteinuria as uPCR of 0.15-1g/g (or its equivalent 0.15-1g/24 hours), significant asymptomatic proteinuria as uPCR of 1-3g/g (or its equivalent: between 1 and 3-3.5g/24 hours), without oedema or the biochemical characteristics of the nephrotic syndrome (hypoalbuminemia, hyperlipidemia), and lastly, nephrotic proteinuria as uPCR greater than 3-3.5g/g (or its equivalent 3-3.5g/24 hours) accompanied by oedema or hypoalbuminemia, hypoproteinemia or hypercholesterolemia. The algorithm to be followed in patients with proteinuria is displayed in Figure 4.
• Recommendations
1. Whatever the cause of proteinuria, patient management must include the withdrawal of nephrotoxic drugs, the control of HBP and DM (if applicable) and, in any case, if the proteinuria is intense, treatment with renin-angiotensin-aldosterone system blockers (ACE inhibitors or ARBs). Recommendation based on consensus.
2. When proteinuria is >1g/24 hours or is accompanied by microhematuria or a GFR <60ml/min/1.73m2, the study should be extended in order to identify the cause. Recommendation based on consensus.
3. Antiproteinuric treatment will mainly be based (except in contraindications due to intolerance) on the blocking of the renin-angiotensin-aldosterone system, with ACE inhibitors, ARBs or aldosterone antagonist diuretics, gradually increasing the dose to achieve proteinuria and BP targets and frequent monitoring of renal function and serum potassium concentration. Quality of the evidence: High. Degree of recommendation: Strong.
4. In patients with proteinuria, the therapeutic target is a uPCR <0.5g/g (or 50mg/mmol) and in patients with a difficult response to the antiproteinuric measures, a uPCR <1g/g (or 100mg/mmol) or at least a reduction of the uPCR >50-75% of the baseline value. Quality of the evidence: Moderate. Degree of recommendation: Strong.
5. In patients with nephrotic syndrome, partial remission (uPCR below 3-3.5g/g or 3-3.5g/24 hours) may be considered to be a satisfactory target. Quality of the evidence: Moderate. Degree of recommendation: Weak.
6. When a HIVAN is suspected, it is recommended to introduce ART. If there is no improvement in renal abnormalities in a period of two or three months and/or other diagnoses are considered, a renal biopsy should be carried out. Quality of the evidence: High. Degree of recommendation: Strong.
6.C. Management of high blood pressure and other cardiovascular risk factors
HBP is frequent both in CKD patients and in HIV-infected patients. As such, blood pressure should be monitored regularly in HIV-infected patients, particularly if they also have CKD. BP control is a major therapeutic objective, since it can significantly decrease mortality and the number of events of cardiovascular origin and it can contribute to reducing proteinuria. Antihypertensive treatment will be adapted to the individual characteristics of each case, bearing in mind albuminuria/proteinuria presence, comorbidities, the effect on many metabolic parameters and concomitant drugs that could favour interactions. Renin-angiotensin-aldosterone system blockers will constitute the mainstay therapy, particularly in patients with albuminuria/proteinuria. Furthermore, due to their excellent tolerance, beneficial effect on the metabolic profile and the practical absence of interactions with ART, these drugs must be considered first-line therapy for HIV-infected patients.
•Recommendations in the management of high blood pressure
1. Non-drug measures in the treatment of HBP are the same as in the general population: salt restriction (<6g/day), control of overweight, physical exercise (at least walking at a brisk pace 30-45 minutes, four days a week). Quality of the evidence: High. Degree of recommendation: Weak.
2. The antihypertensive treatment target will depend on the presence or absence of albuminuria/proteinuria. Quality of the evidence: High. Degree of recommendation: Strong.
3.In patients with an uACR <30mg/g (or its equivalent, a uPCR <0.1g/g), the BP target will be <140/90mmHg. Quality of the evidence: High. Degree of recommendation: Strong.
4. In patients with an uACR >30mg/g (or its equivalent, a uPCR >0.1g/g), the BP target will be <130/80mmHg. Quality of the evidence: High. Degree of recommendation: Weak.
5. In the presence of albuminuria/proteinuria, antihypertensive treatment must begin with renin-angiotensin-aldosterone system blockers (ACE inhibitors or ARBs), when BP values indicate it. In patients with an uACR <30mg/g (or its equivalent, a uPCR <0.1g/g), there is no evidence that supports the use of any particular drug group. Quality of the evidence: High. Degree of recommendation: Strong.
6. Calcium channel blockers must be used with caution in patients who receive PIs (they increase their antihypertensive effect) and non-nucleoside reverse-transcriptase inhibitors (NNRTIs) (they decrease their antihypertensive effect), due to the possibility of drug interaction. If calcium channel blockers are required, raltegavir (RAL) or dolutegravir (DTG) based regimens are recommended. Recommendation based on consensus.
• Recommendations in the approach to other cardiovascular risk factors
1. The prevention and treatment of obesity and overweight is recommended, given their recognised association with the progression of renal failure. Quality of the evidence: Low. Degree of recommendation: Strong.
2. Quitting smoking must be a priority, since it is related to many complications and to the progression of renal failure. Quality of the evidence: Low. Degree of recommendation: Strong.
3. It is recommended to monitor the presence of metabolic acidosis and, if it is severe and progressive, introduce treatment with bicarbonate orally, as with non-infected patients. Quality of the evidence: Low. Recommendation based on consensus.
4. It is recommended to monitor serum uric acid concentration, indicating dietary measures in cases of moderate hyperuricemia and adding hypouricemic drugs in refractory cases or those with very high urate concentrations. Quality of the evidence: Low. Degree of recommendation: Weak.
6.D. Management of dyslipidemia patients with chronic kidney disease
HIV-infected patients very often have lipid metabolism disorders. In addition, CKD is associated with quantitative and qualitative abnormalities in the lipid pattern, which are accentuated as the disease becomes more severe. Dyslipidemia in CKD has similar characteristics to those of HIV-infected patients (increase in triglyceride concentration, decrease in high-density lipoprotein-associated cholesterol [HDL-c]) and as such, HIV-infected patients who develop CKD probably have more pronounced dyslipidemia than in the case of each of the diseases separately.
