About 50% of patients with multiple myeloma (MM) develop various forms of renal injury during the disease1 in, including myeloma kidney (32–48% in MM post-mortem examinations or myeloma cast nephropathy),2 light chain amyloidosis (10–15% of cases1 and monoclonal immunoglobulin deposition disease. Other disorders may be encountered more rarely, such as light chain proximal tubulopathy, light chain-associated acute tubulointerstitial nephritis and glomerular involvement by cryoglobulins. A far less frequently reported lesion is the finding of amyloid casts, which is challenging to comprehend, both in terms of the mechanism of their formation and their clinical significance. We are presenting a case.
He was a 57-year-old man with history of hypertension, hyperuricaemia and stage G3bA3 chronic kidney disease attributed to repeated episodes of obstructive uropathy due to calcium oxalate stones, who consulted for proteinuria. A serum monoclonal IgA lambda component of 0.84 g/dl with lambda free light chains of 1,784 mg/l and Bence Jones proteinuria of 1 g/24 h was identified. Given these findings, the patient was referred to haematology, where a bone marrow aspirate was performed, confirming the diagnosis of IgA lambda MM. It was therefore decided to perform a renal biopsy to assess renal involvement and plan a treatment.
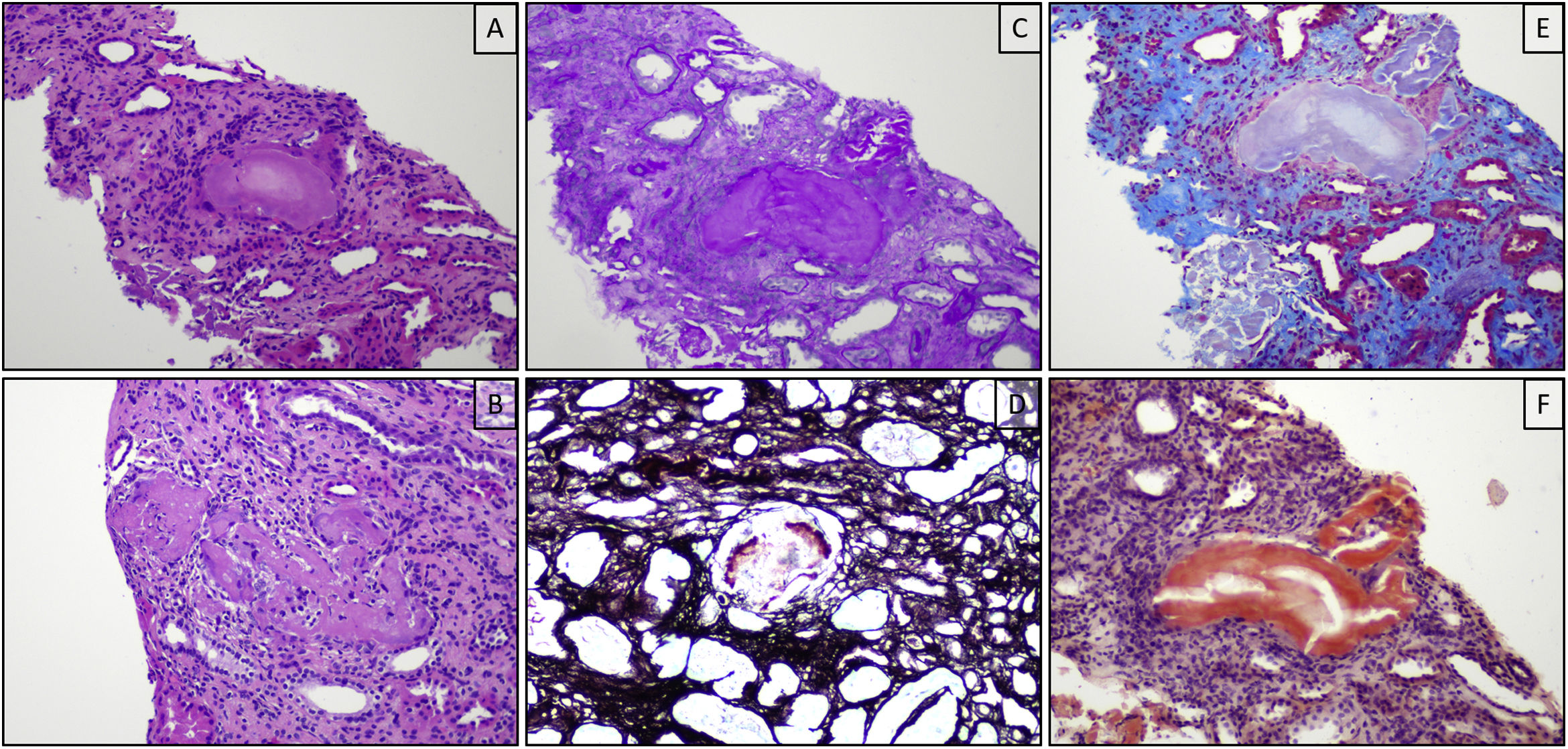
In the renal biopsy, a maximum of three glomeruli per slice plane were counted, none of which was globally sclerosed or ischaemic and all of which retained their normal size and lobulation, and no mesangial expansion, deposits or increased cellularity were identified at any level. In the tubular lumen, casts (up to 3 mm2) of eosinophilic material with