Hypertension (HT) represents a major public health problem affecting many individuals worldwide. It is well known to be an important risk factor for the development of cerebrovascular and cardiovascular diseases. Classifying hypertension as ‘primary’ or ‘secondary’ depends on the underlying mechanism. In 5 to 10% of hypertensive patients, HT develops ‘secondary’ to a separate mechanism that has been encountered with increasing frequency in the tertiary refferral centers. The frequent causes of secondary hypertension include renal parenchymal disease, renal artery stenosis, primary hyperaldosteronism, phaeochromocytoma and Cushing's syndrome. Polyarteritis nodosa (PAN) can involve any organ and in varying degrees. Here we present a young hypertensive patient diagnosed as PAN with the angiographic findings of multiple microaneurysms involving celiac, renal and superior mesenteric arteries and associated with a rarely seen neurological entity-PRES syndrome.
La hipertensión es uno de los principales problemas de salud pública, que afecta a muchas personas en todo el mundo. Se sabe que es un importante factor de riesgo para el desarrollo de enfermedades cerebrovasculares y cardiovasculares. Su clasificación como “primaria” o “secundaria” depende del proceso subyacente. En el 5-10% de los pacientes hipertensos, se trata de un problema “secundario” a otro proceso de creciente frecuencia en los centros de atención terciaria. Las causas más frecuentes de la hipertensión secundaria son: enfermedades del parénquima renal, estenosis de la arteria renal, hiperaldosteronismo primario, feocromocitoma y el síndrome de Cushing. La poliarteritis nodosa puede afectar a cualquier órgano y en diferentes grados. A continuación presentamos a un paciente joven hipertenso al que se le ha diagnosticado poliarteritis nodosa, cuya angiografía muestra múltiples microaneurismas que afectan al tronco celíaco, a la arteria renal y a la arteria mesentérica superior, asociada a un síndrome de encefalopatía posterior reversible de entidad neurológica poco visto.
INTRODUCTION