• Recommendations
1. In patients with CKD and HIV infection, a lipid study is recommended (which includes the measuring of serum cholesterol, triglycerides and HDL-c and LDL-c concentration) every six months. Recommendation based on consensus.
2. The control of dyslipidemia associated with renal disease is a therapeutic objective, given its proven protective effect on cardiovascular complications. The targets will be those set by the guidelines for patients with high cardiovascular risk. Quality of the evidence: High. Degree of recommendation: Strong.
3. Patients with CKD and HIV infection with a GFR <60ml/min/1.73m2 are considered to have very high cardiovascular risk, independently of their scores at traditional risk scales. The therapeutic objective is a LDL-c concentration <70mg/dl or a 50% reduction if this target cannot be achieved. The treatment will include a low-fat diet and drug treatment with statins. Quality of the evidence: Moderate. Degree of recommendation: Weak.
4. The choice of statins in CKD patients with HIV infection will depend on their metabolism (CYP3A4 CYP2C9), their interaction with each antiretroviral drug and the degree of renal failure and maximum dose of each statin. Pitavastatin, atorvastatin, rosuvastatin and pravastatin would be recommended, adjusted to the degree of renal failure. Recommendation based on consensus.
5. If hypertriglyceridemia is detected, lifestyle changes and drug treatment with fibrates are recommended if the triglyceride level is >800-1000mg/dl with the aim of preventing the onset of pancreatitis. If fibrates are used in combination with statins, fenofibrate must be used and must always be adjusted to the degree of renal failure, and the possibility of rhabdomyolysis and renal function deterioration, which is usually reversible, must be monitored. Quality of the evidence: Moderate. Degree of recommendation: Weak.
6. Combined treatment with statins and fibrates is not advised in renal transplantation (RT). Recommendation based on consensus.
6.E. Management of hyperglycemia in HIV-infected patients with chronic kidney disease
Type 2 DM is a growing problem in the HIV-infected population. Although HIV is not involved in the pathogenesis of DM, ART may influence the onset of DM through various mechanisms. The diagnosis of type 2 DM in HIV-infected patients will be based on the same criteria as in the general population. There are no randomised studies in the treatment of hyperglycemia in CKD patients with HIV infection. Consequently, the recommendation will be based o• Recommendations for CKD patients without HIV infection.
• Recommendations
1. The glycated hemoglobin A1c (HbA1c) target in CKD patients with HIV infection will be the same as in the general population. In advanced CKD patients (GFR <30ml/min/1.73m2) on dialysis, the HbA1c target will be <8%. Therapeutic strategies will be implemented in order to avoid episodes of hypoglycemia. Recommendation based on consensus.
2. The use of oral hypoglycemic agents (metformin, sulfonylureas and dipeptidyl peptidase-4 inhibitors) and insulin in CKD patients with HIV infection will be adjusted to the degree of renal function and will follow the same recommendations as for the population without HIV. The potential interactions between hypoglycemic agents and the antiretroviral drugs the patient is taking should be borne in mind. Recommendation based on consensus.
6.F. Management of mineral and bone metabolism disorders
Mineral and bone metabolism disorders in CKD is a complex set of pathologies involving disorders of calcium, phosphorus, parathyroid hormone (PTH), vitamin D and phosphaturic factors, causing abnormalities on skeleton remodelling, mineralisation, volume, growth and fragility and vascular/soft tissues calcification. Hyperparathyroidism secondary to CKD is not only associated with renal function deterioration, but in patients with HIV infection they are also involved in vitamin D deficiency, treatment with some antiretroviral drugs and renal loss of phosphate due to tubular toxicity. This situation of secondary hyperparathyroidism may exacerbate the risk of fractures and other bone complications present in HIV-infected patients.
There are few studies on mineral and bone metabolism disorders in HIV-infected patients with CKD. Consequently, most recommendations are established as opinions and are extrapolated from guidelines for dialysis patients without HIV infection, in whom there are different grades of evidence.
• Recommendations
1. In patients with a GFR lower than 60ml/min/1.73m2 and HIV infection, concentrations of serum calcium, phosphate and calcidiol (25-OH vitamin D) and plasma iPTH will be tested at least once a year. Quality of the evidence: Low. Degree of recommendation: Weak.
2. Treatment of mineral and bone metabolism disorders in CKD patients with HIV infection will be identical to that of patients not infected with HIV: maintaining serum phosphate concentration within the reference range, avoiding calcidiol (25-OH vitamin D) deficiency and correcting iPTH concentration if it is above the reference range. Recommendation based on consensus.
3. When serum iPTH concentration is above the recommended limit, the previously mentioned factors will be corrected and treatment with active vitamin D (calcitriol or selective vitamin D receptor activators [paricalcitol]) will be introduced, preferably the latter because they have a lower incidence of hypercalcemia, hyperphosphatemia and hypercalciuria. Recommendation based on consensus.
4. Hypophosphatemia must always be confirmed with more than one test. Recommendation based on consensus.
5. Other renal markers of proximal tubulopathy must be studied, in particular the presence of proteinuria and glucosuria and plasma concentrations of potassium, urate and bicarbonate. If there are no abnormalities in other tubular dysfunction markers, hormones regulating calcium and phosphorus metabolism must be analysed: iPTH and vitamin D in blood. Recommendation based on consensus.
6. Severe hypophosphatemia (<1mg/dl) requires immediate action, in some cases with the intravenous administration of phosphorus. Quality of the evidence: High. Degree of recommendation: Weak.
7. Mild or moderate hypophosphatemia may resolve with treatment of the aetiology (vitamin D in the case of vitamin D deficiency, surgical resection in primary hyperparathyroidism and withdrawal of the drug or toxin in cases of proximal tubular dysfunction secondary to nephrotoxic drugs). Quality of the evidence: Low. Degree of recommendation: Weak. In some cases, oral phosphate may be used. Recommendation based on consensus.
