A relatively high proportion of deaths in dialysis patients occur suddenly and unexpectedly. The incidence of sudden cardiac death (SCD) in non-dialysis advanced chronic kidney disease (CKD) stages has been less well investigated.
ObjectiveThis study aims to determine the incidence and predictors of SCD in a cohort of 1078 patients with CKD not yet on dialysis.
MethodsProspective observational cohort study, which included patients with advanced CKD not yet on dialysis (stage 4–5). The association between baseline variables and SCD was assessed using Cox and competing-risk (Fine and Gray) regression models. Demographic, clinical information, medication use, and baseline biochemical parameters of potential interest were included as covariates.
ResultsDuring the study period (median follow-up time 12 months), 210 patients died (19%), and SCD occurred in 34 cases (16% of total deaths). All-cause mortality and SCD incidence rates were 113 (95% CI: 99–128), and 18 (95% CI: 13–26) events per 1000patient-years respectively. By Cox regression analysis, covariates significantly associated with SCD were: Age, comorbidity index, and treatment with antiplatelet drugs. This latter covariate showed a beneficial effect over the development of SCD. By competing-risk regression, in which the competing event was non-sudden death from any cause, only age and comorbidity index remained significantly associated with SCD.
ConclusionsSCD is relatively common in non-dialysis advanced CKD patients. SCD was closely related to age and comorbidity, and some indirect data from this study suggest that unrecognized or undertreated cardiovascular disease may predispose to a higher risk of SCD.
Una alta proporción de fallecimientos en pacientes tratados mediante diálisis ocurre de forma súbita e inesperada. La incidencia de muerte súbita (MS) en pacientes con enfermedad renal crónica (ERC) en estadios prediálisis es menos conocida.
ObjetivosDeterminar la incidencia y factores asociados a la MS en una cohorte de 1.078 pacientes con ERC avanzada.
MétodosEstudio de cohortes prospectivo y de observación, que incluyó a pacientes con ERC estadio 4-5 prediálisis. La asociación entre las variables basales y la MS fue analizada mediante modelos de regresión de Cox y de competencia de riesgo (Fine y Gray). Los datos demográficos, clínicos, la medicación y los parámetros bioquímicos basales de potencial interés fueron incluidos como covariables en el análisis predictivo.
ResultadosDurante el periodo de estudio (mediana de seguimiento 12 meses), fallecieron 210 pacientes (19%) y de forma súbita 34 casos (16% total de muertes). Las tasas de incidencia de muerte por cualquier causa y de MS fueron: 113 (IC 95%: 99-128) y 18 (IC 95%: 13-26) eventos por 1.000 paciente-años respectivamente. Mediante análisis de regresión de Cox, la edad, el índice de comorbilidad y el tratamiento con antiagregantes plaquetarios fueron las covariables que se asociaron significativamente con MS. Esta última covariable mostró un efecto beneficioso sobre el desarrollo de MS. En los modelos de regresión por competencia de riesgo, en los que el evento competidor fue la muerte no súbita por cualquier causa, solo la edad y el índice de comorbilidad se asociaron significativamente con la MS.
ConclusionesLa MS es relativamente frecuente en pacientes con ERC prediálisis. La MS se asoció significativamente con la edad y la comorbilidad, y varios datos indirectos de este estudio muestran que un infradiagnóstico o infratratamiento de la enfermedad cardiovascular podría predisponer a un mayor riesgo de MS.
