The neutrophil-to-lymphocyte ratio has demonstrated to be a prognostic inflammatory marker in cardiovascular disease. The objective of this study is to evaluate the association between neutrophil–lymphocyte ratio and pathologic urinary albumin/creatinine ratio as an early marker of cardiovascular risk and systemic endothelial dysfunction, associated with microvascular disease, in asymptomatic subjects.
Materials and methodsA unicenter cross-sectional study was conducted, including 1816 asymptomatic subjects. Patients with previous cardiovascular disease, those who were treated with ACE inhibitors and/or angiotensin II receptor blockers and patients with albumin/creatinine ratio over 300mg/g were excluded. The outcome of the study was the presence of a pathologic urinary albumin/creatinine ratio.
ResultsThe neutrophil–lymphocyte ratio was significantly associated with altered urinary albumin/creatinine ratio in the univariate analysis and after adjustment for other known endothelial and cardiovascular risk factors (age, hypertension, dyslipidaemia, diabetes or altered glomerular filtration rate). Based on the sensitivity and specificity of different neutrophil–lymphocyte ratio thresholds, 3 risk groups were created for altered urinary albumin/creatinine ratio: low risk in those with neutrophil–lymphocyte ratio <1.5, intermediate risk in patients between 1.5 and 3, and high risk in those with neutrophil- to-lymphocyte ratio >3. These groups were found to have a statistically significant and independent prognostic power for altered urinary albumin/creatinine ratio in asymptomatic patients.
ConclusionsThe neutrophil–lymphocyte ratio appears to be a cost-efficient, non-invasive and independent potential marker of systemic endothelial dysfunction in asymptomatic subjects.
El índice neutrófilo/linfocito es un marcador inflamatorio devalor pronóstico en enfermedades cardiovasculares. El objetivo del presente trabajo esvalorar la asociación entre el índice neutrófilo/linfocito y la alteración del cociente albú-mina/creatinina urinario como marcador precoz de disfunción endotelial sistémica asociadaa enfermedad microvascular y riesgo cardiovascular, en sujetos asintomáticos.
Materiales y métodosSe realizó un estudio transversal en 1.816 sujetos asintomáticos. Seexcluyó del a estudio aquellos pacientes que presentaron antecedentes de enfermedad car-diovascular, los que recibían tratamiento con fármacos antiproteinúricos (inhibidores de laenzima conversora de angiotensina y antagonistas de los receptores de la angiotensina II) yaquellos que presentaron un cociente albúmina/creatinina superior a 300mg/dL. La variabledesenlace del estudio fue la alteración del cociente albúmina/creatinina urinario.
ResultadosEl índice neutrófilo/linfocito resultó significativamente asociado a la alteracióndel cociente albúmina/creatinina urinario, tanto en el estudio univariante como en el mul-tivariante, independientemente de otros cofactores como la edad, la hipertensión arterial,la diabetes, la dislipidemia o el filtrado glomerular patológico. El análisis de la sensibilidad yla especificidad de distintos niveles del índice neutrófilo/linfocito permitió generar 3 gruposde riesgo de alteración del cociente albúmina/creatina urinario: riesgo bajo con un cocienteneutrófilo/linfocito < 1,5, riesgo intermedio con cociente neutrófilo/linfocito entre 1,5 y 3 yriesgo alto con un cociente neutrófilo/linfocito > 3. La proporción relativa de alteración delcociente albúmina/creatinina urinario, en los 3 grupos de riesgo, aumentaba en razón delvalor del índice neutrófilo/linfocito de forma independiente al resto de cofactores.
ConclusionesEl índice neutrófilo/linfocito surge como un potencial marcador de disfun-ción endotelial sistémica económico, rápido, no invasivo e independiente de otros factoresconocidos, en sujetos asintomáticos.