6.G. Management of anemia in chronic kidney disease patients
The prevalence of anemia and its intensity may be greater in HIV-infected patients with CKD than in those who are not infected. The direct effect of the virus on erythroid precursors, the presence of opportunistic infections and ART, among others, are factors that may favour anemia. In the absence of randomised studies on anemia in HIV-infected patients with CKD, we report the most up-to-date recommendations for anemia management in CKD.
• Recommendations
1. In HIV-infected patients with CKD, an anemia study will be carried out when hemoglobin concentration is <11g/dl in premenopausal women and prepubescent patients or <12g/dl in adult males and postmenopausal women. Recommendation based on consensus.
2. Ranges of Hb, ferritin and transferrin saturation index are the same as in patients without HIV infection. Recommendation based on consensus.
3. The objective of iron treatment is to achieve a TSI of 20% and a serum ferritin concentration of 100-500ng/ml in pre-dialysis patients or <800ng/ml in dialysis patients. If Hb concentration is lower than 11g/dl (or <10g/dl in diabetic patients), treatment with erythropoiesis-stimulating agents will be indicated, bearing in mind its risks and benefits. Quality of the evidence: Moderate. Degree of recommendation: Weak.
4. Treatment of anemia must include oral or intravenous iron and erythropoiesis-stimulating agents if the target hemoglobin concentrations are not achieved with iron alone. Quality of the evidence: Moderate. Degree of recommendation: Weak.
6.H. Management of coinfection with the hepatitis C virus in chronic kidney disease patients
Coinfection with HCV is common in HIV-infected patients. The risk of developing renal disease and its prognosis are worse in coinfected patients. It is crucial to address HCV coinfection in the early stages of CKD. The complications associated with HCV-induced end-stage liver disease are one of the main causes of morbidity and mortality in patients with HIV coinfection in the era of ART. This negative influence of HIV infection on the progression of hepatitis C is the main argument for recommending HCV treatment in coinfected patients.
There are no specific data on hepatitis C treatment in patients coinfected with HIV who also have renal failure. HCV treatment of coinfected patients on dialysis is not contraindicated; it must be extrapolated from the data obtained in monoinfected patients and it must be carried out by a multidisciplinary team. For more detailed recommendations on HCV treatment in renal failure patients coinfected with HIV, we recommend consulting the guidelines of the corresponding scientific societies.
• Recommendations
1. In patients who are coinfected with HCV, specific treatment will be indicated in accordance with the therapeutic guidelines. In renal diseases that are pathogenically related to HCV (particularly cryoglobulinemic membranoproliferative GN), the treatment of choice is eradication of HCV with the combinations of antiviral drugs that are currently available. Quality of the evidence: Moderate. Degree of recommendation: Strong.
2. In patients with renal complications due to cryoglobulinemia associated with HCV, in whom antiviral treatment against HCV is ineffective, there is little evidence of favourable effects of treatment with steroids, rituximab or plasmapheresis on renal function. Quality of the evidence: Very low. Degree of recommendation: Weak.
3. Treatment of HCV in patients coinfected with HIV must be carried out before RT. Recommendation based on consensus.
4. Given the absence of conclusive studies, HCV treatment regimens for dialysis patients with HIV coinfection will be based on data obtained in patients monoinfected with HCV. Recommendation based on consensus.
5. Interferon use is not advised due to the risk of acute rejection in RT, except in special circumstances. Quality of the evidence: High. Degree of recommendation: Strong.
6. In patients with a GFR lower than 50ml/min/1.73m2 ribavirin is contraindicated, although some studies have obtained good results when it is combined with interferon in low doses. Quality of the evidence: Low. Degree of recommendation: Weak.
7. For more detailed recommendations on HCV treatment in patients with renal impairment coinfected with HIV, we recommend consulting the guidelines of the corresponding scientific societies and updated data on the new antiviral drugs against HCV. Recommendation based on consensus.
6.I. Management of coinfection with the hepatitis B virus in chronic kidney disease patients
Coinfection with HBV is much less common than coinfection with HCV. Its prevalence in dialysis patients in Spain is 8.4%. In all HIV-infected patients coinfected with HBV (with detectable HBV DNA), ART must include drugs that act against HBV with the aim of making the plasma viral load undetectable. TDF has shown to be more effective in controlling viral replication and avoiding resistance development, but there are other suitable alternatives such as lamivudine (3TC), entecavir, adefovir or telbivudine. For more detailed recommendations on management of HBV coinfection we recommend the corresponding clinical guidelines.
• Recommendations
1. In all HIV-infected patients coinfected with HBV (with detectable HBV DNA), ART must include drugs that act against HBV with the aim of suppressing plasma viral load. Quality of the evidence: High. Degree of recommendation: Strong.
7. USE OF ANTIRETROVIRAL DRUGS IN HIV-INFECTED PATIENTS WITH RENAL FAILURE
In HIV-infected patients with CKD, any drug must be used cautiously, avoiding those nephrotoxic and, if a drug is used, observing dose adjustment requirements. In dialysis patients, the degree of drug elimination during dialysis must be known and a supplementary dose must be administered after each session if it is eliminated by dialysis.
There is little clinical evidence about what should be the antiviral regimen of choice and its adequate dosage in CKD patients. NRTIs are eliminated through the kidney, and it is therefore necessary to reduce the dose in patients with impaired renal function. An exception is ABC, whose urinary excretion is low, and as such, a dose adjustment is not necessary. NNRTIs, protease inhibitor, entry inhibitors and integrase inhibitors do not require a dose adjustment in patients with renal function deterioration. In these patients, fixed-dose drug combinations must be avoided due to drug dosage difficulties.
• Recommendations
1. All fixed-dose antiretroviral drug combinations are contraindicated (Atripla®, Eviplera®, Stribild® and Triumeq®) due to dosage difficulties of NRTIs in patients with a GFR <50ml/min. Quality of the evidence: High. Degree of recommendation: Strong.
2. A HLA B57-01 test must be carried out in all patients who are expected to receive ABC, in order to avoid the risk of hypersensitivity to the drug. Quality of the evidence: High. Degree of recommendation: Strong.
