Background: In many centers patients are hospitalised to perform a renal allograft biopsy. Aim: To evaluate the safety and efficacy of outpatient renal allograft biopsies. Methods: Since December 2011 we perform renal allograft biopsies as an outpatient procedure. Exclusion criteria for performing an outpatient biopsy included: 1.) anticoagulant treatment, 2.) thrombocytopenia <50,000/mm3, 3.) body mass index >35kg/m2 and 4.) uncontrolled hypertension. The number and severity of complications were compared with an historical cohort of 124 biopsies done between 2007 and 2011 when all patients were hospitalised for the procedure and with 42 patients biopsied during hospitalisation between 2011 and 2013. Results: Between 2011 and 2013, 210 (95%) out of 230 biopsies indicated in the outclinic were performed as an outpatient procedure (95%). The incidence of major complications (bleeding requiring blood transfusion and/or embolisation) was 0.8% between 2007 and 2011 and 2.4% in biopsies between 2011 and 2013 in hospitalised patients (p=0.475). No major complications were observed in the outpatient biopsy group. Minor complications (hematuria, hematoma or fistula not requiring transfusion or embolisation) were also not different between groups (3.2%, 7.1% and 2.7%; respectively). Sample size adequacy according to the Banff criteria was not different among groups (p=0.052). Conclusion: Ambulatory renal allograft biopsy is a safe and efficient procedure.
Antecedentes: La biopsia del aloinjerto renal se realiza habitualmente con el paciente hospitalizado. Objetivo: Evaluar la seguridad y eficacia de un programa de biopsias ambulatorio en receptores de trasplante renal. Métodos: En diciembre de 2011 se inició un programa ambulatorio de biopsias en trasplante renal. Se contraindica la biopsia ambulatoria en los casos siguientes: 1) tratamiento anticoagulante, 2) trombocitopenia < 50 000/mm3, 3) índice de masa corporal > 35 kg/m2, 4) hipertensión arterial no controlada. Se compara la seguridad y eficacia de las biopsias realizadas bajo hospitalización en el período 2007-2011 (n = 124) con las biopsias ambulatorias realizadas durante el período 2011-2013 (n = 219) y las realizadas en este mismo período bajo hospitalización (n = 42). Resultados: Entre diciembre de 2011 y diciembre de 2013 se han indicado 230 biopsias desde la consulta externa y se han realizado 219 (95 %) en régimen ambulatorio. La incidencia de complicaciones mayores (necesidad de transfusión y/o embolización) ha sido de 0,8 % para el período 2007-2011 y de 2,4 % para las realizadas bajo hospitalización del período 2011-2013 (p = 0,475). No se observaron complicaciones mayores en el grupo de pacientes con biopsias realizadas de forma ambulatoria. La tasa de complicaciones menores (hematuria macroscópica, hematoma o fístula que no requirieron transfusión ni embolización) no ha sido distinta entre los grupos (3,2 %, 7,1 % y 2,7 %, respectivamente). La adecuación de la muestra obtenida según los criterios de Banff no ha sido distinta entre los grupos (p = 0,052). Conclusión: La realización ambulatoria de la biopsia de injerto renal es un procedimiento seguro y eficaz.
INTRODUCTION
Over the past decade, the need to meet a growing surgical waiting list for low or medium complexity processes has encouraged the development of outpatient intervention programmes, complying with efficiency and safety conditions, with a high degree of patient and professional satisfaction1.
Percutaneous renal biopsy is a diagnostic procedure used since 1944 and a fundamental tool for the knowledge of renal pathology and to guide decision making in clinical practice2,3. Traditionally, this procedure required patient hospitalisation. However, over the last two decades the performance of automatic needle biopsy guided by ultrasound has reduced procedure related serious complications (death and/or graft loss)4,5. These changes have favoured ambulatory biopsy of native kidneys and renal allografts, making it a safe procedure in a selected population of patients6,7.
The implementation of a programme of outpatient biopsies improves patients’ quality of life by minimizing their hospital stay. In addition, it results in a significant decrease in the cost of the procedure and a shorter waiting list1.