Cardiovascular disease is a leading cause of mortality in patients with chronic kidney disease (CKD), and a large proportion of these deaths occur suddenly and unexpectedly.1–4 The pathogenetic mechanisms behind the association between CKD and sudden cardiac death (SCD) are incompletely understood. Ischemic, structural and electro-physiologic changes of the heart are common in CKD, and all of these conditions predispose to a higher risk of developing lethal arrhythmias.1–4 Furthermore, hyperkalemia, major shift of electrolytes, and hemodynamic instability associated with haemodialysis may trigger cardiac arrhythmias.1–4 In fact, the majority of studies about the association of SCD and CKD have been focused on patients undergoing haemodialysis.5–10
The incidence of SCD in pre-dialysis stages of CKD has been less well investigated. In patients diagnosed with ischemic heart disease or heart failure, a decreased GFR is associated with an increased risk of SCD.11–14 However, to our knowledge, there have been no studies addressing the incidence of SCD in unbiased pre-dialysis CKD populations.
Therefore, we conducted a study to determine the incidence of SCD in a prospective incident cohort of 1078 patients with advanced CKD patients not yet on dialysis. Factors associated with SCD were determined using prediction models, which accounted for competing causes of death.
Material and methodsPatientsBetween January 2000 and October 2013, 1078 incident patients admitted to the advanced chronic kidney disease outpatient clinic of our hospital were included in this prospective observational cohort study. All patients were older than 18 years, they had CKD stage 4 or 5 pre-dialysis, and none of them had previously received a kidney transplant. There were no other exclusion criteria. Demographic, clinical information, and medication use were obtained through the medical records and anamnesis. Co-morbidity was assessed at baseline, using the Davies index,15 and patients were categorized according to their comorbidity scores as having: absence, mild-moderate or severe co-morbid conditions.
The following baseline biochemical parameters, measured by standard laboratory techniques, were included: serum creatinine, potassium, phosphate, calcium, bicarbonate, PTH, albumin, and C-reactive protein. The abbreviated Modification of Diet in Renal Disease equation (MDRD) was used to estimate GFR.16 Chronic hyperkalemia was considered when serum potassium levels were greater than 5.5meq/l in at least 50% of samples from an individual patient, measured throughout the study period.
Outcome measuresCauses of death were categorized as: sudden cardiac death, non-sudden cardiac death, and non-cardiac death.
Sudden cardiac death (SCD) was defined as an unexpected natural death due to cardiac or unknown causes, occurring within 1h after the onset of symptoms; or sudden unexplained death during sleep.
Sudden deaths in patients with end-stage renal disease who rejected dialysis or were on palliative therapy were not considered as SCD.
All SCD were confirmed in interviews with the patient's physician or family members.
Non-sudden cardiac deaths included those due to ischemic heart diseases or heart failure, which had an expected course of events. The rest of causes of death were grouped as non-cardiac death.
Patients in pre-dialysis CKD stages were followed from the time of the study entry until death, loss to follow-up, initiation of dialysis, or end of follow-up on February 2014.
Statistical analysisRates of all-cause and specific causes of mortality are expressed as events per 1000patient/years and their 95% confidence intervals (CI). Parametric and non-parametric tests were chosen as appropriate for descriptive comparisons of continuous variables, and Pearson's chi-squared or Fisher exact test for categorical variables.
The association between baseline variables and SCD in pre-dialysis patients was assessed using Cox proportional hazards models that censored for other causes of death. Age, gender, comorbidity index, diabetes, serum potassium, chronic hyperkalemia, serum levels of phosphate, calcium, bicarbonate, albumin, PTH, C-reactive protein, and medication use (statin, aspirin, betablockers, etc.) were the covariates selected according to their clinical interest and plausible potential to act as confounders. Due to the skewed frequency distribution of serum C-reactive protein values, they were analyzed as their logarithm (log)-transformed values, and also as a dichotomous variable (upper tertile vs. mid or lower tertiles).
Models were fit using Efron approximation for handling ties. The proportional hazard assumption was checked graphically (log–log Kaplan–Meier curves) for all covariates. The age of patients did not satisfy the assumption of linear relationship with log cumulative hazard. All patients who died from SCD were older than 65 years. Thus, additional analyses with models stratified according to the age of the study patients (above or below the median), were carried out. To estimate the cumulative incidence of SCD while accounting for the competing risks of dying from other causes, a competing-risk proportional hazards regression model was built using the method of Fine and Gray.17 The same baseline covariates selected in the Cox model were also re-analyzed with the competing-risk model, and the subhazard ratios (SHR) were estimated.