3. ART in CKD patients must follow the same recommendations as in patients without renal involvement, although it is not advised to administer the TDF/FTC/COBI/EVG coformulation (Stribild®) in patients with a GFR <70ml/min. If there are no contraindications, the combination of ABC (or TDF adjusted to the GFR as an alternative) plus 3TC (adjusted to the GFR) with an NNRTI, a PI boosted with RTV or an INSTI other than EVG may be used. Quality of the evidence: Moderate. Degree of recommendation: Strong.
4. If TDF or ABC cannot be administered, the combination of protease inhibitors boosted with RTV and 3TC (adjusted to the GFR) or RAL may be used, or in very selective cases, it may be simplified to a monotherapy: protease inhibitors boosted with RTV. Quality of the evidence: Low. Degree of recommendation: Strong.
8. MANAGEMENT OF HIV-INFECTED PATIENTS ON DIALYSIS
The prevalence of HIV infection in dialysis patients and the frequency of CKD in these patients are low (about 0.5%). In recent decades, the prognosis of these patients has improved notably, and current survival rates are similar to those of general population on dialysis. The management of HIV-infected patients who require renal replacement therapy is particularly complex and requires a multidisciplinary approach.
• Recommendations
1. Treatment with dialysis cannot be contraindicated due to HIV infection in any patient. Recommendation based on consensus.
2. In HIV-infected patients on dialysis, the universal prevention and disinfection measures must be strictly followed in both hemodialysis and peritoneal dialysis, and are the same as for patients not infected with HIV. Quality of the evidence: Moderate. Degree of recommendation: Strong.
3. There are no contraindications for these patients receiving dialysis in a general unit, once the recommended universal protective precautions are followed. Quality of the evidence: Moderate. Degree of recommendation: Weak.
4. There is no evidence for isolation in different rooms in the case of coinfection with HCV and HIV. Quality of the evidence: Moderate. Degree of recommendation: Weak.
5. In the case of coinfection with HBV, the patient should receive dialysis in a special HBV unit. Quality of the evidence: High. Degree of recommendation: Strong.
6. The method of dialysis in HIV-infected patients will be selected in line with the same recommendations as for the population without HIV infection. Patients will decide the method once they have been informed about the advantages and disadvantages of each one, provided that the nephrologist does not consider that a particular method is contraindicated. Recommendation based on consensus.
7. If the treatment chosen is hemodialysis, the first vascular access option must be arteriovenous fistula, the second option is polytetrafluoroethylene prosthesis and lastly, the tunnelled catheter. In peritoneal dialysis, the catheter will be inserted taking the universal precautions. Recommendation based on consensus.
8.In the case of peritoneal dialysis, it is suitable for the patient to carry it out and handle all the material themselves at home. It is recommended that after eliminating the dialysate in the lavatory, a disinfectant such as bleach be added, waiting 30 minutes before flushing to the general network. Likewise, the lines and bags of peritoneal fluid should be deposited in bins for contaminating material after use, which the patient may bring to the health centre for elimination. Recommendation based on consensus.
9. If there is accidental percutaneous exposure or exposure through mucous, prophylactic treatment will be introduced as soon as possible following exposure in accordance with the indications of the corresponding specialist. Recommendation based on consensus.
10.Patients with CKD and HIV infection must be vaccinated against hepatitis A and B if they are not immunised, preferably before the start of dialysis. Recommendation based on consensus.
9. REGIMENS FOR THE ANTIRETROVIRAL THERAPY OF CHOICE AND ITS DOSE ADJUSTMENT IN DIALYSIS
The ART regimens of choice in dialysis patients are the same as for CKD patients, but some considerations should be taken into account with regard to administration of the drugs in hemodialysis. The information on ART dosage and administration in peritoneal dialysis patients is very poor. NRTIs are eliminated through the kidneys and are therefore eliminated by dialysis, and as such, they must be administered after dialysis. NNRTIs do not require a dose adjustment, but in the specific case of NVP, an additional 200mg dose is indicated after each hemodialysis session. There is also a particular recommendation in the case of protease inhibitors: Atazanavir (ATV) administration must be boosted with RTV in dialysis patients, since they have low ATV concentrations.
The ideal ART regimen in naïve HIV-infected patients on dialysis must include ABC or TDF with 3TC/FTC, in combination with a third drug, which may be EFV, a protease inhibitor boosted with RTV or RAL/DTG. In patients who are on the waiting list and are close to receiving a renal transplant, with the aim of avoiding drug interactions with the immunosuppressants and renal toxicity of the graft, the combination of choice would be ABC with 3TC and RAL (or EFV as an alternative).
• Recommendations
1. The same recommendations as in the previous section (on the use of antiretroviral drugs in HIV-infected patients with renal failure).
2. In patients who are on the waiting list and are likely to receive soon a renal transplant, with the aim of avoiding drug interactions with immunosuppressants and renal graft toxicity, the combinations of choice would be ABC with 3TC (adjusted to the GFR) and RAL/DTG (or EFV as an alternative). Quality of the evidence: Low. Degree of recommendation: Strong.
10. CRITERIA FOR INCLUSION of HIV-INFECTED PATIENTS WITHOUT HEPATITIS C OR B VIRUSES COINFECTION IN THE RENAL TRANSPLANTATION WAITING LIST
HIV-infected patients on dialysis or in pre-dialysis must not be excluded a priori from receiving a RT. The criteria for RT in HIV-infected patients are the same as in the general population, but they must also meet criteria related to HIV: 1) clinical criteria: not have had any AIDS-defining event, with the exception of some opportunistic infections that are potentially preventable or treatable (tuberculosis, oesophageal candidiasis and pneumonia due to Pneumocystis jiroveci) and, in the case of tumours, there should be a five year disease-free period before transplantation; 2) immunological criteria: the CD4+ lymphocyte count must be greater than 200 cells/ml; 3) virological criteria: HIV RNA must be undetectable in plasma (<50 copies/ml) before transplantation and patients should have the opportunity to receive an ART regimen that is effective in the long-term in the post-transplantation period; 4) other criteria: all patients must have a positive psychiatric evaluation, a drug-free period of two years is recommended for heroin and cocaine and six months for other drugs, including alcohol. Lastly, patients must have an adequate degree of social stability.