PAN is a necrotizing vasculitis involving medium and small arteries of almost any organ with an unknown cause. The characteristic pathologic findings are fibrinoid necrotizing inflammatory foci in the walls of arteries with multiple small aneurysms. Organ involvement in PAN is usually represented by HT, renal impairment, peripheral neuropathy, abdominal pain and involvement of the musculoskeletal system. The kidney is the most frequently affected organ. The finding of multiple aneurysmal dilatations up to 1cm in size on visceral angiography is considered to be pathognomonic, even in the absence of histological evidence of the disease.1 Diagnosis should be done according to the clinical, laboratory and imaging findings and biopsy of affected organs should be performed whenever possible for definitive diagnosis.
CASE
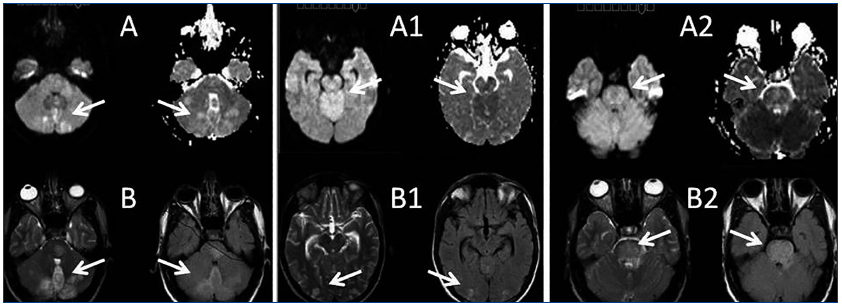
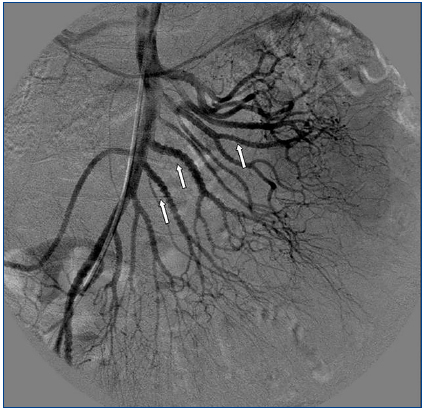
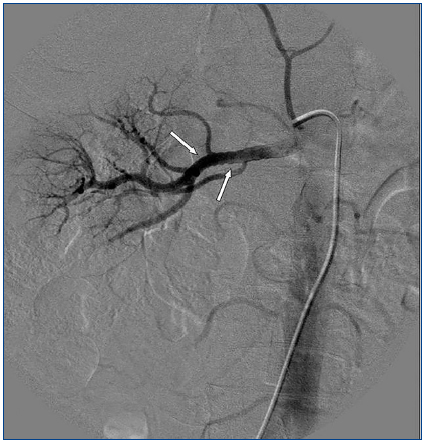
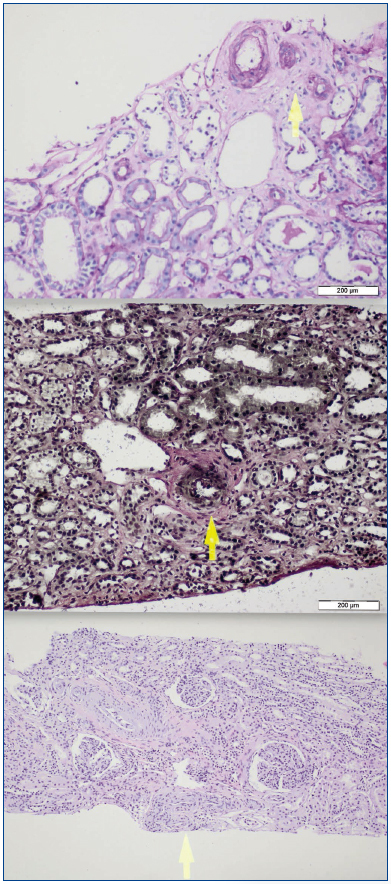
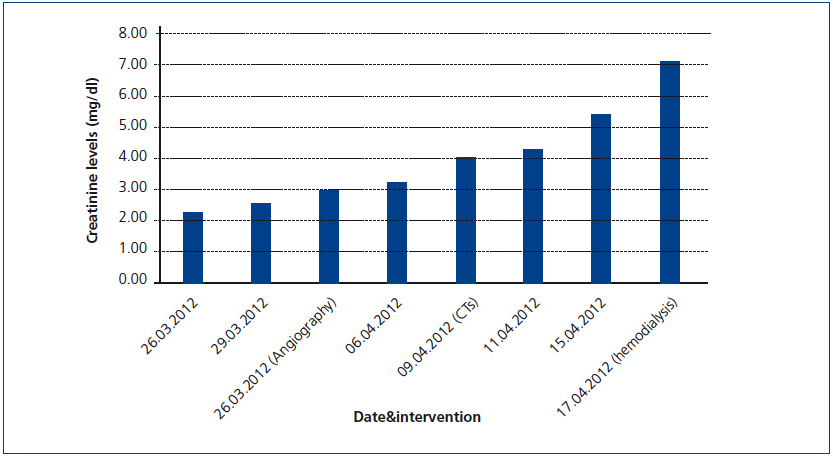
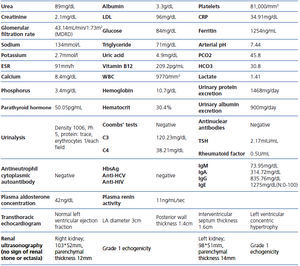
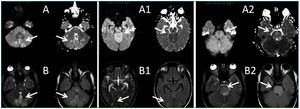
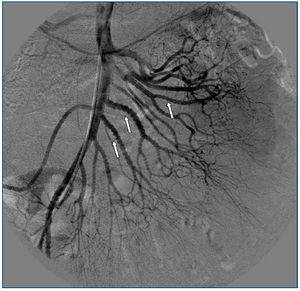
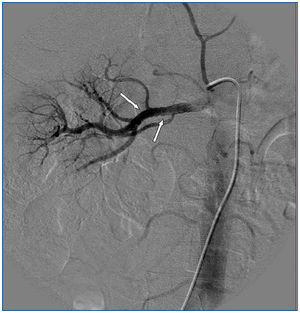
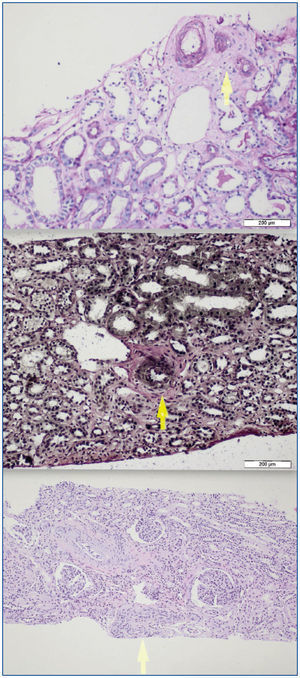
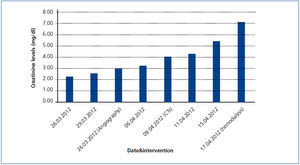
A 26-year-old man presented with a few weeks history of nausea, malaise, blurred vision, a brief episode of visual loss and hypertension. While working, he experienced loss of sight but regained it after a couple of minutes. He had no history of chronical disease. Family history of patient was not contributory-without a family history of vascular disease or hypertension. He was not taking any medications and denied any heavy alcohol, cocaine or intravenous drugs use, but had a 10-pack-year tobacco history. On review of systems, he reported 6-7 kilograms of unintentional weight loss in the past one year. Blood pressure was 150/70mm/Hg, a temperature of 36.8oC, heart rate of 64/min, and respiratory rate of 16/min. Cardiovascular and respiratory system examination was not remarkable. Indirect ophthalmoscopy was performed; retinal detachment (exudative) in both eyes, raised papilla limits, retinal vessel tortuosity, choroidal hypopigmented lesions under retina and superficial haemorrhages were detected. Exudative retinal findings defined as Vogt-Koyanagi-Harada syndrome. Laboratory and ultrasonographic findings were shown at Table 1. Peripheral blood smear revealed; enough platelets with normal morphology, schistocytes were rare, reticulocytes <2%, normochromic normocytic red blood cells. Primary hematological disease was not considered. He was started to be investigated for primary hyperaldosteronism, Cushing's disease and pheochromocytoma. During the follow up in nephrology