Descriptive statistics is presented as mean and standard deviation, or median and interquartile ranges (IQR) for continuous variables, and absolute values and percentages for categorical variables. A p-value p<0.05 was considered to be significant. All p-values are reported two-sided. Analyses were performed using IBM SPSS Statistics 21.0 (IBM Corp. Armonk, NY, USA), and STATA 11.1 (StataCorp, TX, USA).
ResultsPatient characteristics and mortalityThe clinical characteristics of the whole group and subgroups according to outcome status in the pre-dialysis follow-up period are listed in Table 1.
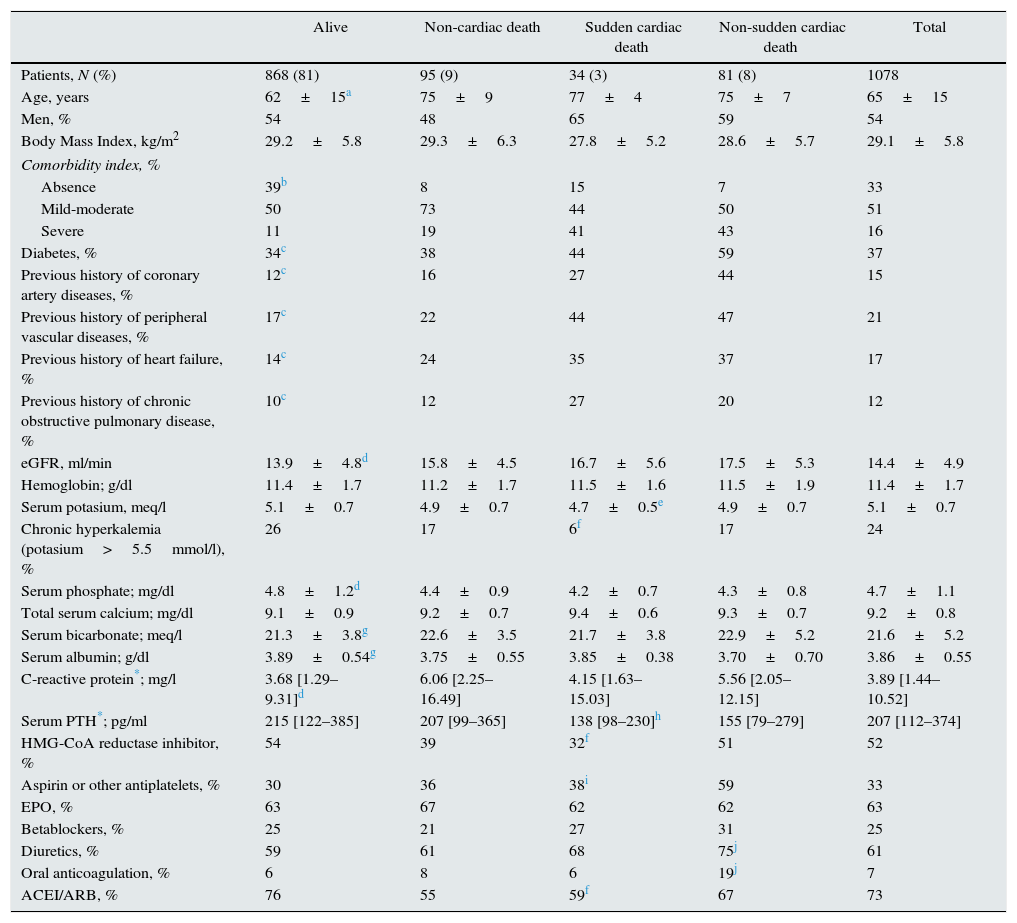
Clinical and biochemical characteristics of patients according to outcome status.
