We present the case of a male patient with severe SARS-CoV-2 pneumonia, with simultaneous onset of p-ANCA positive rapidly progressive glomerulonephritis. We discuss the different therapeutic possibilities, emphasising the appropriateness of their administration according to the time in the course of the infection.
Presentamos el caso de un varón afecto por neumonía SARS-CoV-2 grave, que a la vez comienza con una glomerulonefritis rápidamente progresiva p-ANCA positiva. Se comentan las distintas posibilidades terapéuticas haciendo hincapié en la idoneidad de su administración según el momento evolutivo de la infección.
The current SARS-CoV-2 pandemic causes harm by itself, and by altering the procedures and treatments that the patient must receive for other diseases. We report a complex case, highly relevant at this time of the health emergency that we are experiencing. This is a man who was admitted with respiratory failure due to severe SARS-CoV-2 pneumonia and who simultaneously developed acute renal failure due to ANCA positive rapidly progressive A glomerulonephritis. The therapeutic possibilities in the current epidemiological context are discussed.
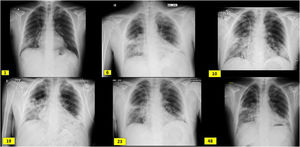
Case descriptionA 60-year-old male from Ecuador who was admitted in August 2020 due to few days of dyspnea, without any other referred symptoms. Upon arrival, he was normotensive and afebrile with basal oxygen saturation greater than 94%, but tachypneic at 16 breaths per minute. SARS-CoV-2 RT-PCR in pharyngeal exudate was positive. Laboratory results revealed usual parameters of COVID infection with a clear renal involvement: Plasma creatinine 3.94 mg/dl, eGFR (CKD-EPI): 16 ml/min/1.73 m2, microhematuria and moderate proteinuria (Table 1). Chest X-ray with bilateral interstitial pneumonia (Fig. 1). The patient worked as a painter in construction, had no toxic habits or contact with animals, and had long-term hypertension.
Evolution of blood analysis.
| Days | Hb | Nf/Lf | Cr/eGFR | CRP | GPT/GOT | DD | Ferritin | IL-6 | pANCA (anti-MPO) | Urine | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| −6months | 1.3/64 | 100.4 | ACR > 30 | ||||||||
| RBC-U 200 (10-20 pc) | |||||||||||
| 1 | 9.6 | 5.25 | 3.94/ | 59 | 59/40 | 164 | 114.23 | RBC-U U-PCR 418.8 | |||
| 2 | 9.3 | 13.5 | 4.59/ | 74 | 74/41 | 160 | PCR Cov (+) | ||||
| 4 | 8.1 | 6.25 | 5.26/ | 70 | 70/43 | 134 | 41 | ||||
| 6 | 11.4 | 11.5 | 5.58/ | 124 | 124/123 | 140 | Bolus of MPR | ||||
| 7 | 11.0 | 13 | 5.21/ | 433 | 433/334 | 150 | PCR Cov (+) | ||||
| 9 | 10.8 | 12.3 | 5.45/ | 647 | 647/324 | 170 | 1,867 | 55 | Prd 1 mg/kg/day | ||
| 10 | 10.8 | 20.2 | 121 | 306/76 | 186 | Kidney Bx | |||||
| 12 | 9.1 | 17.25 | 3.8/ | 47 | 47/27 | 182 | 2,955 | 785 | |||
| 16 | 9.8 | 6 | 3.49/ | 34/36 | 385 | Ig IV/5 days | |||||
| 19 | 9.3 | 7.7 | 2.93/ | 92 | 92/37 | 2. 3. 4 | TCZ/2 days | ||||
| 21 | 10.8 | 4 | 2.59/ | 107 | 107/69 | 400 | RBC-U 200 | PCR Cov (+) | |||
| IgG (+) | |||||||||||
| 23 | 8.8 | 5.75 | 2.78/ | 180 | 180/68 | 568 | RTXMB | ||||
| 24 | 11.2 | 7.6 | 2.8/ | 90 | 90/45 | 410 | |||||
| 28 | 11.3 | 8.8 | 3.01/ | 49 | 49/24 | RBC-U 80 | IgG (+) | ||||