service, the patient had presyncope, convulsion, hypertensive emergency and altered mental function. His blood pressure was 220/110mm/Hg. Blood pressure was controlled via sodium nitroprusside infusion for 48 hours and amlodipine 10mg, nebivolol 5mg, doxazosin mesylate 8mg at following days in intensive care unit. After the neurology consultation was performed the patient was evaluated with magnetic resonance imaging (MRI). Bulbus, pons and left mesencephalon, basal ganglia, peri-supraventricular white matter areas of increased T2 signal is considered to be consistent with vasogenic edema. Subretinal haemorrhages were detected at posterior parts of both orbit. It was reported as posterior reversible encephalopathy syndrome (Figure 1). After 5 days of intensive care follow up the patient had normal blood pressure. After excluding frequently seen secondary hypertension causes, renal and mesenteric angiography was performed at the same time suspecting renovascular hypertension. Angiography revealed multiple microaneurysms involving the parenchymal branches of the celiac artery, bilateral renal artery and superior mesenteric artery (Figure 2 and Figure 3). After the angiography, eventually, ultrasound-guided renal biopsy was performed. Light microscopy revealed 9 glomeruli with no specific patterns except a slight increase in mesangial matrix. Normal interstisium, tubular focal atrophy and intimal-medial thickening of blood vessels was apparent. Due to increased cellularity obstruction and perivascular fibrosis were found in blood vessels. Histochemically, periodic acid-Schiff, M. Silver, Masson’s Trichrome staining was used. Congo red staining was negative. With direct immunofluorescence method, there was no specific deposition of IgA, IgG, IgM, C3, C4, C1q, fibrinogen, kappa and lambda. Histological diagnosis was reported as signs of vascular myointimal hyperplasia (Figure 4). With all these findings (clinical characteristics such as severe hypertension and renal insuficiency, constitutional symptoms such as malaise and weight loss, inflammatory parameters like elevated erythrocyte sedimentation rate-ESR and C-reactive protein-CRP, angiographic findings) the patient was diagnosed as PAN. Oral prednisolone therapy as 0.5mg/kg/day had been given. But as creatinine levels raised up and hypertension could not be well controlled with drug therapies, eventually hemodialysis was started (Figure 5).
