Alogliptin is one of the dipeptidyl-peptidase 4 (DPP4) inhibitors used in the treatment of type 2 diabetes mellitus. Acute pancreatitis, as well as hypersensitivity reactions and allergic reactions such as rash or pruritus have been reported as side effects. However, there are very few reported cases of renal adverse effects. Renal adverse reactions include isolated cases of acute interstitial nephritis associated with DPP4 inhibitors.1,2
We present the case of a 57-year-old female patient with a history of type 2 diabetes mellitus, being evaluated in the outpatient internal medicine department for constitutional syndrome of one and a half month duration. She was urgently admitted to the nephrology department after detecting creatinine levels of 5.1 mg/dl in a blood test carried out prior to the consultation. The patient exhibited no cardiovascular symptoms and was haemodynamically stable with a tendency to hypertension, 160/80 mmHg, heart rate 96 bpm and normal blood volume. In the Emergency room, impairement of renal function was confirmed, with potassium 4.6 mmol/l, sodium 138.6 mmol/l, pH 7.27, bicarbonate 18.4 mmol/l, pCO2 40 mmHg and lactate 0.9 mmol/l. The patient’s urinalysis and urinary sediment were normal, with proteinuria of less than 0.3 g/24 h, suggestive of tubulointerstitial damage.
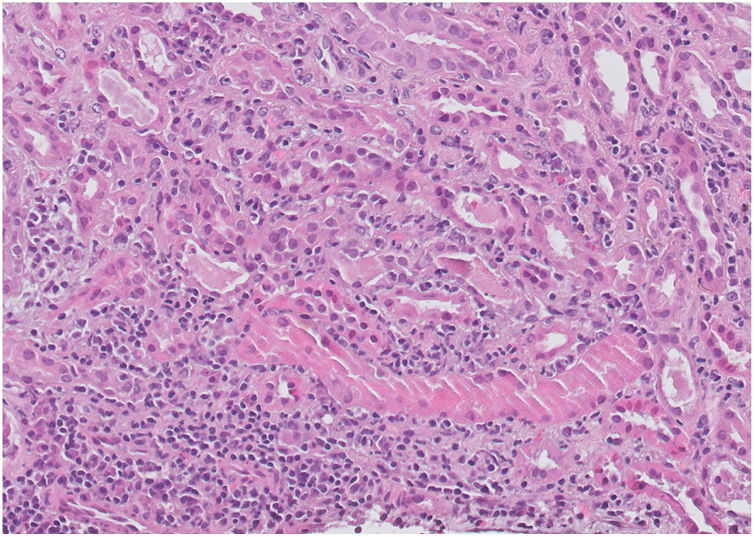
Investigation of impaired renal function (autoimmunity, serology, complement and immunoglobulins) was extended and all were negative. Renal biopsy confirmed morphological data consistent with acute tubulointerstitial nephropathy and acute tubular necrosis (Fig. 1). With these findings, pulses of 250 mg methylprednisolone were started for three days followed by oral prednisone at a tapering dose of 1 mg/kg. After ruling out other possible causes of tubulointerstitial nephritis and conducting a thorough review of the patient’s usual medication, we were able to relate the onset of symptoms and impaired renal function to the start of treatment with alogliptin. After starting corticosteroid therapy and discontinuing alogliptin, the patient’s renal function progressively improved, with Cr 2.5 mg/dl upon hospital discharge. She is currently on outpatient follow-up at our clinic; renal function has been restored to previous values, recording 1.2 mg/dl at the last creatinine check (Table 1). We classified this adverse drug reaction as probable, according to the WHO causality criteria, as it had a reasonable time relationship between the clinical manifestations and drug intake, could not be attributed to other causes or medications, and responded favourably to drug withdrawal.
The interstitium shows a prominent polymorphous inflammatory infiltrate consisting of abundant polymorphonuclear leucocytes, lymphocytes, plasma cells and occasional eosinophils with images of tubulitis. Renal parenchyma with morphological data consistent with acute interstitial nephropathy and acute tubular necrosis with regenerative changes. PAS (periodic acid-schiff) stain, ×400.
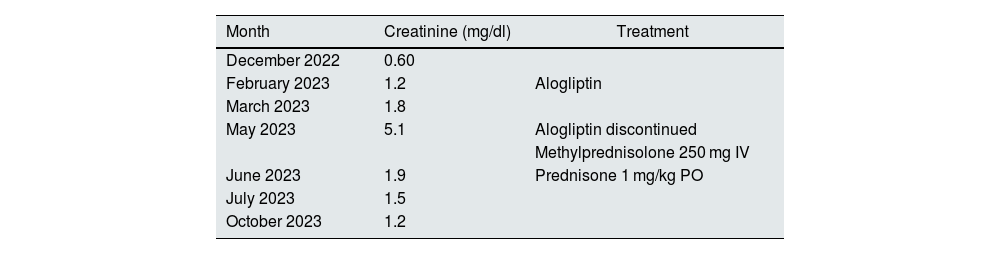
Changes in renal function.
| Month | Creatinine (mg/dl) | Treatment |
|---|---|---|
| December 2022 | 0.60 | |
| February 2023 | 1.2 | Alogliptin |
| March 2023 | 1.8 | |
| May 2023 | 5.1 | Alogliptin discontinued |
| Methylprednisolone 250 mg IV | ||
| June 2023 | 1.9 | Prednisone 1 mg/kg PO |
| July 2023 | 1.5 | |
| October 2023 | 1.2 |
The table shows changes in the patient’s renal function and treatment received in chronological order.
In our patient’s case, the clinical presentation of tubulointerstitial nephritis was not classic, as she did not have skin rash, eosinophilia or joint pain.3–5 Our patient’s condition began with rapidly progressive renal failure accompanied by weight loss and asthenia. Other diseases and recent medication that could have triggered the condition were ruled out, with the most likely cause of tubulointerstitial nephritis being the initiation of alogliptin as an oral antidiabetic agent.5,6
Reviewing the literature, there are very few reported cases of adverse reactions to alogliptin compared to other DPP4 inhibitors, such as sitagliptin. Evidence supports starting corticosteroid therapy when renal function does not improve despite drug withdrawal and while awaiting renal biopsy results.5–7 In our case, given the severity of the patient’s renal failure, it was decided to start corticosteroid therapy early8,9 after withdrawal of alogliptin and until the result was confirmed by the renal biopsy report. As a result, the patient experienced a marked clinical improvement and her renal function returned to its previous values.
Given that this is a rarely described adverse reaction and that these are commonly-used drugs, we believe it is important to consider the involvement of these drugs in patients presenting with acute renal failure in relation to tubulointerstitial nephritis.10