We report a case of the onset of lupus nephritis (LN) in the form of fibrillary glomerulonephritis (FGN).
In August 2021 a 17-year-old girl of Romanian origin developed malar erythema and inflammatory gonalgia. Her primary care physician confirmed a preserved renal function with positive ANA and anti-DNA, microhematuria and albuminuria. The patient was referred to our clinic. Fever, weight loss, other arthralgias, arthritis, uveitis, Raynaud’s and aphthae were all absent. She had regular menstruation and was not obese. She had no other relevant medical history. Her mother suffered from arterial hypertension, psoriasis and autoimmune hypothyroidism. A skin biopsy was performed on the malar erythema and results confirmed acute cutaneous lupus. A complete blood count and biochemistry study were ordered. The hemogram and biochemistry results were all within normal values except for total protein: 5.6 g/dl and serum albumin: 2.5 g/dl. Urinalysis showed proteinuria (30 mg/dl), red blood cells (3–10 per high power field); CrCl: 246 ml/min/1.73 m2 and proteinuria: 2.4 g/24 h.
Autoimmunity: positive for a homogeneous ANA pattern AC1 420 titer 1:160, Anti-DNA native antibodies: positive (titer 243.3). Anti-double stranded DNA antibodies (Crithidia luciliae): positive. The rest, including antiphospholipid antibodies and ß-2 glycoprotein 1, were negative. C3: 61 mg/dl, C4: 9 mg/dl. Thyroid profile with normal TSH and antimicrosomal antibodies (TPO) 107.60 IU/mL (normal values: 0.0–60.0). Neck ultrasound was normal. Immunoglobulin (Ig) G positive and IgM negative for EBV, rubella, HHV type 6, varicella zoster. Quantiferon and serology negative for Leishmania, Leptospira, Trypanosoma cruzi, Borrelia, Strongyloides, parvovirus, lupus, cytomegalovirus, toxoplasma, HBV, HCV, HIV, Brucella and Bartonella.
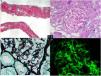
A renal biopsy was performed (Fig. 1). The results showed 19 glomeruli per sample, 1 with global sclerosis of the capillary mass (5%). The preserved glomeruli were normal in size and lobulation. Fine and permeable capillary loops. There were no wire loop deposits, hyaline pseudothrombi, necrotizing lesions, karyorrhexis, neutrophils or endo- or extra-capillary hypercellularity. Slight mesangial expansion without increased cellularity. In 2 glomeruli, with segmental distribution, images suggest projections without basement membrane (BM) vacuolization or clear subepithelial deposits by direct immunofluorescence (DIF). No significant alterations of the tubule. No lesions on the muscle wall vessels or arterioles. No intimal fibrosis, arteriolar hyalinosis or amyloid material with Congo red. DIF: mesangial and subendothelial granular deposits of IgG (++) and C3 (+++) in capillary loops, and mesangial granular deposits of IgM (+) and C1q (++), with a slight predominance of kappa light chains over lambda. Diagnosis: class III LN. Activity score: 0/24. Chronicity score: 1/12.
Renal biopsy. Optical microscopy. (A) Renal cortical parenchyma with preserved general architecture and without significant chronic changes (Masson’s trichrome). (B) Glomeruli with no mesangial expansion, proliferative lesions or other morphological alterations (PAS, 20×). (C) Glomerulus with very focal spikes of basement membrane (silver, 40×). (D) DIF: IgG and granular mesangial C3 deposits in capillary loops and IgM and mesangial C1q deposits (IgG, 40×).
We started treatment with ACE inhibitors, hydroxychloroquine, corticosteroids and mycophenolate. After 6 months of follow-up treatment, renal function remains stable and proteinuria has decreased to 0.65 g/24 h.
The results of electron microscopy were then received (Fig. 2): There were 4 glomeruli with distorted general architecture. Podocytes presenting villous transformation in the cytoplasm; no intracytoplasmic deposits. Focal pedicel fusion affecting 30% of the total pedicellar extension and rearrangement of the intermediate filaments towards the cell’s basal pole. BM with irregular contours and diffusely thickened, lamina densa thickness between 700 and 925 nm (average 863 nm, NV: 300−400 nm), moderately electrodense diffuse deposits, arranged as fibrils ranging in diameter from 10.60 to 35.65 nm (average 18.32 nm), embedded in the lamina densa and causing a spiculate reaction of the glomerular BM, but not embedded in the lamina densa. Few subendothelial deposits. Endothelia with a normal shape, clearly visible fenestrations and tubuloreticular inclusions. Moderately enlarged mesangium at the expense of the matrix and with frequent electrodense deposits of the same characteristics described above. No ultrastructural lesions of tubules or vessels. No deposits in the tubular or vascular BM. In view of these findings, an immunohistochemical staining for DNAJB9 was performed. The result was positive for a granular pattern in the subepithelial aspect of the BM, which led to a diagnosis of fibrillary glomerulonephritis.
Electron microscopy. (A) Glomerulus with massive pedicel fusion (black arrows). (B) Presence of tubuloreticular inclusions in the endothelium. (C) Moderately electrodense deposits in the subepithelium (*) with spiculated reaction of the lamina densa (white arrows). (D) Deposits organized in the form of fibrils. Transmission electron microscopy A and C: 5,000×, B: 8,000×, D: 1,2000×. CL: capillary lumen; GBM: glomerular basement membrane; ECN: endothelial cell nucleus; P: podocyte.
Given the good initial response, immunosuppressive treatment was maintained pending evolution of the patient’s condition.
The DNA protein JB9 is a cochaperone located in the endoplasmic reticulum of many cells. It is activated by stress and is involved in protein folding. In FGN1,2 with immunocomplex deposition, Ig has been shown to be restricted for IgG4 and IgG1. This explains why Ig is the protein deposited or whyis deposits a misfolded JB9 DNA protein at glomerular level, which will trigger the subsequent inflammatory response. The mechanism depends on complement activation through the classical pathway (C4 positive in generalized staining). The largest published series of FGN3 describes 23% of cases associated with tumors that started between 15 years before and 10 years after (multiple myeloma, chronic myelomonocytic leukemia; thyroid, hepatocellular, lung, uterine, prostate, colon or renal cancer; melanoma), 15% associated with unspecified autoimmune diseases (lupus, Sjogren’s, Crohn’s disease, Graves-Basedow, primary biliary cholangitis, idiopathic thrombocytopenic purpura and ankylosing spondylitis), 3% with chronic hepatitis C, 20% with diabetes, 9% with chronic ischemic heart disease, 5% with COPD and 17% with monoclonal gammopathy. Clinically, 71% of cases presented with HTN, 59% with edema, 41% with proteinuria (mean 3.1 g/day), 62% with hypoalbuminemia, 38% with hypercholesterolemia, 62% with full-blown nephrotic syndrome, 52% with hematuria, 29% with leukocyturia and 2% with hypocomplementemia.
The association of FGN with LN4–10 has only been reported rarely in relapses following renal transplantation in cases of LN without systemic lupus and in one case of neuropsychiatric lupus, which resulted in a diagnosis of class 4 L N.
In a review of 223 renal biopsies of LN with organized electrodense deposits11, investigators identified 3 cases with tubular deposits and 2 cases with fibril deposits, with the structural features of immunotactoid glomerulopathy and FGN.
Regarding the prognosis, there is no conclusive data given the low number of cases, although FGN progresses to end-stage renal disease in 50% of cases after 5 years. For the same reason, there is no evidence regarding treatment, although the use of rituximab is recommended.
In our case, and given the short time of evolution, we can report a partial response after 2 months of treatment.
FundingThis article has no source of funding.