Combining with his clinical presentation of mesenteric and renal arterial angiographic findings, and hypertensive emergency with renal involvement, the diagnosis of PAN was established. In this patient, abdominal visceral and renal arteriography was performed because of concern for systemic vasculitis or renovascular hypertension, just before the renal biopsy because of the possible diagnosis of PAN. Now he continues to do well under hemodialysis treatment thrice-a week.
DISCUSSION
Secondary hypertension is frequently associated to vascular and parenchymal lesions of the kidney. Vasculitis can affect blood vessels of all sizes as large vessels. The clinical presentation of disease depends on the size and location of the affected vessel.2
PAN is characterized by focal, segmental lesions of the small and medium-sized arteries, beginning in the adventitia or media and later involving the intima. It is a panmural, progressive, inflammatory necrotizing vasculitis. The vascular lesions may be seen in different stages of development and have a predilection for branch points of vessels.3 The frequency of organ involvement is given as follows: kidney 85%; heart 76%; gastrointestinal tract 51%; skeletal muscle 39%; peripheral nerves 27%; mesenteric arteries 25%; skin 20%.4 Approximately 30-40% cases have been related with hepatitis B virus infection.5 It generally affects people in their fourth and fifth decades of life; the male-to-female ratio is 2-3:1. There is no specific laboratory test for the diagnosis of PAN. The most frequent clinical and biochemical findings are irregular fever, abdominal pain, edema, hypertension, loss of weight, leucocytosis, anemia, albuminuria, thrombocytosis, elevation in CRP and erythrocyte sedimentation rate. Blood urea nitrogen (BUN) and creatinine increase as renal involvement progresses. Three of the 10 diagnostic criteria that have been classified by the American College of Rheumatology (ACR) must be present for the diagnosis of PAN. A positive angiogram with typical findings is one of the 10 criteria. The presence of any three or more criteria yields a sensitivity of 82.2% and a specificity of 86.6%.6 Our patient fullfilled four of ten classification criteria for PAN (weigt loss, HT, the visceral microaneurysms and the elevated serum creatinine).
Renal involvement is the most important visceral finding. Renal PAN may manifest as acute or chronic renal failure, nephritic syndrome or perirenal hemorrhage.7 With renal complications, HT often develops rapidly. Approximately 30-40% of the patients may have HT.8 PAN does not usually affect arterioles, venules, capillaries and not associated with glomerulonephritis, differentiating it from microscopic polyangiitis.9 The arteritis is frequently seen in PAN and primarily involves the arcuate vessels and consists of fibrinoid necrosis and vascular thrombosis. The aneurysm, forming as a result of focal necrosis of the vessel wall, may undergo necrosis and healing with fibrosis, but occasionally will rupture and produce perirenal and retroperitoneal hemorrhage.10
PAN apparently has two effects on the eye; typical thickening of the wall and diminution of the lumina of the arteries of the various ocular coats and as retinal edema, transudates, hemorrhages and cytoid body formation; these changes are probably effected through renal alteration leading to HT.3 Our patient also had some visual complaints at initial presentation.