For these reasons, since December 2011 we have implemented a renal transplant outpatient biopsies programme in our service. The aim of our study is to evaluate the safety and efficacy of the procedure, comparing the incidence and severity of complications with a historical cohort in which the procedure was performed with the patient hospitalised.
PATIENTS AND METHOD
This is a cohort study in which the incidence and severity of complications associated with kidney biopsy performed on an inpatient basis during the period January 2007-November 2011 and December 2011-December 2013 are compared with those performed on an outpatient basis during the period December 2011-December 2013.
Biopsy procedure
Outpatient renal biopsy is indicated in the Outpatient Consulting Offices. Its performance is contraindicated under the following circumstances: 1) anticoagulant warfarin treatment, 2) thrombocytopenia <50 000/mm3, 3) obesity with BMI> 35 kg/m2, 4) hypertension uncontrolled with medical treatment. In patients receiving antiplatelet medication, this was discontinued a week before and reintroduced after 72 hours if there were no complications. The patient was admitted on the same day of the procedure to the haemodialysis unit. After obtaining informed consent, a peripheral vein was channelled and samples were obtained for a complete blood count with platelet count, determination of coagulation parameters and blood bank reserves. Patients who had to be hospitalised due to contraindications for performing an outpatient biopsy were admitted the day before the procedure and discharged after 24 hours of observation. If the patient continued treatment with oral anticoagulants, these were discontinued a week before and low molecular weight heparin was given, which was discontinued 24 hours before biopsy.
In patients with a glomerular filtration rate <30ml/min/1.73 m2 estimated by CKD-EPI formula (Chronic Kidney Disease Epidemiology Collaboration) and/or who have a known coagulation disorder, desmopressin was administered at 0.3mg/kg one hour before the procedure. In addition, all patients received 5mg diazepam orally 30 minutes before the procedure and atropine 0.5mg subcutaneously at the time of the biopsy.
Renal biopsy was performed by the radiologist using an automatic 16-gauge needle (BARD® MONOPTY®) under real-time ultrasound guidance and routinely two cores are obtained for study by light and immunofluorescence microscopy. In addition, in all follow-up biopsies a third core for molecular biological studies was obtained. In a group of 42 biopsies performed during the period 2007-2011, an additional core for molecular biology8 was also obtained. After the procedure, the patient remained at rest for 6 hours while diuresis and blood pressure were checked, and a control blood count was performed after 4 hours. If no immediate complications were detected, the patient is discharged, with a recommendation of relative rest for 72 hours.
If immediate complications are observed such as subcapsular haematoma with no active bleeding during post-biopsy ultrasound and/or there is post-puncture renal macroscopic haematuria, observation time is extended or hospitalisation is indicated according to severity of symptoms.
Assessment of complications
After biopsy the following complications were evaluated: a) major complications: gross haematuria, haematoma and/or arterio-venous fistula, requiring blood transfusion or interventional treatment; b) minor complications: self-limited gross haematuria and/or subcapsular haematoma without haemodynamic complications.
Statistical analysis
Results are expressed as frequencies for qualitative variables and as mean ± standard deviation for continuous variables. Comparison between groups is performed with the Χ2 test for qualitative variables and analysis of variance for comparison of means with the Scheffe post hoc test for individual comparisons between groups. All analyses are two sided and a P value of <.05 is considered to be significant.