associated histiocytic reaction were identified, which were weakly positive with periodic acid-schiff (PAS) staining, negative with the silver technique, polychromatophilic with Masson's trichrome and positive with the Congo red technique, with no positivity identified with this technique at other levels. At the tubulointerstitial level, chronic lymphocytic inflammatory infiltrate, tubular atrophy and interstitial fibrosis were also identified affecting 40% of the cortical surface. Direct immunofluorescence techniques showed strong positivity for lambda light chains in the cytoplasm of proximal tubule epithelial cells (Fig. 1).
Renal biopsy, optical microscopy: histologically, intratubular eosinophilic casts are observed (A), which are focally associated with inflammatory response (B). The casts are weakly positive with the PAS technique (C), negative with silver (D) and polychromatophilic with Masson's trichrome (E). Positivity was also observed in this case with the Congo red technique (F).
The observed casts preented positivity for IgA, kappa and lambda light chains. Chronicity score: 4, mild chronic changes, glomerular sclerosis 0 (<10%), tubular atrophy 2 (26–50%), interstitial fibrosis 2 (26–50%), atherosclerosis 0 (intimal fibrosis < average). Ca1 (<5 casts/mm2) T2 (atrophy/fibrosis 25–50%) according to the classification proposed by Royal et al.3,4
In view of the findings described above, the patient was diagnosed with myeloma cast nephropathy and chronic tubulointerstitial nephritis with mild chronic changes. A further study was performed with a fat biopsy, which did not identify an amyloid deposit, and the D-CVD regimen (daratumumab, bortezomib, cyclophosphamide and dexamethasone) was administered.