Each year, cardiovascular diseases (CVDs) cause around 4million deaths in Europe and 1.9million deaths in the European Union, accounting for 47% of all deaths in Europe and 40% of all deaths in the European Union. CVDs are the most common cause of death in women in all European countries and the most common cause of death in men in at least 6 of them. CVDs cost €196,000million each year in Europe (54% in healthcare spending, 24% in lost productivity and 22% in the care of patients with CVD).1
The high prevalence of cardiovascular risk factors, especially smoking, obesity and diabetes, promotes the development of CVDs, and therefore detection and management of cardiovascular risk factors is very important. The World Health Organisation believes that 75% of deaths due to CVDs could be prevented with suitable changes in lifestyle and modification of risk factors.2 Therefore, early detection of patients with cardiovascular risk is an objective of major importance. To this end, an abnormal urinary albumin/creatinine ratio (UACR) has been shown to be an early marker of cardiovascular morbidity and mortality.3
Noteworthy among the mechanisms involved between an abnormal UACR and CVDs is endothelial dysfunction, at both the microvascular level (decrease in nitric oxide, increase in von Willebrand factor, vascular endothelial growth factor, asymmetric dimethylarginine [ADMA]) and the macrovascular level (vessel dilatation).4 This damage is due in part to inflammation of the endothelium of the microvasculature, which promotes the accumulation of lipids and the development of atherosclerosis.5
Inflammation plays a central role in the physiopathology of diseases that are considered to be non-inflammatory, such as cancer and atherosclerosis.6–11 The determination of circulating peripheral blood leukocytes is a simple, inexpensive and widely available method that helps to assess inflammation. Among the various leucocyte parameters, the ratio absolute neutrophil count to the absolute lymphocyte count (neutrophil–lymphocyte ratio [NLR]) is significantly associated to elevation pro-inflammatory cytokine concentration and development of CVD and progression.12–14 This parameter has been shown to be an inflammatory marker with a high predictive power of death, acute myocardial infarction and severity of coronary heart disease.14–16 In addition, various studies have investigated the link between NLR and diabetes mellitus, insulin resistance as assessed by the Homoeostasis Model Assessment of Insulin Resistance (HOMA-IR), hypertension, obesity, hyperlipidaemia, lifestyle and endothelial dysfunction.17–19
To date, studies about the association between NLR and CVDs have been performed in patients with symptomatic CVD. The objective of this study was to evaluate whether this association is observed in asymptomatic subjects without a prior history of CVD, using an abnormal UACR as an early marker of systemic endothelial damage and cardiovascular risk.
Materials and methodsA cross-sectional study was conducted with patients from several regions of Spain who visited the Clínica Universidad de Navarra for routine medical check-up and an estimation of cardiovascular risk. A total of 2246 clinically asymptomatic patients were evaluated since May 1999–January 2011. Each patient underwent a complete medical history that included the collection of family histories of CVD, as well as general laboratory testing with a complete blood count, blood chemistry, cholesterol fractions and the determination of albumin and creatinine in urine from first urination. Patients were excluded if they had a personal history of cardiovascular events (acute myocardial infarction, stroke or peripheral artery disease), were on antiproteinuric drugs (angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists) or had a UACR >300mg/g. The 1816 remaining subjects were enrolled in the final cohort. The study was designed in accordance with the Declaration of Helsinki and the protocol was approved by the Clínica Universidad de Navarra Ethics Committee.
Cardiovascular risk factors were defined according to the guidelines of the modified Adult Treatment Panel (ATP)-III.20 Laboratory determinations were made using standard laboratory techniques. To calculate the NLR, the ratio absolute neutrophil count to absolute lymphocyte count was obtained from the complete blood count. To evaluate insulin resistance, the HOMA-IR was used as both a continuous variable and as values above or below the cut-off point of 2.19 The Modification of Diet in Renal Disease formula was used for the estimated glomerular filtration rate (eGFR),, and it was considered to be pathological below 60ml/min/1.73m2. A urinary albumin/creatinine ratio between 30 and 300mg/g was used as a marker of endothelial dysfunction and cardiovascular risk.21
The results were expressed as the value of the arithmetic mean±standard deviation for continuous quantitative variables. Qualitative variables were presented in the form of proportions. Univariate statistical analysis was performed using hypothesis-corroborating tests: the chi-squared test for qualitative variables, Student's t test for comparisons of quantitative variables with homogeneity of variances in Levene's test and the Mann–Whitney U test in the rest. Multivariate analysis was performed using logistic regression models. A sensitivity analysis was performed to seek the values with the greatest discriminatory capacity. In the results of this analysis, the parameters with the highest sensitivity and specificity were considered to be clinically useful.22 Statistical calculations were performed using the SPSS version 20.0 program.