RESULTS
Patients
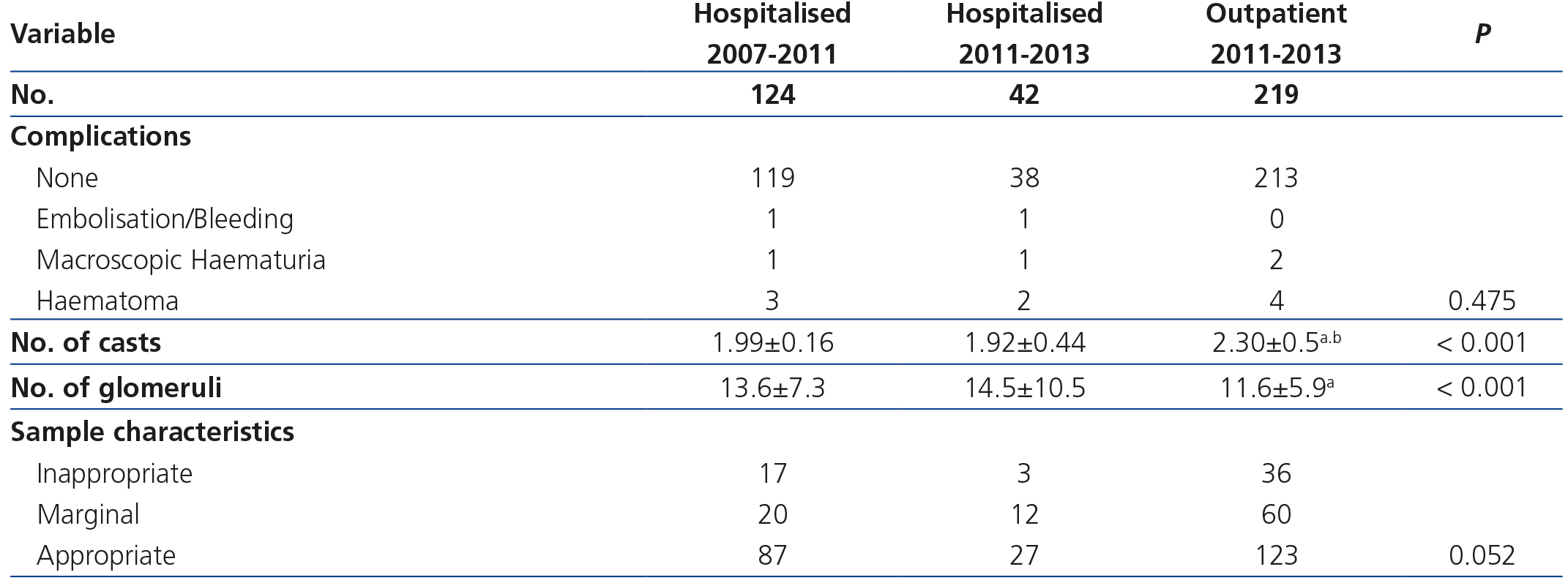
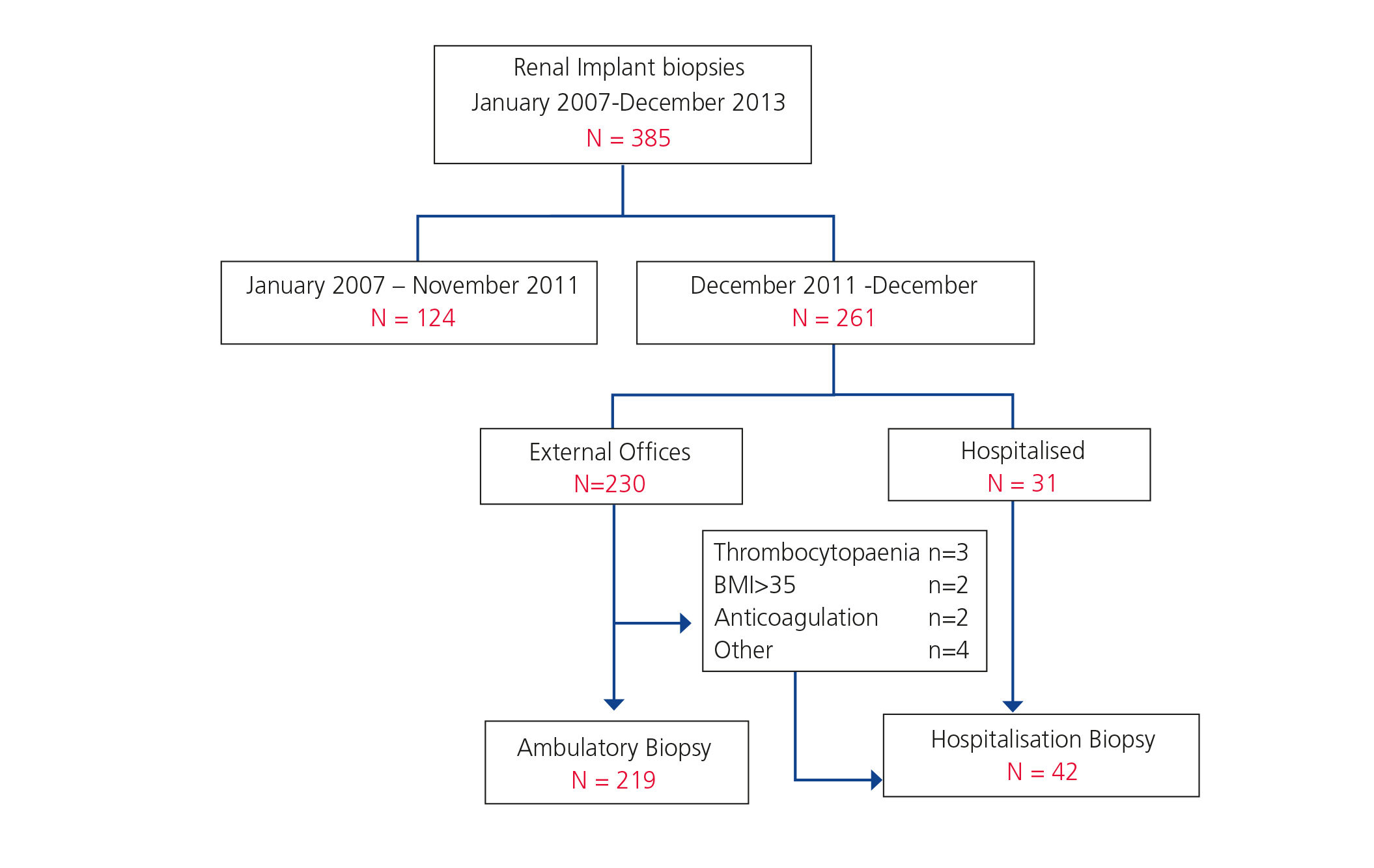
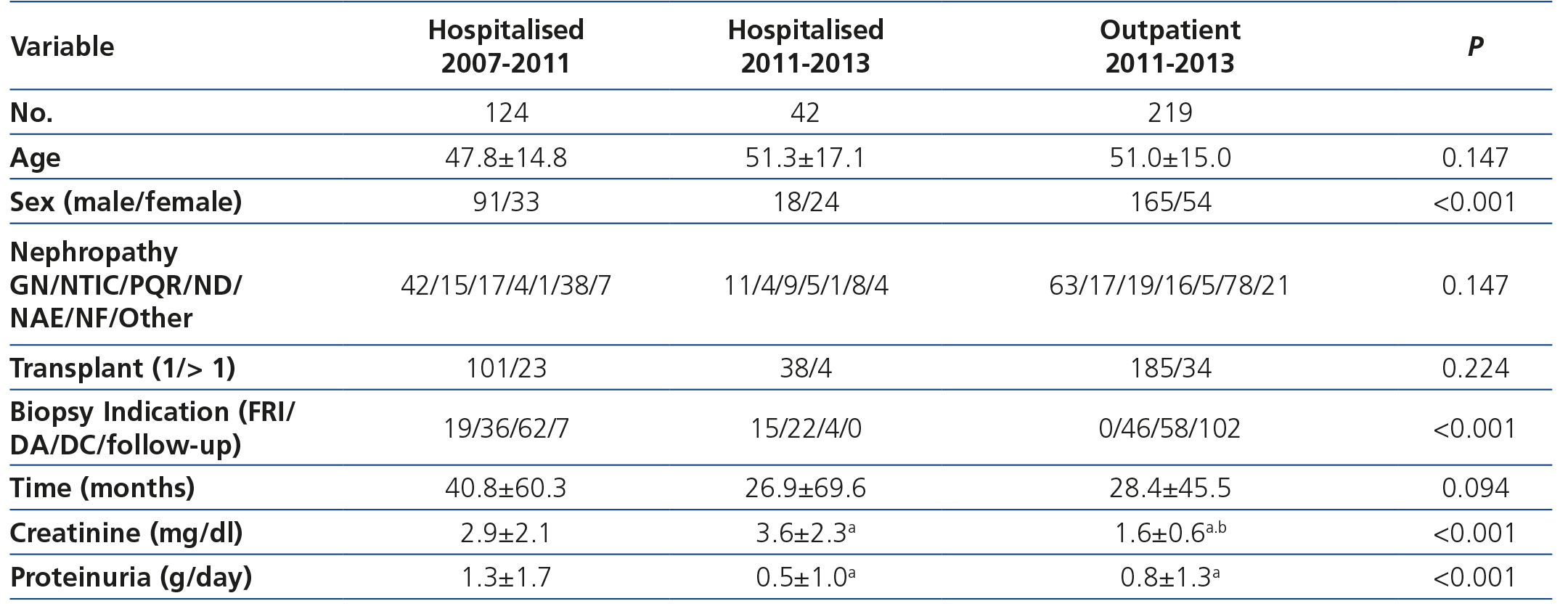
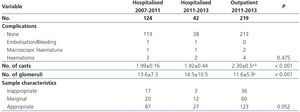
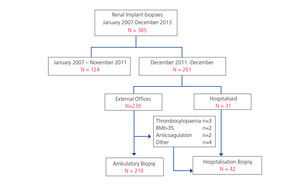
During the study period, 385 renal graft biopsies were performed. In the period between January 2007 and November 2011, 124 biopsies were performed on an hospitalisation basis. In the period between December 2011 and December 2013 a total of 261 biopsies, of which 219 (84%) were on an outpatient basis were performed. During this same period, biopsies on an hospitalisation basis were performed in 42 cases (16%) for the following reasons: Biopsies are indicated in hospitalised patients either due to delayed graft function or to acute impairment of renal function (n = 31), b) patient hospitalisation is programmed because there is a contraindication for an outpatient biopsy (n = 11), as shown in Figure 1. Contraindications for ambulatory biopsies are the following: treatment with systemic anticoagulants (n=2), obesity (n=2), thrombocytopenia (n=3), inadequate social and/or family environment for ensuring patient monitoring 24 hours post-biopsy (n=3) and insular residence (n=1).
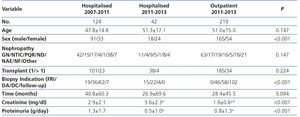