| Alive | Non-cardiac death | Sudden cardiac death | Non-sudden cardiac death | Total | |
|---|---|---|---|---|---|
| Patients, N (%) | 868 (81) | 95 (9) | 34 (3) | 81 (8) | 1078 |
| Age, years | 62±15a | 75±9 | 77±4 | 75±7 | 65±15 |
| Men, % | 54 | 48 | 65 | 59 | 54 |
| Body Mass Index, kg/m2 | 29.2±5.8 | 29.3±6.3 | 27.8±5.2 | 28.6±5.7 | 29.1±5.8 |
| Comorbidity index, % | |||||
| Absence | 39b | 8 | 15 | 7 | 33 |
| Mild-moderate | 50 | 73 | 44 | 50 | 51 |
| Severe | 11 | 19 | 41 | 43 | 16 |
| Diabetes, % | 34c | 38 | 44 | 59 | 37 |
| Previous history of coronary artery diseases, % | 12c | 16 | 27 | 44 | 15 |
| Previous history of peripheral vascular diseases, % | 17c | 22 | 44 | 47 | 21 |
| Previous history of heart failure, % | 14c | 24 | 35 | 37 | 17 |
| Previous history of chronic obstructive pulmonary disease, % | 10c | 12 | 27 | 20 | 12 |
| eGFR, ml/min | 13.9±4.8d | 15.8±4.5 | 16.7±5.6 | 17.5±5.3 | 14.4±4.9 |
| Hemoglobin; g/dl | 11.4±1.7 | 11.2±1.7 | 11.5±1.6 | 11.5±1.9 | 11.4±1.7 |
| Serum potasium, meq/l | 5.1±0.7 | 4.9±0.7 | 4.7±0.5e | 4.9±0.7 | 5.1±0.7 |
| Chronic hyperkalemia (potasium>5.5mmol/l), % | 26 | 17 | 6f | 17 | 24 |
| Serum phosphate; mg/dl | 4.8±1.2d | 4.4±0.9 | 4.2±0.7 | 4.3±0.8 | 4.7±1.1 |
| Total serum calcium; mg/dl | 9.1±0.9 | 9.2±0.7 | 9.4±0.6 | 9.3±0.7 | 9.2±0.8 |
| Serum bicarbonate; meq/l | 21.3±3.8g | 22.6±3.5 | 21.7±3.8 | 22.9±5.2 | 21.6±5.2 |
| Serum albumin; g/dl | 3.89±0.54g | 3.75±0.55 | 3.85±0.38 | 3.70±0.70 | 3.86±0.55 |
| C-reactive protein*; mg/l | 3.68 [1.29–9.31]d | 6.06 [2.25–16.49] | 4.15 [1.63–15.03] | 5.56 [2.05–12.15] | 3.89 [1.44–10.52] |
| Serum PTH*; pg/ml | 215 [122–385] | 207 [99–365] | 138 [98–230]h | 155 [79–279] | 207 [112–374] |
| HMG-CoA reductase inhibitor, % | 54 | 39 | 32f | 51 | 52 |
| Aspirin or other antiplatelets, % | 30 | 36 | 38i | 59 | 33 |
| EPO, % | 63 | 67 | 62 | 62 | 63 |
| Betablockers, % | 25 | 21 | 27 | 31 | 25 |
| Diuretics, % | 59 | 61 | 68 | 75j | 61 |
| Oral anticoagulation, % | 6 | 8 | 6 | 19j | 7 |
| ACEI/ARB, % | 76 | 55 | 59f | 67 | 73 |
Median pre-dialysis follow-up time was 12 months (IQR 5–27 months). During this period, 210 patients died (19%), and 63 were lost to follow-up (6%).
Sudden cardiac death (SCD) occurred in 34 cases (3% of total study patients; 16% of total deaths in pre-dialysis CKD stages).