• Recommendations
1. HIV-infected patients with CKD on dialysis or in pre-dialysis (GFR <20ml/min/1.73m2) who meet the general criteria for renal transplantation and the specific HIV infection criteria must be included on the renal transplantation waiting list. Quality of the evidence: High. Degree of recommendation: Strong.
11. CRITERIA FOR INCLUSION of HIV-INFECTED PATIENTS WITH HEPATITIS C OR B VIRUSES COINFECTION IN THE RENAL TRANSPLANTATION WAITING LIST
HBV and, in particular, HCV coinfections are very prevalent, since they share transmission routes with the HIV. Management of HBV virus infection is currently not a problem, given that there are different drugs that effectively slow down viral replication. HCV infection is a major problem, given the high prevalence, mainly after RT, when chronic HCV infection is associated with a greater risk of infections and is a risk factor for mortality and graft loss. Likewise, immunosuppressant treatment may reactivate the HCV infection and antiviral treatment with IFN is not advised after transplantation due to the high risk of triggering acute rejection with renal graft loss. HCV infection also behaves more aggressively in HIV-infected patients, which may be an additional risk in coinfected patients who receive transplants.
• Recommendations
1. In patients who meet the criteria of the previous section (with regard to recommendations on the criteria for inclusion on the renal transplantation waiting list in HCV or HBV coinfected patients) and who are coinfected with HCV and/or HBV, a complete evaluation of their liver disease (viral load, an ultrasound, a hepatic hemodynamic study and a transjugular liver biopsy) must be carried out. Quality of the evidence: High. Degree of recommendation: Strong.
2. In patients with HIV and HCV coinfection who are candidates for an isolated renal trasplant on its own, antiretroviral treatment must be assessed before performing transplantation. Quality of the evidence: High. Degree of recommendation: Strong.
3. Patients who are coinfected with HIV and HBV who are candidatesfor an isolated renal trasplant on its own must receive antiviral treatment before and after transplantation. Quality of the evidence: High. Degree of recommendation: Strong.
4. If the patient has advanced chronic liver disease, an isolated renal trasplant on its own is not advised and the possibility of combined liver and renal transplantation (LRT) will be evaluated, although the experience is very limited and its indication should be individualised in accordance with indications in the next point. Quality of the evidence: Moderate. Degree of recommendation: Weak.
12. CRITERIA FOR INCLUSION of HIV-INFECTED PATIENTS WITH END-STAGE LIVER DISEASE iN THE COMBINED LIVER AND RENAL TRANSPLANTATION WAITING LIST
The criteria that indicate a combined LRT have beeng discussed in patients who are not infected with HIV. Advanced liver disease is a contraindication for isolated renal transplant and chronic renal failure is a contraindication for isolated liver transplantation (LT) on its own. Combined LRT improves the survival of patients with the indication of LT when they are on dialysis or have advanced renal failure. However, it has not been demonstrated that this model benefits survival with respect to isolated LT on its own in patients with mild-moderate renal failure. The degree of interstitial fibrosis and of flomerulosclerosis in renal biopsy is very useful for establishing the irreversibility of renal failure and for indicating combined LRT or isolated LT on its own.
The current recommendations for indicating combined LRT in patients with ESLD and renal failure are based on populations without HIV infection. The experience of LRT in HIV-infected patients is very limited. The following indications for LRT are currently established: 1) end-stage liver disease and CKD on dialysis; 2) end-stage liver disease and acute renal failure (ARF) on dialysis for a minimum of 8 weeks; 3) end-stage liver disease and CKD with a GFR <30ml/min/1.73m2; 4) end-stage liver disease and ARF of unknown origin with a GFR <30ml/min/1.73m2 and fibrosis and/or glomerulosclerosis >30% in the biopsy.
• Recommendations
1. For combined LRT, it is necessary to assess HIV-infected patients with CKD and chronic liver disease who meet the general renal transplantation criteria, as well as criteria specific to HIV infection and those specific to LRT. Quality of the evidence: Low. Recommendation based on consensus.
2. The experience of combined LRT in patients with HIV infection is very limited, and as such its indication must be individualised. Quality of the evidence: Low. Recommendation based on consensus.
13. CRITERIA FOR INCLUSION of HIV-INFECTED PATIENTS WITH DIABETES MELLITUS IN THE COMBINED RENAL AND PANCREAS TRANSPLANTATION WAITING LIST
Combined renal and pancreas transplantation (RPT) is indicated in patients younger than 50 years of age, with type 1 diabetes mellitus and CKD (on dialysis or in predialysis) and without severe vascular disease. Pancreas transplantation may be carried out simultaneously or subsequently to renal transplantation. The results of RPT have been improving, with renal graft survival rates being comparable to those of renal transplants in patients without DM. Pancreas survival is better in simultaneous transplantation and very significant results have also been achieved. The main risk of RPT is surgical complications (thrombosis, fistulae, pancreatitis), infections and a higher risk of pancreatic and renal rejection. The experience of RPT in HIV-infected patients is very limited.
• Recommendations
1. For RPT, all patients with HIV infection, type 1 DM and advanced CKD who meet the general renal transplant criteria, specific HIV infection criteria and RPT criteria must be assessed. Quality of the evidence: Low. Recommendation based on consensus.
2. The experience of RPT in HIV-infected patients is very limited, there is a higher risk of infective or surgical complications, and its indication must be individualised. Quality of the evidence: Low. Recommendation based on consensus.