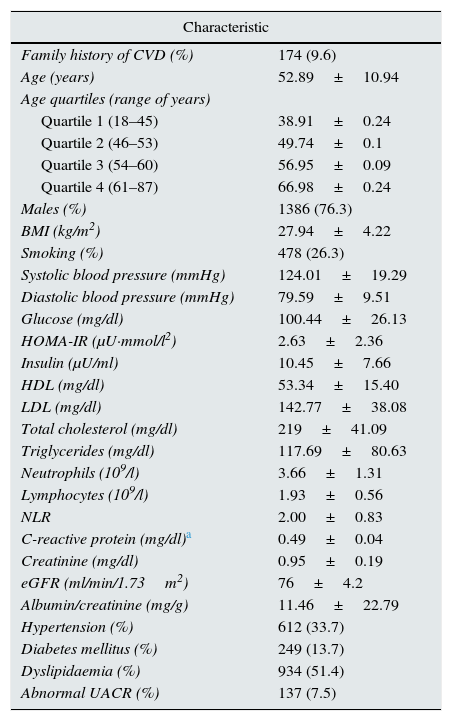
ResultsTable 1 shows the clinical characteristics of the study population. Mean NLR was 2±0.83. Abnormal UACR was observed in 7.5% of patients.
Clinical characteristics of the population (n=1816).
| Characteristic | |
|---|---|
| Family history of CVD (%) | 174 (9.6) |
| Age (years) | 52.89±10.94 |
| Age quartiles (range of years) | |
| Quartile 1 (18–45) | 38.91±0.24 |
| Quartile 2 (46–53) | 49.74±0.1 |
| Quartile 3 (54–60) | 56.95±0.09 |
| Quartile 4 (61–87) | 66.98±0.24 |
| Males (%) | 1386 (76.3) |
| BMI (kg/m2) | 27.94±4.22 |
| Smoking (%) | 478 (26.3) |
| Systolic blood pressure (mmHg) | 124.01±19.29 |
| Diastolic blood pressure (mmHg) | 79.59±9.51 |
| Glucose (mg/dl) | 100.44±26.13 |
| HOMA-IR (μU·mmol/l2) | 2.63±2.36 |
| Insulin (μU/ml) | 10.45±7.66 |
| HDL (mg/dl) | 53.34±15.40 |
| LDL (mg/dl) | 142.77±38.08 |
| Total cholesterol (mg/dl) | 219±41.09 |
| Triglycerides (mg/dl) | 117.69±80.63 |
| Neutrophils (109/l) | 3.66±1.31 |
| Lymphocytes (109/l) | 1.93±0.56 |
| NLR | 2.00±0.83 |
| C-reactive protein (mg/dl)a | 0.49±0.04 |
| Creatinine (mg/dl) | 0.95±0.19 |
| eGFR (ml/min/1.73m2) | 76±4.2 |
| Albumin/creatinine (mg/g) | 11.46±22.79 |
| Hypertension (%) | 612 (33.7) |
| Diabetes mellitus (%) | 249 (13.7) |
| Dyslipidaemia (%) | 934 (51.4) |
| Abnormal UACR (%) | 137 (7.5) |
UACR, urinary albumin/creatinine ratio; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; BMI, body mass index; NLR, neutrophil–lymphocyte ratio; LDL, low density lipoprotein.
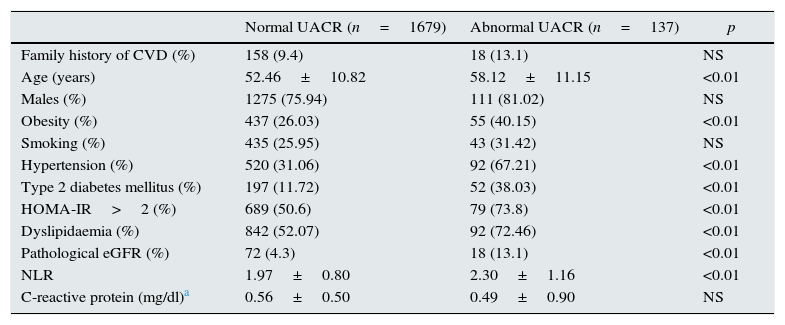
As shown in Table 2, abnormal UACR was significantly associated with age, obesity, hypertension, diabetes, insulin resistance (HOMA-IR>2), dyslipidaemia and <60ml/min/1.73m2 of eGFR. The same table shows a significant increase in NLR in patients with an abnormal UACR. C-reactive protein (CRP) was used to evaluate an abnormal UACR's capacity for predicting other markers of inflammation. This analysis was performed in a subgroup of the patients in which this determination was available (n=439). In this subgroup, the prevalence of an abnormal UACR was 8%, which was similar to that of the general population (p>0.5). No statistically significant differences were found in CRP levels in patients with an abnormal UACR as compared with a UACR within normal limits (p<0.45). Nevertheless, in this subgroup of 439 patients, NLR remained significantly associated with an abnormal UACR (p<0.05).