All-cause mortality, SCD, and non-sudden cardiac death incidence rates were: 113 (95% CI 99–128), 18 (95% CI 13–26) and 44 (95% CI 35–54) events per 1000patient-years, respectively.
Patients who died from SCD were predominantly elderly men with abundant comorbid conditions, mainly diabetes, cardiovascular disease and COPD (Table 1). There were no statistically significant differences in the medians of plasma C-reactive protein levels across subgroups. Median PTH level in the SCD subgroup was slightly lower, but statistically significant, than that of survivors (Table 1).
Despite the fact that patients who died from SCD had high scores of comorbidity, they seemed to be less frequently treated with HMG-CoA reductase inhibitors, antiplatelets drugs, diuretics or ACE inhibitors and/or ARBs than the subgroup of patients who died from non-sudden cardiac diseases. In the SCD subgroup, baseline serum potassium levels were lower and the percentage of patients with chronic hyperkalemia smaller than the rest of subgroups, although this difference was only statistically significant with respect to survivors (Table 1).
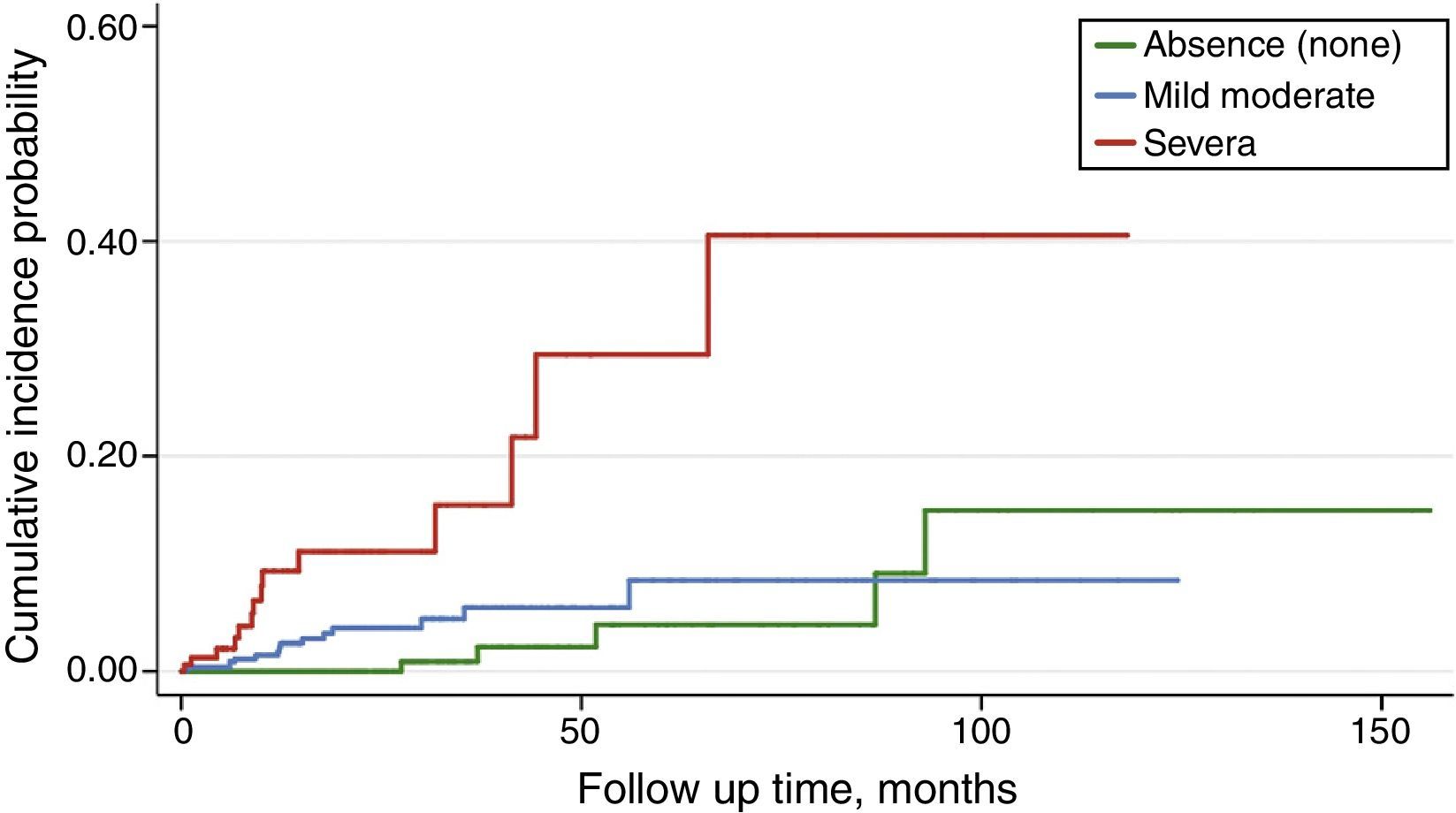
Predictors of sudden cardiac death in pre-dialysis CKD stagesBy non-stratified Cox regression analysis, covariates significantly associated with SCD were: age, comorbidity index, and treatment with antiplatelet drugs (Table 2) (Fig. 1). This latter covariate showed a weak, although significant beneficial effect over the development of SCD.
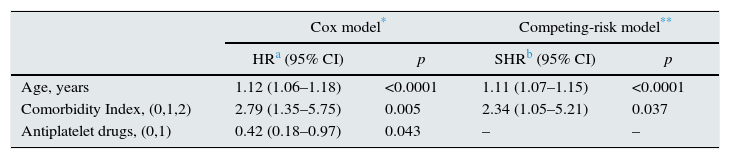
Cox regression and competing-risk regression models for sudden cardiac death in the pre-dialysis study population.
| Cox model* | Competing-risk model** | |||
|---|---|---|---|---|
| HRa (95% CI) | p | SHRb (95% CI) | p | |
| Age, years | 1.12 (1.06–1.18) | <0.0001 | 1.11 (1.07–1.15) | <0.0001 |
| Comorbidity Index, (0,1,2) | 2.79 (1.35–5.75) | 0.005 | 2.34 (1.05–5.21) | 0.037 |
| Antiplatelet drugs, (0,1) | 0.42 (0.18–0.97) | 0.043 | – | – |
Neither log-transformed C-reactive protein levels, nor their upper values (third tertile) were associated with SCD.