| 29 | 11.9 | 9.25 | 3.0/ | 37 | 37/16 | 1,120 | 130 | 17.88 | CLCR 18 | ||
| UPr 0.3 g/24 h PCR (+) | |||||||||||
| 35 | 11.3 | 8.7 | 3.0/ | 35 | 335/16 | RBC-U 80 | PCR(−) | ||||
| 37 | 12.0 | 17.3 | 2.85/ | 35 | 35/15 | ||||||
| 41 | 12.0 | 11 | 2.92 | 88 | 88/29 | RBC-U 25 (isolated) | PCR(−) | ||||
| U-PCR 310.59 | |||||||||||
| 48 | 12.1 | 13.4 | 2.5 | 92 | 92/23 | 570 |
ACR: albumin/creatinine ratio; CLCR: creatinine clearance in mL/min; U-PCR: protein creatinine ratio in mg/Cr; Cr/eGFR: blood creatinine in mg/dl and estimated glomerular filtration rate CDK-EPI; DD: D-dimer (turbidimetric) in µg/l; Ferritin in ng/mL; GPT/GOT: transaminases in U/l; Hb: hemoglobin in g/dl; RBC-U: elementary urine red blood cells; IgG: immunoglobulin G against SARS-CoV-2; Ig IV: intravenous immunoglobulins; IL-6: interleukin 6 in pgr/mL; MPR: methylprednisolone at a dose of 250 mg/d/3 days; Nf/Lf: neutrophil/lymphocyte ratio; pANCA: anticytoplasmic antibodies, myeloperoxidase standard (MPO) in U/l; CRP: C-reactive protein in mg/l; PCR CoV: RT-PCR against SARS-CoV-2; UPr: 24 h urine proteinuria in g/day; RTX: rituximab; TCZ: tocilizumab.
Radiological evolution since admission (day 1), boluses of methylprednisolone (day 6), kidney biopsy (day 10), initiation of tocilizumab (day 19), initiation of rituximab (day 23) and hospital discharge (day 48). It shows subpleural peripheral reticular thickening of the middle fields, right basal field and lingula and interstitial alveolar opacities in the middle and lower fields of the right lung and lower fields of the left lung.
Seven years earlier, he had been studied in another center for dyspnea, identifying multiple pulmonary infiltrates and pulmonary thromboembolism. Thrombophilia study was negative. He was on oral anticoagulation for 6 months with acenocoumarol.
Three years ago, again had dyspnea and ventilation-perfusion scintigraphy showed, multiple filling defects compatible with multiple pulmonary thromboembolisms, and oral anticoagulation was reintroduced.
Seven months earlier, he returned to hospital after presenting a cough of several months’ duration with purulent expectoration, self-limited hemoptysis, and thoracic pain. Chest X-ray and chest computed tomography revealed multiple consolidative opacities in the SLL, ML, and upper segment of the RIL with bilateral peri bronchial-vascular inflammatory nodules, one of them cavitated in the left apex. With suspicion of active post-primary tuberculosis, he was discharged and scheduled for additional complementary evaluations but the patients did not return.
Five months before, he came to our center for the first time due to chest pain with dyspnea. Signs of diffuse interstitial lung disease were identified, along with new pulmonary infarcts. The patient also had antiphospholipid syndrome, livedo reticularis, and positivity for p-ANCA (anti-MPO) and anticentromere antibodies. Paresthesia of the lower limbs, pericardial effusion, and mural thickening of the aorta and supra-aortic trunks (aortitis) compatible with vasculitis of the aortic arch and supra-aortic trunk. CT/PET without signs of activity. At that time, a moderate renal involvement is already detected.