14. MANAGEMENT OF HIV-INFECTED PATIENTS RECIPIENTS OF A RENAL TRANSPLANT
Until a few years ago, HIV infection was an absolute contraindication for any type of transplantation. The fear of accelerating progression to AIDS and development of opportunistic infection, in addition to the poor prognosis of HIV infection, resulted in a high rate of rejection of this kind of therapy. In the last ten years, renal transplantation experience has notably increased in HIV-infected patients, and medium-term survival (patient and graft) is similar to medium-term survival in patients without HIV infection. In the post-transplant period, patients may have good virological and immunological control and it has not been demonstrated that immunosuppressant treatment leads to a greater progression to AIDS or a higher number of opportunistic infections or tumours related to AIDS. The complexity of patients with HIV who undergo renal transplantation requires the multidisciplinary collaboration of many specialists and involves a series of particular characteristics, both in the pre-transplantation and post-transplantation periods, which it is essential to understand and evaluate in order to obtain the best results.
• General recommendations
1. Renal transplantation should be considered as a valid treatment in adequately selected HIV-infected patients, since patient and graft survival is similar to that of patients without HIV infection and there is no evidence of a poor outcome of the HIV infection in the post-transplantation period. Quality of the evidence: High. Degree of recommendation: Strong.
2. It is recommended to create multidisciplinary teams in renal transplantation and HIV-infectious diseases for the clinical follow-up of these patients. These teams will together analyse the potential pharmacological interactions of antiretroviral or immunosuppressant drug dose changes, or any introduction or discontinuation of any of these drugs. Recommendation based on consensus.
14.A. Type of donor for renal transplantation in HIV-infected patients
HIV-infected patients on the transplantation waiting list may receive a renal graft from a cadaveric donor or a living donor who is seronegative for HIV. In previous studies, graft loss frequency was considerably lower when the organ was from a living donor. There is not sufficient information on safety and long-term efficacy, and as such, donor kidneys from HIV-infected patients currently must not be accepted.
• Recommendations
1. Kidney donor selection criteria for HIV-infected patients are similar to those of the general population (cadaveric donor or living donor). Quality of the evidence: Moderate. Degree of recommendation: Strong.
2. The use of renal grafts from HIV-infected donors is contraindicated. Quality of the evidence: Low. Degree of recommendation: Based on consensus.
14.B. Regimens for the antiretroviral therapy of choice in renal transplant recipients
It has not been established what the ideal ART regimen in renal transplant recipients should be, but the recommended treatment regimen should have the following characteristics: sufficient potency to maintain long-term suppression of a viral load (HIV RNA) and an adequate CD4+ lymphocyte count, few drug interactions with immunosuppressants that are metabolised by cytochrome p450 enzymes, low renal toxicity and an adequate cardiovascular safety profile with low likelihood of developing dyslipidemia or insulin resistance. The general recommendations for treating HIV infection in renal transplant recipients are almost the same as those in the general population with HIV infection, but some considerations must be done.
• Recommendations
1. HLA B57-01 typing should be carried out in transplant donors and recipients. If positive, treatment with ABC will be avoided in the organ recipient due to the risk of hypersensitivity to the drug. Quality of the evidence: Low. Degree of recommendation: Strong.
2. It is recommended to introduce ART as soon as possible after transplantation. Quality of the evidence: Low. Degree of recommendation: Based on consensus.
3. If there is no contraindication or risk of virologic failure, the antiretroviral regimen of choice in renal transplant recipients would include the combination of ABC (or TDF adjusted to the GFR as an alternative) plus 3TC (or FTC adjusted to the GFR) plus RAL/DTG (or EFV as an alternative). Quality of the evidence: Moderate. Degree of recommendation: Strong.
4. If NNRTIs must be used, it will be necessary to increase the dose of immunosuppressants, since they are cytochrome CYP450 inducers: cyclosporine, tacrolimus, sirolimus and everolimus. Quality of the evidence: High. Degree of recommendation: Strong.
5. If PIs/r must be used, it will be necessary to reduce the dose of immunosuppressants, since they are potent cytochrome P4503_A4 inhibitors: cyclosporine, tacrolimus, sirolimus and everolimus. Quality of the evidence: High. Degree of recommendation: Strong.
6. It is recommended to carry out more frequent viral load tests in the initial period after transplantation (3-6 months). Quality of the evidence: Low. Degree of recommendation: Based on consensus.
14.C. Pre-transplant vaccinations
Due to the increased risk of infections after transplantation, it is important to prevent against them as far as possible, with additional immunizations being recommended (in addition to the regular vaccination schedule immunisations).
• Recommendations
1. HIV-infected CKD patients who are candidates for receiving a renal transplant must be vaccinated against Haemophilus influenzae b, hepatitis B (in all patients without immunity), Streptococcus pneumoniae (in patients not vaccinated or those vaccinated more than three years ago), flu (yearly, at the start of Autumn), chicken pox in seronegative patients (delaying transplantation by one month) and hepatitis A. Recommendation based on consensus.
14.D. Regimens for the immunosuppressants of choice in renal transplant recipients
No specific immunosuppressant treatment regimens exist for patients with HIV infection and the immunosuppressant treatment regimens used in renal transplant recipients in the ART era are not significantly different to those used in patients without HIV infection. It is advised to create teams of specialists who will together assess the potential pharmacokinetic and clinical consequences of any modification of treatment, both in the field of antiviral efficacy and in the field of immunosuppression, due to the complexity of potential interactions between antiretroviral drugs and immunosuppressants.
• Recommendations
1. The immunosuppressant treatment of choice in HIV-infected patients are monoclonal anti-lymphocyte antibodies (basiliximab) as induction treatment and the combination of tacrolimus and mycophenolate mofetil (or mycophenolic acid) and corticosteroids as maintenance treatment. Quality of the evidence: Low. Degree of recommendation: Recommendation based on consensus.
2. Polyclonal anti-lymphocyte antibodies must be used with caution in low doses adjusted to CD3 levels, due to an increased risk of developing prolonged lymphocytopenia and infection. Likewise, they must be assessed individually in high immunological risk renal transplantation or in treatment of severe acute rejection or corticosteroid resistance. Quality of the evidence: Low. Recommendation based on consensus.
14.E. Drug interactions between antiretroviral drugs and immunosuppressants
Some antiretroviral drugs may inhibit or induce metabolism of some immunosuppressants due to interference of the CYP3A4 isoenzyme of the p450 enzyme system (involved in the metabolism of cyclosporine, tacrolimus, sirolimus or everolimus) and, as such, close monitoring of immunosuppressant treatment levels in renal transplantation patients with ART is essential.