Univariate analysis of the association of different cardiovascular risk factors with an abnormal UACR.
| Normal UACR (n=1679) | Abnormal UACR (n=137) | p | |
|---|---|---|---|
| Family history of CVD (%) | 158 (9.4) | 18 (13.1) | NS |
| Age (years) | 52.46±10.82 | 58.12±11.15 | <0.01 |
| Males (%) | 1275 (75.94) | 111 (81.02) | NS |
| Obesity (%) | 437 (26.03) | 55 (40.15) | <0.01 |
| Smoking (%) | 435 (25.95) | 43 (31.42) | NS |
| Hypertension (%) | 520 (31.06) | 92 (67.21) | <0.01 |
| Type 2 diabetes mellitus (%) | 197 (11.72) | 52 (38.03) | <0.01 |
| HOMA-IR>2 (%) | 689 (50.6) | 79 (73.8) | <0.01 |
| Dyslipidaemia (%) | 842 (52.07) | 92 (72.46) | <0.01 |
| Pathological eGFR (%) | 72 (4.3) | 18 (13.1) | <0.01 |
| NLR | 1.97±0.80 | 2.30±1.16 | <0.01 |
| C-reactive protein (mg/dl)a | 0.56±0.50 | 0.49±0.90 | NS |
UACR, urinary albumin/creatinine ratio; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; NLR, neutrophil–lymphocyte ratio.
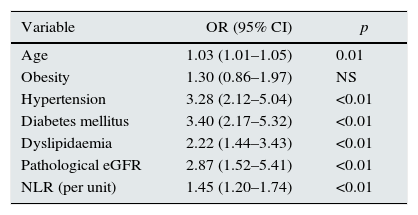
After the univariate analysis, a logistic regression model was prepared including all the previously significant variables. As shown in Table 3, all the vascular risk factors except obesity remained significantly and independently associated with an abnormal UACR. The NLR value remained an independent marker of an abnormal UACR (OR: 1.45. p<0.01). In an alternative model, the HOMA-IR was changed by diabetes to avoid collinearity. The HOMA-IR was significant in identifying an abnormal UACR (OR: 1.11; p<0.01), and the predictive power of NLR's persisted. Table complementary 1 presents this model. In the subgroup of 439 patients whose CRP levels were available, 2 multivariate models were tested with the same adjustment variables. The first model used the NLR to predict inflammation; it was significantly associated with an abnormal UACR (OR: 1.6; p<0.01; Table complementary 2). By contrast, the second model that used CRP as a marker of inflammation, it was not significantly associated with an abnormal UACR (OR: 0.94; p>0.05; Table complementary 3).
Multivariate analysis of the association of different cardiovascular risk factors with an abnormal UACR.
| Variable | OR (95% CI) | p |
|---|---|---|
| Age | 1.03 (1.01–1.05) | 0.01 |
| Obesity | 1.30 (0.86–1.97) | NS |
| Hypertension | 3.28 (2.12–5.04) | <0.01 |
| Diabetes mellitus | 3.40 (2.17–5.32) | <0.01 |
| Dyslipidaemia | 2.22 (1.44–3.43) | <0.01 |
| Pathological eGFR | 2.87 (1.52–5.41) | <0.01 |
| NLR (per unit) | 1.45 (1.20–1.74) | <0.01 |
eGFR, estimated glomerular filtration rate; NLR, neutrophil–lymphocyte ratio; OR, odds ratio.
Subsequently, the relationship between the NLR and the different cardiovascular risk factors was studied. In our study, hypertension, diabetes, smoking and obesity were not associated with the NLR. Moreover, the anti-inflammatory effect of statins on the endothelium could affect the relationship between the NLR and an abnormal UACR.23,24 In our series, the 196 patients being treated with statins had lower NLR values, although the difference was not statistically significant, (1.89±0.85 for the group with statins vs 2.01±0.83 for the group without statins, p>0.05). However, statistically significant differences were observed between the NLR of patients with a HOMA-IR>2, as compared to patients without insulin resistance (1.94±0.78 vs 2.05±0.89 p<0.05).