Pulmonary artery stenosis and new bilateral pulmonary infiltrates are also observed, in addition to the previously known cavitated one. He was discharged on treatment with colchicine, isoniazid, rifampicin, pyrazinamide, and ethambutol, which he maintained for 3 months, until receiving negative results for BAAR, PCR for Mycobacterium tuberculosis, and IGRA (QuantiFERON-TB Gold In-Tube®).
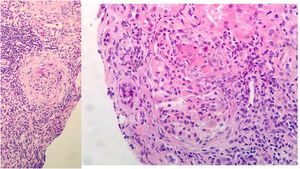
Evolution and complementary testsUpon admission, he began treatment with levofloxacin, prophylactic anticoagulation with LMWH, and respiratory support. Both renal insufficiency and respiratory failure worsen. Thus a renal biopsy was performed 10 days after admission, with a clinical judgment of pauci-immune mixed extracapillary proliferative glomerulonephritis associated with ANCA with moderate chronic changes (Fig. 2).
Renal biopsy: 11 glomeruli, 3 were globally sclerosed. Five with minor congestive changes and 3 with extracapillary proliferation and cell crescent formation. Tubulointerstitial compartment with chronic and acute inflammation, 30% tubular atrophy, tubular thyroidization, and 30% interstitial fibrosis. No changes in medium size vessels, or thickening of the media layer of the arteries. No congophilic deposits. Direct immunofluorescence with slight granular mesangial deposit of C3+, without deposits of IgG, IgM, IgA or restriction of light chains.
At the same time, he developed multifactorial parenchymal liver disease (quinolone, viral hepatotoxicity and hepatic vasculitis), so levofloxacin was replaced by ceftriaxone. On day 11, he developed anemia that required blood transfusion without data of post-biopsy renal bleeding by ultrasound; the patient also had self-limited hemoptysis with a fiberoptic bronchoscopy without findings, for which he received 3 doses of 500 mg of methylprednisolone. He evolved poorly, with radiological progression of the infiltrates, greater respiratory and renal failure, so on day 16 we started IV immunoglobulins at a dose of 0.2 mg/kg/day for 5 days and on day 19, after recovering liver function, he received 2 doses tocilizumab, 600 mg on the first day and 400 mg on the second day. He was admitted to the ICU and treated with continuous veno-venous hemodiafiltration (VVCHDF) (3 sessions) and respiratory support with high-flow NIV. Glomerular filtration recovered slowly and respiratory function improved with a better oxygenation. After verifying positive COVID IgG serology on day 21, treatment with rituximab was scheduled, 4 doses of 375 mg/m2/week IV. It was proposed to complement the treatment with a new batch of immunoglobulins, but it was not held since levels in plasma were maintained adequately. Renal function stabilized with GFR 20−30 m/min/1.73 m2, but respiratory infiltrates and ventilatory dependency did not improve during the following 3 weeks despite physical therapy and adequate support. Due to this torpid respiratory evolution, fiberoptic bronchoscopy was repeated without relevant findings. Finally, he could be discharged after 50 days of hospitalization with chronic home oxygen and an eGFR 27 ml/min.
Discussion-conclusionsThis case illustrates the difficulty that COVID-19 infections pose in patients with active vasculitis. The patient went through a period of admissions and previous unfinished studies and repeated non-appearances in part justified by the instability of the a emergency health situation in which we operate. Diffuse interstitial lung disease (DILD) was initially suspected, then tuberculosis and finally large-vessel vasculitis despite having a positive p-ANCA determination. He lost follow-up and at the time of admission we found severe acute renal failure of unclear origin in the context of a positive COVID-19 infection with bilateral pneumonia. In addition, he developed acute liver disease that contraindicates the use of tocilizumab and the very nature of the viral infection contraindicates the use of cyclophosphamide and rituximab at a time of SARS-COVID-19 infection in phase 1.