• Recommendations
1. Protease inhibitors boosted with RTV are very potent enzyme inhibitors of cyclosporine, tacrolimus, sirolimus and everolimus metabolism, increasing significantly their plasma concentrations, and posing a high risk of nephrotoxiticy. A dose reduction in these immunosuppressant drugs is required. Quality of the evidence: High. Degree of recommendation: Strong.
2. NNRTIs are moderate enzyme inducers of cyclosporine, tacrolimus, sirolimus and everolimus metabolism, which significantly reduce their plasma concentrations, and as such, there is an increased risk of acute rejection. A dose increase in these immunosuppressant drugs is required. Quality of the evidence: High. Degree of recommendation: Strong.
3. If using NNRTIs or PIs/r, plasma levels of these immunosuppressant drugs must be monitored during treatment and if there is a change in dose or discontinuation of treatment. Quality of the evidence: Moderate. Degree of recommendation: Strong.
4. The websites available must be consulted for the potential drug interactions existing between immunosuppressant treatment, antiretroviral treatment and other drugs that are prescribed to transplant patients. Quality of the evidence: Low. Degree of recommendation: Weak.
14.F. Acute rejection of renal transplant in HIV-infected patients
A higher frequency of acute rejection was reported in renal transplantation among HIV-infected patients (30%-40%) in comparison with patients who were not infected by HIV (15%-20%). The exact mechanism is unknown, although different causes have been suggested (dysfunction of the immune system associated with HIV, inadequate immunosuppression due to drug interaction with antiretroviral drugs, racial factors, cyclosporine use and grafts from deceased donors).
• Recommendations
1. Renal transplantation in HIV-infected patients is associated with a greater risk of acute rejection. Quality of the evidence: Moderate. Degree of recommendation: Weak.
Acknowledgements
The boards of directors of GESIDA-SEIMC, SEQC and S.E.N. are grateful for the contributions and opinions of the members who contributed to improving the drafting and enriching of the content of the consensus document: Drs. Anunciación González y Ángel Chocarro (Hospital Virgen de la Concha, Zamora), Dr. Antonio Buño (Hospital Universitario La Paz, Madrid), Dr. Carmen Mar (Hospital de Galdakano, Vizcaya). We are in debt with Dra. Marina Pontello Cristelli (Hospital do Rim, Sao Paulo, Brasil) who have reviewed the English version of this guidelines.
Drs. José Luis Górriz, Alberto Martínez-Castelao, Manuel Praga, Carlos Quereda, Silvia Gràcia y Rosario Montañés are members of RETIC/REDinREN (RD12/0021/0019), ISCIII-Subdirectorate General of Evaluation and the European Regional Development Fund (FEDER). Drs Félix Gutiérrez, José R. Arribas, Pere Domingo, José A. Iribarren, José López-Aldeguer, Fernando Lozano, Esteban Martínez, Eugenia Negredo, María J. Pérez-Elías, Joaquín Portilla, Antonio Rivero, and José M.ª Miró are members of the AIDS Research Network (RIS) (ISCIII-RETIC RD12/0017) of the Ministry of Health and Consumption, Instituto de Salud Carlos III. Madrid.
Conflicts of interest
José Luis Górriz has received fees from Abbvie for meetings he attended with regard to the creation of a working group on renal disorders in HIV and he received fees for Abbvie, ViiV Healthcare, Bristol-Myers-Squibb and Merck conferences.
Félix Gutiérrez states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for the Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Janssen-Cilag and ViiV Healthcare, laboratories and received fees for carrying out educational presentations or speaking at scientific meetings organised by the Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Janssen-Cilag and ViiV Healthcare laboratories.
Joan Carles Trullas has received fees from Abbvie for participating in the working group on renal disorders in HIV.
Piedad Arazo states that she has not received any financial support or grant related to this document. In the past, she carried out consultancy work in the following laboratories: Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; she received payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; she received payment for writing manuscripts for Abbvie and Janssen laboratories, as well as payment for educational presentations for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
José R. Arribas has carried out consultancy work in the following laboratories: Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Tobira and ViiV Healthcare; he was given grants for clinical research by Janssen, MSD and Gilead; he received payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Guillermina Barril received fees from Abbvie for participating in the working group on renal disorders in HIV and received fees for consultancy and/or courses or conferences for Abbvie and Gilead Sciences,
Miguel Cervero received fees from Abbvie for participating in the working group on renal disorders in HIV, he carried out consultancy work in Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck and ViiV Healthcare, laboratories and received financial support for attending Gilead, Janssen-Cilag, Boehringer Ingelheim and Bristol-Myers Squibb conferences.
Frederic Cofán received fees from Abbvie for participating in the working group on renal disorders in HIV and received fees for consultancy and conferences from Fresenius, Novartis, Roche, Bristol-Myers-Squibb and Abbvie.
Pere Domingo received fees from Abbvie for the creation of a working group on renal disorders in HIV and he has carried out consultancy work in the following laboratories: Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck and ViiV Healthcare; he was given grants for clinical research by Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare and he received payment for talks for Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare.
Vicente Estrada states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for the Abbvie, Gilead Sciences, Janssen and MSD laboratories. He received grants for clinical research by Janssen, MSD and Abbvie and payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Xavier Fulladosa received fees from Abbvie for participating in the working group on renal disorders in HIV, carried out consultancy work in Gilead Sciences laboratory and received payment for Gilead Sciences conferences.
María J. Galindo states that she has not received any financial support or grant related to this document. In the past, she carried out consultancy work for the Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, and Merck laboratories; she was given grants for clinical research by Abbvie, Boehringer Ingelheim, Glaxo and Janssen; she received payment for talks for Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and Roche, and collaborated in the preparation of educational material for Janssen, Pfizer, ViiV, Glaxo and Abbvie.
Silvia Gràcia received fees from Abbvie for participating in the working group on renal disorders in HIV.