When Cox regression was stratified by the median age of patients (above or below 69 years), age was no longer a significant predictor (HR 1.06; 95% CI 0.98–1.15; p=0.09), but comorbidity index (HR 2.59; 95% CI 1.25–5.36; p=0.010) and antiplatelet drug therapy (HR 0.39; 95% CI 0.16–0.92; p=0.031) remained significantly associated with SCD.
By competing-risk regression based on Fine and Gray's proportional subhazards model, in which the competing event was non-sudden death from any cause, only age and comorbidity index remained significantly associated with SCD (Table 2).
DiscussionIn this study, we show that the incidence of SCD among advanced CKD population not yet on dialysis is relatively high, accounting for 16% of total deaths and more than 29% of cardiovascular mortality.
Elderly male patients with high comorbidity burden, mainly diabetes and cardiovascular diseases, were the major clinical characteristics associated with SCD in pre-dialysis CKD stages.
Previous information about SCD incidence in nondialysis CKD population has been derived from subgroup analyses of clinical trials designed to evaluate the efficacy of implantable cardioverter-defibrillators,11,12 or from CKD patients diagnosed with ischemic heart disease o heart failure.13,14 Although the total number of participants with CKD stage 4–5 included in these studies has been rather small, the unanimous conclusion of all of these studies has been that SCD risk increases in parallel with the worsening of renal function.11–14
In the study of Pun et al.,14 which included 175 patients with eGFR <15ml/min not on dialysis, the SCD rate was of 12.6 events per 1000patient-years, incidence lower than that we have observed in our patients. Notwithstanding, patients included in the Pun et al. study were younger (median age 61 years) than our study population (median age 69 years old). The age of patients is a key determinant of SCD risk, as we have demonstrated in our study, and this feature may explain the variability of SCD rates between studies.