Imaging studies in PAN include evaluation of vasculature with MRA/MRI and/or angiographies showing tortuous patterns and microaneurysms.11 As Miller stated, biopsies of symptomatic sites may have the greatest diagnostic yield, however, confirmation of the specific vascular lesion by biopsy is not always successful. Biopsy results may only provide information about the small arteries, thereby missing the pathologically involved medium sized vessels. Multiple aneurysm formation is a characteristic finding in PAN (50-60% of cases), also may be seen in patients with neurofibromatosis or they may be congenital. In both of these conditions aneurysms occur on the main renal arteries and are extraparencyhmal. Mycotic aneurysms have different clinical and bacteriologic findings, so aneurysm of the main renal artery only is most likely of congenital or atherosclerotic etiology. Congenital aneurysms occur usually at bifurcations, are frequently multiple and do not involve the intraparenchymal arteries. The diagnosis of PAN is suggested by an angiographic finding of aneurysms up to 1cm in diameter within the renal, mesenteric, and hepatic vasculature; however, it is also seen in necrotizing angiitis associated with drug abuse and other vasculitides such as Wegener granulomatosis and systemic lupus erythematosus. The diagnosis is ideally made by means of biopsy of involved tissue in a patient with the appropriate clinical symptoms and laboratory data but an angiogram provides the proof in some cases. According to Hekali et al. who analyzed 17 patients with PAN and 21 with many other disorders showing multiple aneurysms, angiography had a sensitivity of 89% and a specificity of 90% for the diagnosis.12 Although negative findings on angiography do not exclude the presence of PAN, about 80% of all patients with PAN may be diagnosed angiographically.13 On angiography, typical aneurysms in patients with PAN are 2-5mm and are multiple (>10), affecting the branch points of arteries. The presence of numerous aneurysms (>30) is itself almost diagnostic.12 Without angiography, the correct diagnosis is established only by obtaining a biopsy of subcutaneous nodules or skeletal muscle; however, the diagnostic success rate of the biopsy may not be satisfying always.14 Therefore, a negative biopsy does not rule out the condition. Predominantly the visceral arteries are involved but also arteries of the extremities and small branches of the aorta may be affected.15
Currently, the recommended treatment for PAN is prednisone 0,5-1mg/kg/day(40 to 60mg/day) depending on the severity for 4 weeks with subsequent taper over 9 months. This approach achieves remission in 50% of cases, and the rest may require the addition of oral cyclophosphamide.
Malignant HT is defined as severe hypertension in association with retinal hemorrhages and/or exudates with or without papilloedema. Hypertensive encephalopathy has been described in the literature as a presenting feature of PAN.16,17 Central nervous system (CNS) signs/symptoms develop in about 20 to 45% of patients, typically in the form of cerebral infarction, which may cause hemiplegia and disturbance of consciousness.18 The most common reported CNS manifestation is diffuse encephalopathy, followed in frequency by focal deficits and seizures.19 Posterior reversible encephalopathy syndrome (PRES) is a clinical entity characterized with headache, nausea, vomiting, seizures, consciousness disturbance, and frequently visual disorders associated with neuroradiological findings, predominantly white matter abnormalities of the parieto-occipital lobes. PAN-associated PRES has been rarely reported.20 Stanzani et al. reported a case of a female patient affected by PAN, presenting with seizures, during an acute hypertensive state and MRI imaging showed increased T2 and FLAIR signal in posterior regions. Papanikolaou reported a case of a female patient with acute renal failure due to PAN. The authors stated that the patient’s clinical course was complicated by an unusual form of hypertensive encephalopathy (as reversible posterior leukoencephalopathy syndrome: RPLS) and cardiogenic shock.21
Here, we report a case of a male patient with PRES in PAN with the involvement of parietal and occipital cortical areas during an acute hypertensive state. The differential diagnosis indicated malignant arterial hypertension with severe retinopathy, hypertensive encephalopathy and renal insufficiency which contained sclerodermal renal crisis, hemolytic uremic sydrome, polyarteritis and systemic fibromuscular dysplasia. Since, PRES was reported as the most common brain imaging abnormality in severe manifestations of thrombotic thrombocytopenic purpura, TTP might also be considered in the differential diagnosis. A hemolytic-uremic syndrome (HUS) can sometimes be presented with malignant hypertension.22 However, the absence of microangiopathic hemolytic anemia and the renal histology prompted us to exclude HUS. The absence of sclerodermal cutaneous lesions disagrees this hypothesis, but it can’t be completely overlooked since the renal involvement may be incipient. Neverthless, in this patient such a diagnosis is less likely on account of his gender, the seronegative immunological tests and the absence of distinctive renal histological signs such as marked adventitial fibrosis, arterial and arteriolar lesions. Also, normal renal doppler ultrasonography and the values for plasma renin activity excluded the presence of renovascular HT.