For all these reasons, we decided to start a sequential treatment that initially included corticosteroid therapy, supplemented with IV immunoglobulins, and in a second stage, tocilizumab, which seemed to us to be the most appropriate drug because it combines its positive effect against the cytokine storm that develops in SARS. -CoV-19 and its proven efficacy in ANCA-positive vasculitis.1,2 Once seroconversion occurred, we started full-dose rituximab with a good overall outcome.
The literature is scant regarding the attitude to follow in similar cases. Aortitis in cases of p-ANCA vasculitis has been sufficiently described.3–5 It is not an overlap of large and small vessel vasculitis, but rather a part of their spectrum of clinical manifestations that may appear before or at the same time as typical small vessel vasculitis. A stenosis of the vasa vasorum occurs due to intimal hyperplasia, the number of elastic fibers in the media decreases and it evolves to stenosis as a late consequence. Cases have been reported in association with Wegener's disease and microscopic polyangiitis but not with eosinophilic granulomatosis with polyangiitis. Chirinos et al.6 collect the 13 cases published up to 2002 of large-vessel involvement (aortitis) in ANCA (+) vasculitis, with an age range between 27–71 years, mean age 44.7 years, M/F ratio: 1.1. A 76% had constitutional symptoms, 38% arthralgia and weight loss, 46% upper airway involvement, 53% hypertension, 53.8% proteinuria in the range of 1.8–4.5 g/d, 76.9% hematuria, 8 biopsied of whom 7 had pauci-immune GN and 5 extracapillary proliferation. A 46% had purpura, 30% ocular involvement (conjunctivitis, necrotizing sclerokeratitis with corneal ulcers and episcleritis) and 23 % GI symptoms (dyspepsia and abdominal pain).
The use of tocilizumab in p-ANCA vasculitis is supported by various publications prior to the COVID era.2 Takenaka et al.1 describe a 47-year-old Japanese woman with p-ANCA vasculitis, hypertrophic pachymeningitis, alveolar hemorrhage, and aortitis who did not respond to prednisolone and cyclophosphamide, but did respond to tocilizumab at a dose of 400 mg/month that was maintained for one year. It is not the only case.7–11 Sakai et al.7 presented 2 more cases and makes a literature review up to year 2017 describing 17 cases that were treated with tocilizumab, of which 15 (88.2%) achieved complete remission with the use of tocilizumab 8 mg/kg/month for one plus prednisolone 1 mg/kg/day/for 2 weeks with a progressive dose reduction until discontinuation at 24 weeks.
There are no clear recommendations about the best treatment strategy for a patient with vasculitis and active COVID- 19 infection. The English guidelines16 suggest that rituximab is preferable to cyclophosphamide, but without a compelling justification. In some reported cases, immunosuppressive treatment did not appear to increase the severity of COVID-19.12–14 They expose the argument that the action of rituximab on depleting peripheral B cells, including memory B cells and the modulation of the antibody-dependent cytotoxic response, the complement-dependent response and apoptosis, but not the precursor B cells that do not express CD20. They do state that it can minimize the immunogenic response after vaccination. By contrast, other articles15–17 suggest that it can induce serious complications or prolong the viremia. To avoid this, it is suggested to supplement the treatment with fresh plasma in patients who have received B-cell depleting drugs.18,19
In the patient that is presented, the sequential regimen used managed to control the vasculitis, not without risks. The healing process of COVID-19 was slowed down and RT-PCR remained positive for 35 days, which demonstrates the complexity of these cases and the difficulty they pose when prioritizing therapeutic strategies.
FinancingThis article has no funding sources.
Conflict of interestsThe authors have no conflicts of interest to declare.
ThanksDr. Eduardo Gutiérrez Martínez from the Nephrology Department of Hospital 12 Octubre for his good advice.
Please cite this article as: Martín Navarro JA, Cintra Cabrera M, Proccacini F, Muñoz Rodríguez J, Roldán Cortés D, Lucena Valverde R, et al. Más difícil todavía: tratar una glomerulonefritis rápidamente progresiva grave en el seno de una neumonía por COVID-19. Nefrologia. 2022;42:94–98.