José Antonio Iribarren states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for the Gilead Sciences and Janssen-Cilag laboratories; he received clinical research grants from the following laboratories and organisations: Abbvie, Bristol-Myers Squibb, the Basque Government, FIPSE (Foundation for Research and Prevention of AIDS) and FISS (Health Research Fund), and financial support for attending Abbvie, Gilead, Janssen-Cilag and ViiV conferences, and he participated in educational activities, talks or symposia sponsored by Abbvie, Bristol-Myers Squibb, Gilead, Merck, Novartis Janssen, Pfizer and ViiV.
Hernando Knobel states that he has not received any financial support or grant related to this document. In the past, he received payment for consultancy work and talks for the following laboratories: Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
José López-Aldeguer states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for the following laboratories: Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare; he was given grants for clinical research by Bristol-Myers Squibb, Merck and ViiV Healthcare. and he received payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Fernando Lozano states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithkline, Janssen, Merck-Sharp & Dome, Pfizer, Roche Pharmaceuticals and ViiV Healthcare, and he received payment for talks for Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithkline, Jansen, Merck-Sharp & Dome, Pfizer, Roche Pharmaceuticals and ViiV Healthcare.
Alberto Martínez-Castelao received fees from Abbvie for the creation of a working group on renal disorders in HIV; he received fees for conferences for Abbvie, Amgen, Boehringer-Ingelhein). Esteve, Novartis and Roche, and he participated in consultancy work in laboratories of Abbvie, Amgen, Boehringer-Ingelheim, Esteve, Janssen-Cilag and Novartis.
Esteban Martínez states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec and ViiV Healthcare laboratories; he received payment for talks for Abbvie, Boehringer-Ingelhein). Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec and ViiV Healthcare, as well as payment for educational presentations for Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline and ViiV Healthcare.
María A. Mazuecos coordinated a research group in renal transplantation in HIV patients and received financial support for carrying out this research from Astellas Pharma.
Celia Miralles states that she has not received any financial support or grant related to this document. In the past, she carried out consultancy work for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare laboratories; she received payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; she received payment for writing manuscripts for Abbvie, Bristol-Myers Squibb, Gilead Sciences and ViiV Healthcare laboratories, as well as payment for educational presentations for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Rosario Montañés received fees from Abbvie for participating in the working group on renal disorders in HIV.
Eugenia Negredo states that she has not received any financial support or grant related to this document. In the past, she carried out consultancy work for Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Janssen, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme and ViiV Healthcare laboratories; she received payment for talks for Abbvie, Boehringer-Ingelheim, Roche, Janssen, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme and ViiV Healthcare laboratories, as well as payment for educational presentations for Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline and ViiV Healthcare.
Rosario Palacios states that she has not received any financial support or grant related to this document. In the past, she has carried out consultancy work in Boehringer Ingelheim laboratories and she received payment for talks for Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare and Roche.
María J. Pérez Elías states that she has not received any financial support or grant related to this document. In the past, she carried out consultancy work for Abbott Laboratories, Bristol-MyersSquibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare laboratories; she was given grants for clinical research by Abbott Laboratories, Gilead Sciences, ViiV Healthcare and Janssen laboratories; she received payment for talks for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare, as well as payment for educational presentations for Abbott Laboratories, Bristol-Myers Squibb, Janssen, Merck Sharp Dome and ViiV Healthcare.
Joaquín Portilla received fees from Abbvie for participating in the working group on renal disorders in HIV and he has carried out consultancy work in the following laboratories: Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; he was given grants for clinical research by Abbvies, Janssen, Merck and ViiV Healthcare and he received payment for talks for Abbvies, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Roche and ViiV Healthcare.
Manuel Praga received fees from Abbvie for participating in the working group on renal disorders in HIV and received financial support for research and fees for consultancy and conferences for Novartis, Astellas, Gambro, Fresenius, Alexion, Abbvie, Glaxo, Gilead, Roche, Merck, Janssen and Pfizer.
Carlos Quereda states that he has no conflicts of interest in this project.
Antonio Rivero has carried out consultancy work in Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare laboratories; he was given grants for clinical research by Abbvie, Gilead Sciences, Merck and ViiV Healthcare laboratories; he received payment for talks for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare, as well as payment for educational presentations for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Juan M. Santamaría received fees from Abbvie for participating in the working group on renal disorders in HIV.
Jesús Sanz states that he has not received any financial support or grant related to this document. In the past, he carried out consultancy work for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, ViiV Healthcare and Boehringer Ingelheim laboratories; he received payment for talks for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, ViiV Healthcare and Boehringer Ingelheim and received payment for educational presentations for ViiV Healthcare.
José Sanz states that he has not received any financial support or grant related to this document. In the past, he gave informative talks and carried out consultancy work, for which he received fees from ViiV Healthcare, Bristol-Myers Squibb, Gilead Sciences, Janssen, MSD, Boehringer-Ingelheim and Abbvie.
José M. Miró received fees from Abbvie for creating a working group on renal disorders in HIV and he has carried out consultancy work in Abbvie Laboratories, Bristol-Myers Squibb, Gilead Sciences, Merck, Novartis and Sanofi laboratories; he was given grants for clinical research by Cubist, Novartis, Merck, the Health Research Fund (FIS) of the Instituto de Salud Carlos III (Madrid), the Foundation for Research and Prevention of AIDS in Spain (FIPSE, Madrid), the Ministry of Health, Social Services and Equality (MSSSI, Madrid), National Institutes of Health (NIH, Bethesda, MA, USA) and NEAT and he received payment for talks for Novartis and ViiV Healthcare.
Finally, the logistics of the meetings attended by the members of this panel who participated in a working group on renal disorders in HIV was financed by Abbvie Spain laboratories.
Table 1. Basic and comprehensive renal studies that must be carried out in HIV-infected patients
Table 2. Regularity of renal assessment in HIV-infected patients
Figure 1. Algorithm for the initial renal evaluation of HIV-infected patients
Figure 2. Algorithm for the evaluation of renal function impairment in HIV-infected patients
Figure 3. Algorithm for the haematuria study in HIV-infected patients
Figure 4. Algorithm for evaluating proteinuria in HIV-infected patients