In the general population in Europe, the incidence of SCD is approximately of 4 cases per 1000patient/years,18 and among the population aged 60 years or over the incidence increases up to 8 cases per 1000patient/years.19 The incidence of SCD in our study patients was much higher than that observed in the general population, but lower than that observed in patients undergoing haemodialysis (17–28 cases per 1000patient-years).
Both subgroups of patients who died from cardiovascular diseases (SCD and non-SCD) showed similar co-morbidity burden. However, those who died from SCD were being treated less frequently with drugs commonly used for preventing acute cardiovascular events. This finding was especially remarkable with the use of aspirin or other antiplatelet drugs, covariate that eventually became a significant predictor of SCD in Cox regression models.
Underutilization of antiplatelet drugs in these patients may be related to contraindications for their use, or due to a lack of an appropriate diagnosis of ischemic heart disease, as it is suggested by the smaller percentage of patients with previous history of coronary artery disease in those who died from SCD than that of those who died from other cardiovascular diseases. All these data suggest that unrecognized or undertreated cardiovascular disease in CKD patients may predispose to a higher risk of SCD.
Twenty-four percent of our study patients showed chronic hyperkalemia, however, those who died from SCD had lower mean baseline serum potassium levels, and had less frequently chronic hyperkalemia than survivors.
Although hyperkalemia is a dreadful complication in end-stage renal disease that can lead to cardiac arrhythmias and SCD, mild to moderate hyperkalemia, commonly seen in nondialysis CKD patients, does not seem to be associated with serious adverse events.20 Unlike hyperkalemia, hypokalemia has been shown to be associated with greater all-cause mortality in nondialysis CKD patients.20 In the present study, hypokalemia was only observed in 19 patients and none of them died from SCD.
In this study, we have not found a significant association between C-reactive protein (CRP) and SCD. The medians of CRP levels were higher in the subgroups of patients who died than that of survivors, however, CRP levels were not significantly different among subgroups according to the cause of death. In other studies in patients on dialysis,6,10 CRP and serum albumin concentrations have been shown to be associated with a higher risk of SCD.
Age and comorbidity are variables strongly related with all-cause mortality, and therefore, their association with a specific cause of death can be overestimated if competing risk is not taken into account. In this study, we applied a competing-risk analysis using the Fine and Gray method as described by Putter et al.,17 which estimates the cumulative incidence of SCD while accounting for the competing risks of dying from other causes. With this more rigorous method of analysis, age and comorbidity index remained significantly associated with SCD, although treatment with antiplatelet drugs lost the statistical significance.
Potential limitations of this study need to be acknowledged. The study was single center, and the cohort included, although largely representative of the local population, was, however, ethnically homogenous (Caucasian). Some clinical characteristics that could be important in predicting SCD, such as electrocardiogram abnormalities, left ventricular hypertrophy, etc., were not available in all patients. With the exception of serum potassium levels, we only used baseline measurements to determine the association with SCD and not changes in clinical parameters over time. Necropsies were not performed in those who died from SCD, thus precluding more accurate information about the cause of death.
In conclusion, SCD accounted for 16% of global mortality of patients with advanced CKD not yet on dialysis, with an incidence rate much higher than that of the general population. SCD was closely associated with age and comorbidity, and some indirect data from this study suggest that unrecognized or undertreated of cardiovascular disease may predispose to a higher risk of SCD.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Caravaca F, Chávez E, Alvarado R, García-Pino G, Luna E. Muerte súbita en pacientes con enfermedad renal crónica avanzada. Nefrología. 2016;36:404–409.