Secondary causes are more common in malignant hypertension. Renal arteriography magnetic resonans angiography should be considered to exclude renal artery stenosis in patient with the evidence of vascular disease or in young female patients who might have fibromuscular dysplasia. Classic PAN can originate from severe ischemic nephropathy with malignant hypertension secondary to renal arteritis. The specificity of the characteristic angiographic features was found to be 95%.6 Fibromuscular displasia (FMD) is a non-inflammatory and non-atherosclerotic abnormality which like PAN affects small and medium-sized arteries and whose pathogenesis is unclear. FMD is a well-known cause of HT in young Caucasian females usually under the age of 35 years when the renal artery is involved. Other vascular structures may also be involved in 28% of the patients with FMD.23 Severe HT and cerebrovascular symptoms are the most common problems. The diagnosis is mainly based on the characteristic angiographic appearance of the ‘string of beads’.24 PAN and FMD can both be diagnosed by angiography without histopathological confirmation. This established diagnostic criterion raises the question that the arteriographic profiles of those diseases are truly neither patognomonic neither characteristic. Finding a visceral aneurysm or angiographic appearance of the ‘string of beads’ can cause confusion between both diseases.25 It should be kept in mind that FMD may present as systemic vascular disease mimicking PAN and being called pseudovasculitis. Visceral angiography is an important diagnostic tool, but it lacks specificity. Characteristically, the biological signs of inflammation is absent in FMD except in cases of associated infarction. On the other hand, the biological signs of inflammation are absent in about one-third of cases of vasculitis.26 The diagnosis of vasculitis is usually based on the recognition of characteristic clinical pictures, such as fever, night sweats, malaise, weight loss, arthralgia and myalgia. It is also important to realize the limitations of angiography in the diagnosis of both vasculitis and FMD. The final diagnosis was renal insufficiency possibly due to PAN with putative but not proven with renal biopsy. The presence of vaso-occlusive conditions in different arterial beds should draw our attention not only to true vasculitis, but also to other non-inflammatory conditions that mimic vasculites.
PRES is a specific form of hypertensive encephalopathy that can be associated with vasculitis and is characterized by transient clinical and neuroradiological findings mainly located in the parietal-occipital areas.27
Acknowledgement
We are grateful to Dr. Nefati Kıylıoğlu for his support.
Conflict of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
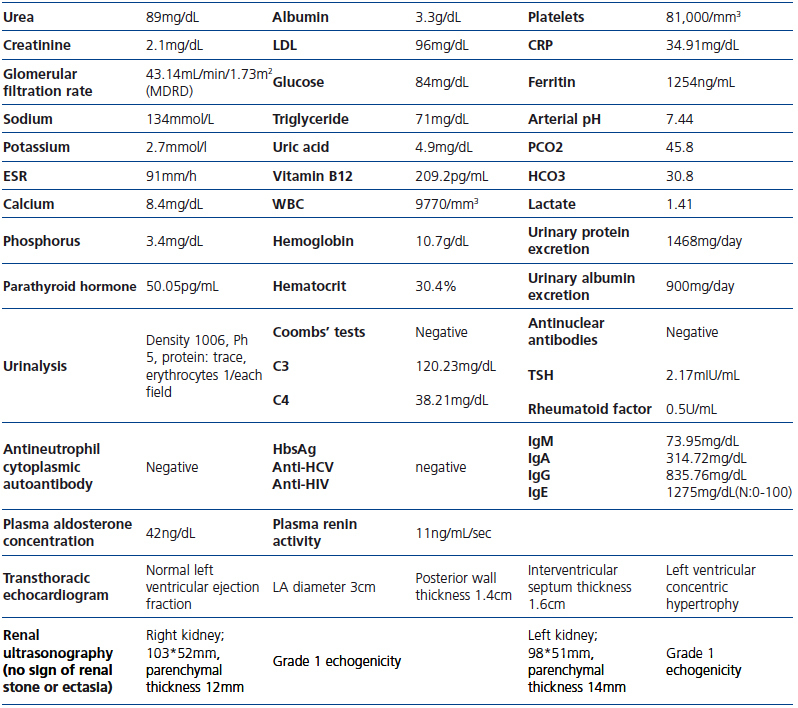
Table 1.
Figure 1.
Figure 2. Multiple microaneurysms involving the parenchymal branches of the celiac artery, bilateral renal artery and superior mesenteric artery
Figure 3. Multiple microaneurysms involving the parenchymal branches of the celiac artery, bilateral renal artery and superior mesenteric artery
Figure 4.
Figure 5. Increase in serum creatinine levels during the follow-up of the patient