Table 1 shows the demographic and clinical characteristics of the three groups of patients. Differences in renal function and proteinuria between groups are related to the indication for biopsy. It is noteworthy that in the group of outpatient biopsies 53% were indicated due to graft dysfunction and 47% were follow-up biopsies at 3 or 12 months after transplantation.
Post-biopsy complications
During the period between January 2007 and November 2011 one major complication (0.8%) was recorded in a patient requiring puncture embolisation of the epigastric artery with intra-abdominal bleeding and hypotension. Minor complications were three bruises without haemodynamic complications and without transfusion requirements and one patient suffered a self-limiting gross haematuria (3.2%).
During the period between December 2011 and December 2013, 219 patients that underwent biopsy on an outpatient basis with 6 (2.7%) minor complications were recorded (2 self-limiting haematurias and 4 subcapsular haematomas without haemodynamic complications). One patient with self-limiting haematuria and two patients with subcapsular haematomas were discharged 6 hours after the biopsy. One patient with gross haematuria and 2 patients with perirenal hematoma were hospitalised. Patients were discharged at 24 hours and one patient with perirenal hematoma after 48 hours, as his home was 200km from the hospital.
Of the 42 hospitalised patients who underwent biopsy during 2011-2013 one major complication was seen (2.4%) that required embolisation for abdominal wall bleeding with haemodynamic complications and 3 patients (7.1%) suffered minor complications: uncomplicated subcapsular hematoma (n=2) and self-limited haematuria (n=1). Table 2 summarises the complications of the three patient groups.
Number of cores obtained and sample adequacy
The number of cores obtained in the outpatient biopsies was significantly higher than in hospitalised patients, since in follow-up biopsies an additional core for molecular biology was obtained. Although in the core obtained for light microscopy the number of glomeruli was significantly lower in the outpatient biopsies, this difference did not attain statistical significance when the adequacy of the sample was evaluated according to Banff criteria9, as shown in Table 2.
DISCUSSION
Renal allograft biopsy is a useful method for understanding and characterising what happens to the graft and provides guidance for therapeutic interventions. The introduction of ultrasound-guided automatic needle puncture has made biopsing allografts, for either diagnostic reasons or during follow-up, a safe procedure3,6.