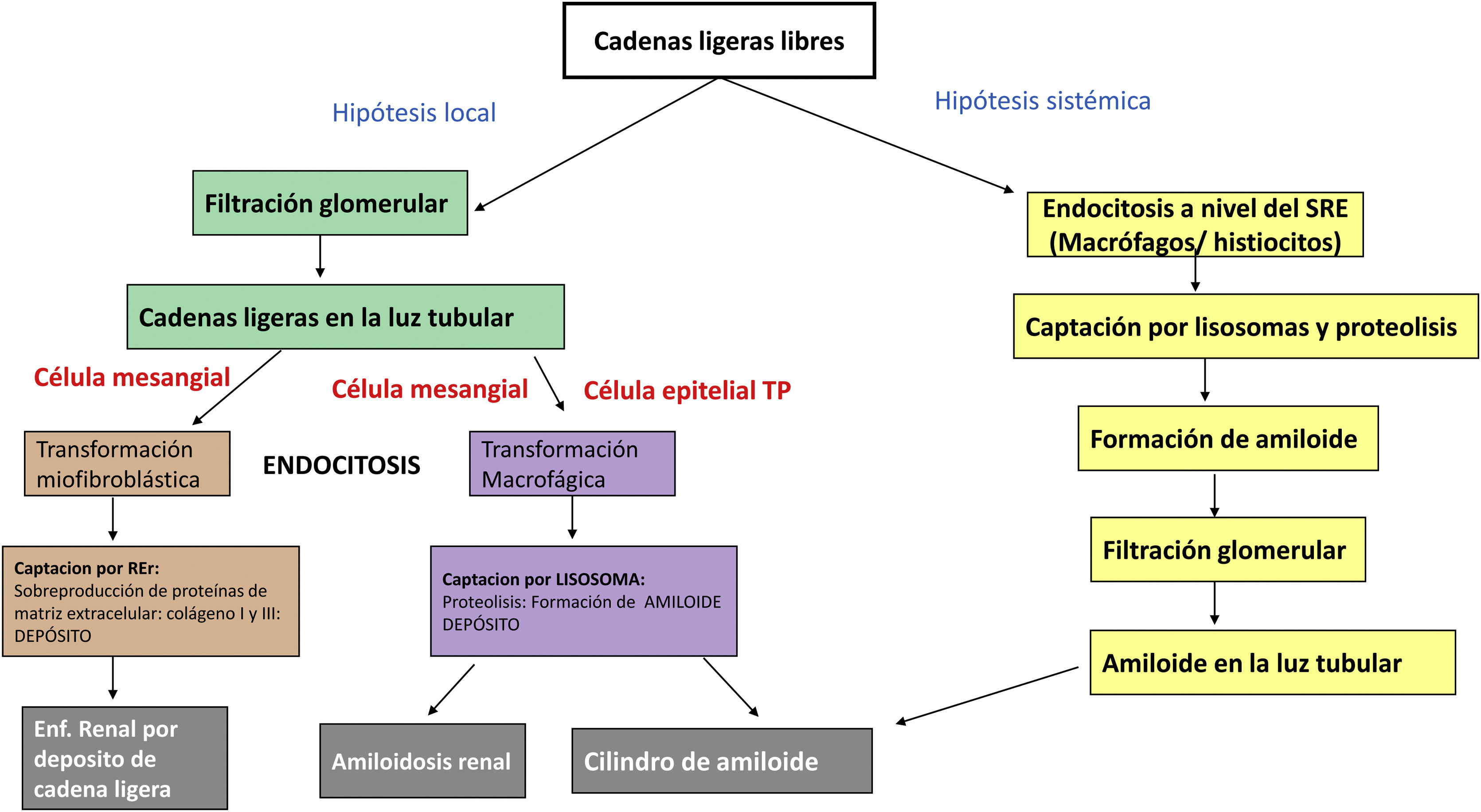
The pathogenesis of renal light chain damage involves the transformation of mesangial and tubular cells into cells of other lineages. If the transformation is to the myofibroblastic lineage, light chain disease will develop, whereas if the transformation is to the macrophage-histiocyte lineage, renal amyloidosis will develop. In the case of amyloid casts, there are two hypotheses for their formation: systemic and local via endocytosis of the megalin/cubilin receptor in tubular cells (Fig. 2).5,6
Pathophysiology of AL renal amyloidosis versus light chain renal injury: in the first hypothesis, systemic endocytosis of light chains occurs by macrophage cells, which take them up by lysosomes, where their proteolysis occurs and amyloid fibrils are formed, which will be filtered by the glomerulus and engulfed in the casts. In the local hypothesis, they leak freely through the glomerulus and are taken up by mesangial cells that have undergone myofibroblastic transformation with the production of extracellular matrix proteins that will be deposited, leading to renal light chain deposition disease. However, if the mesangial cells have undergone macrophage transformation, they will give rise to renal amyloidosis. The epithelial cell of the proximal tubule (PT) undergoes macrophage transformation and the light chains are endocytosed by means of the megalin and cubilin receptors, with subsequent uptake by the lysosomes, which undergoes proteolysis, resulting in the formation of amyloid fibrils that will finally form the amyloid casts.