In our study, in which we retrospectively evaluated complications associated with graft biopsies in hospitalised patients (periods 2007-2011 and 2011-2013) and outpatients (2011-2013), it has been demonstrated that there are no differences in the incidence of complications between the two periods of time and that the rate of serious complications was less than 1%, as described in other series of biopsies. There were no cases of death or loss of transplant5,6,10.
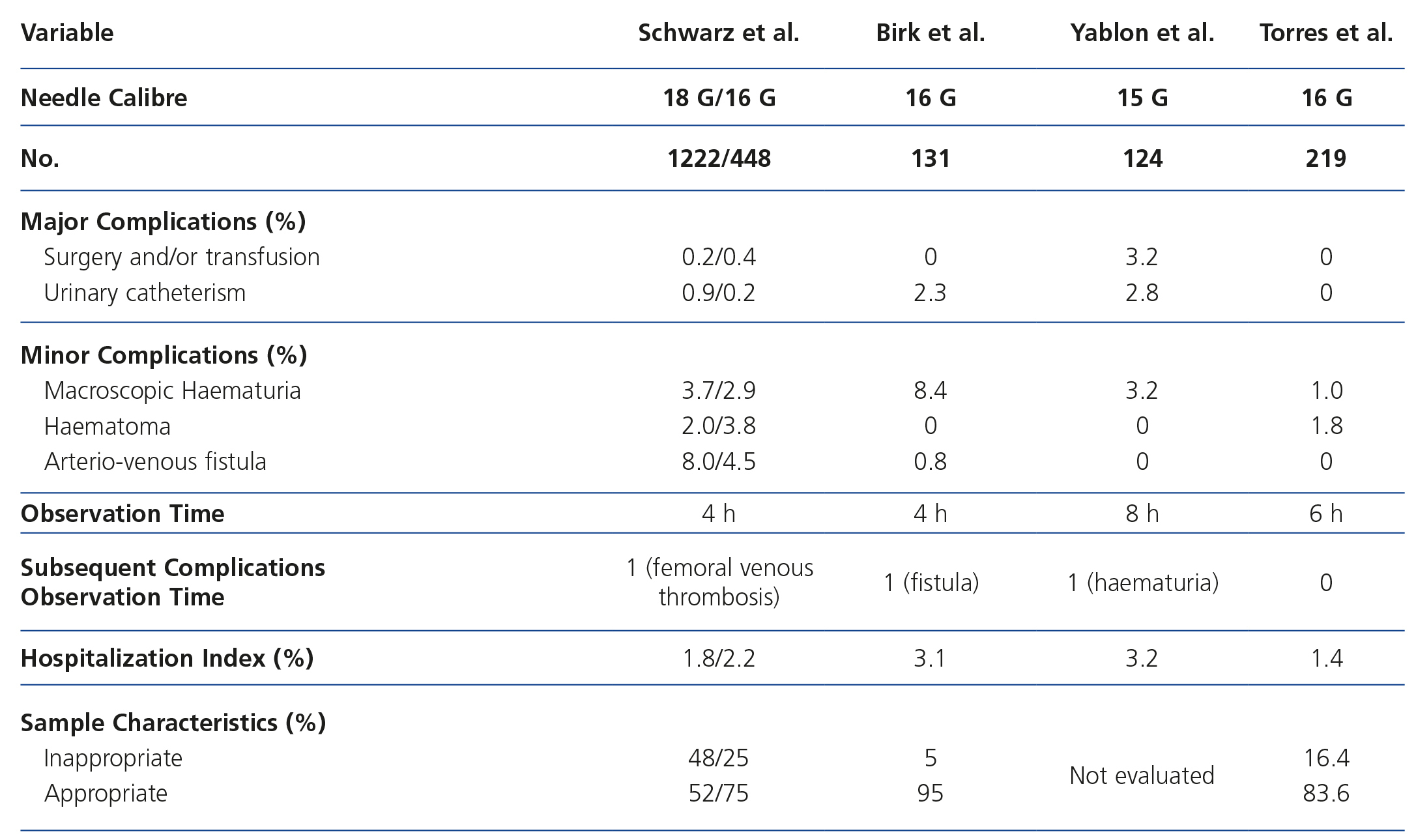
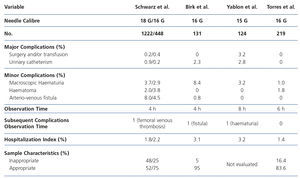
The safety of outpatient procedures has been evaluated in retrospective studies of different series of renal allograft biopsies performed in adult and paediatric recipients. In one of the most comprehensive reviews in 1670 biopsies, Schwarz et al. showed that graft biopsy performed as an outpatient procedure is safe and that there are no differences in the complication rate regardless of indication (clinical or follow-up). In addition, according to other authors, using a 16 G gauge needle it is possible to obtain the same safety profile and improved sample adequacy for histological evaluation6. Despite the absence of homogeneity between centres on the criteria for contraindication of outpatient procedures and patient observation time after performance of biopsies to monitor complications, the results obtained in different series (Table 3 ) suggest that a window period of between 4 and 8 hours post-biopsy during which evaluation of vital signs and urine monitoring, with or without analytical and/or ultrasound control, is enough to observe any procedure associated bleeding complication6,7,11,12.
The successful implementation of a low or medium complexity outpatient procedure programme is based on reduction of waiting time, as well as achieving efficiency and safety. In addition, it is of benefit to the patient, who perceives better quality of life and degree of care, to professionals as it simplifies the clinical process, and to the institution, as it increases process efficiency and reduces hospitalisation costs. It requires coordination between different specialties and a high degree of protocolisation of the clinical process applicable to a low-risk group of similar patients. All of this allows a minimum time of observation and a low rate of hospitalisation due to complications7,13.
According to our experience, we have to highlight that since the implementation of outpatient biopsies only one major complication occurred in 261 (0.38%) procedures; this was in a patient who was already hospitalised and who was biopsied for acute graft dysfunction. During this same period, we should note that 95% of biopsies indicated in consulting offices were performed as outpatient procedures. None of these patients suffered a major complication. Two patients with subcapsular hematoma and one with gross haematuria were hospitalised due to minor complications detected during the monitoring period (6 hours), representing a hospitalisation rate of 1.37%, similar to that observed in other series (Table 3). In patients without complications during the monitoring period no late complications were observed.
The most frequent indications for biopsy in patients hospitalised during 2011-2013 were delayed graft function and acute dysfunction. Only 11 of 230 patients (5 %) in which biopsies were indicated in the external consulting offices were hospitalised due to clinical or social/family contraindications.
Of the 219 biopsies performed on an outpatient basis, a total of 102 (47%) were follow-up biopsies. In these cases a third core for molecular biology studies was obtained, and it is for this reason that the average number of cores in outpatients was significantly higher. Despite obtaining an additional core in these low-risk patients, no increase in complications was observed.
However, the number of renal tissue samples defined as marginal and adequate for diagnosis was slightly lower in outpatients, although this difference did not reach statistical significance and was not clinically relevant, since in all cases it was possible to perform a diagnosis.
CONCLUSION
Ambulatory renal allograft biopsies are a safe procedure when applied to a low-risk subgroup of patients, improving their quality of life, decreasing the waiting list and reducing hospitalisation costs.
Conflicts of interest
Receive Scholarships: IB Torres received a predoctoral fellowship from the Institut de Recerca Vall d'Hebron (VHIR). Azancot MA has received a FIS predoctoral fellowship (FI11/0246). This study received support from the Carlos III Institute (grants PI10/2496, PIE13/00027) of the Renal Research Network (REDinREN 12/0021/0013) and a grant from the Spanish Society of Transplant-Novartis.
We acknowledge the cooperation of the nursing staff of the Haemodialysis Unit of the Vall d'Hebron Hospital who have helped care for the patients.
Table 2. Complications associated with renal biopsy and efficiency of this in each of the groups studied
Table 3. Percentage of complications, observation time and complications subsequent to the observation period, hospitalisation rate and sample adequacy in different series of outpatient renal allograft biopsies.
Figure 1. Flowchart of biopsies performed during 2007-2013
Table 1. Demographic variables, indication for biopsy and laboratory data at the time of biopsy