Most of these casts appear in lambda-type gammopathy, with some series reporting a percentage of 76.4% compared to kappa-type gammopathy of 26.4%.8
The series with the highest number of cases are those of Vassar and Culling in 19629 with 57 cases and 58% amyloid casts, Limas et al. in 197310 with 43% in 35 cases, Hill et al. in 198311 with 39% in 33 cases and Gibier et al.8 between 2002 and 2012, with 60 cases, with 28% intratubular amyloid.
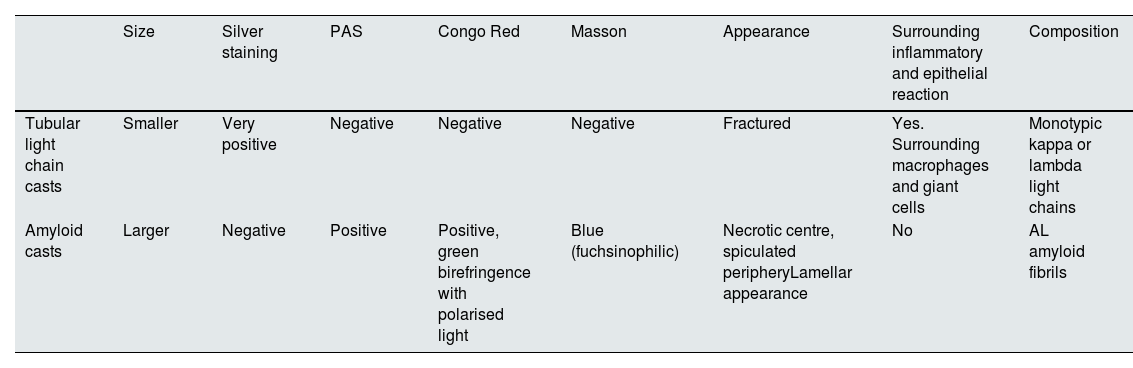
Morphologically, the material is described as homogeneous and laminated in appearance.7 The tubular casts show a unique morphology, with a central paler nest-like area and a periphery that is spiculated, PAS-positive, argyrophilic, congophilic and blue with Masson's trichrome stain.12,13 The central zone, with necrotic features, has cellular debris that is arranged alongside Tamm-Horsfall proteins. In the peripheral zone: amyloid, which in some series makes it possible to see a specific peripheral staining,8 with morphology of disordered fibrils in clusters and the presence of amyloid in intracytoplasmic vacuoles measuring 100−600 nm in diameter14 under electron microscopy (Table 1). In our case, unusually, the findings were mixed, between those described as amyloid casts and conventional myeloma casts.
Differential characteristics between light chain tubular casts and amyloid casts.
| Size | Silver staining | PAS | Congo Red | Masson | Appearance | Surrounding inflammatory and epithelial reaction | Composition | |
|---|---|---|---|---|---|---|---|---|
| Tubular light chain casts | Smaller | Very positive | Negative | Negative | Negative | Fractured | Yes. Surrounding macrophages and giant cells | Monotypic kappa or lambda light chains |
| Amyloid casts | Larger | Negative | Positive | Positive, green birefringence with polarised light | Blue (fuchsinophilic) | Necrotic centre, spiculated peripheryLamellar appearance | No | AL amyloid fibrils |
AL: amyloidosis; PAS: periodic acid-schiff.
Some series have found that their presence compared to non-congophilic casts is a risk factor for systemic amyloidosis (38.46% vs 9%), with no differences in survival, renal survival, mortality rates or the need for renal replacement therapy.8 In other cases it is considered as a finding that could precede the formation of light chain casts15 and systemic amyloidosis. As such, this finding would make it necessary to rule out amyloid deposits at other levels, which we were unable to identify in our case.
FundingThere was no funding for this article.