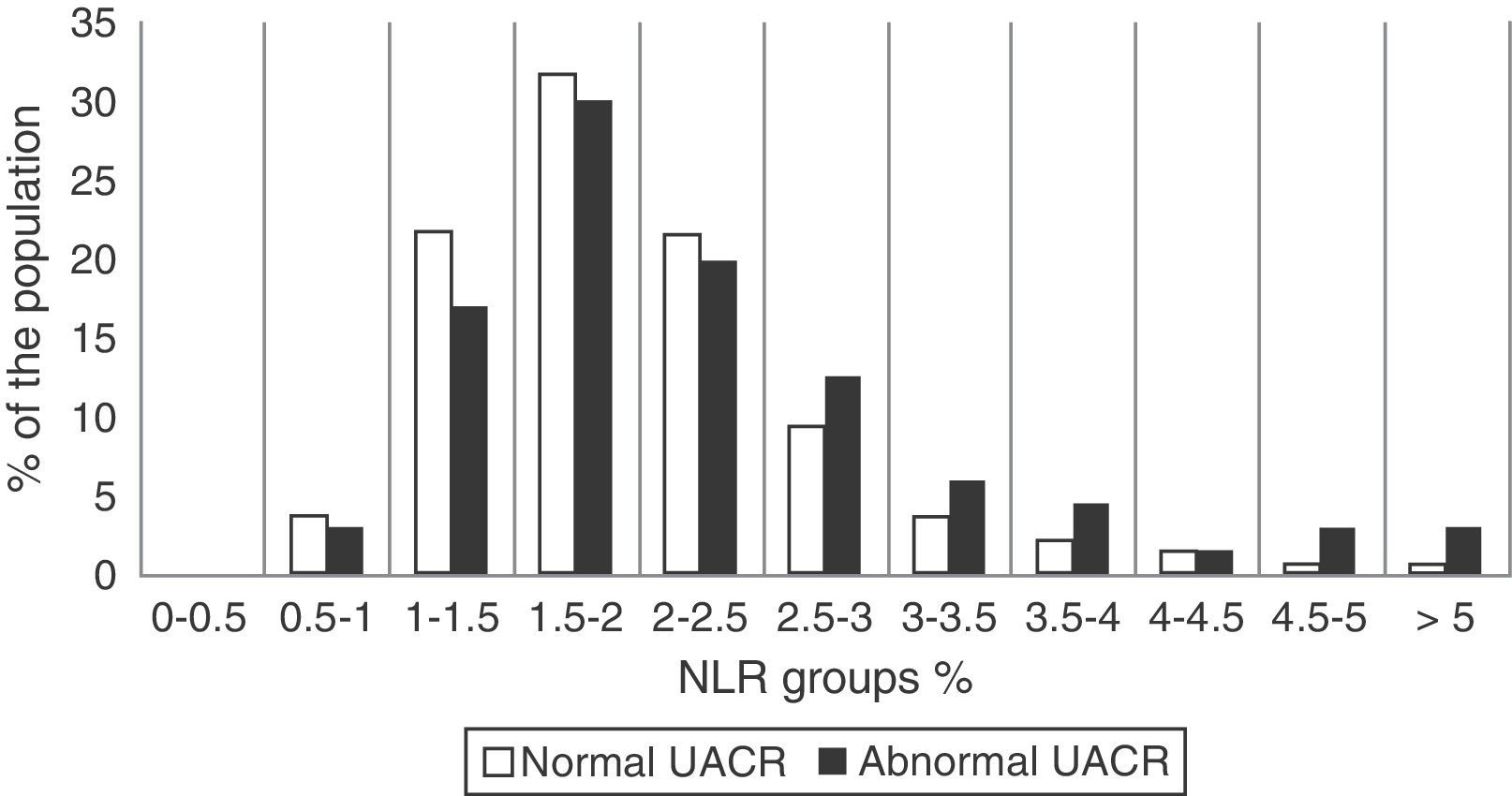
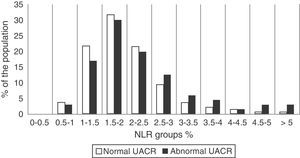
Next, the distribution of the NLR in our sample was analysed. To do this, the population was divided into 2 groups depending on whether or not they had an abnormal UACR. Then, patients in both subgroups were divided into 11 NLR strata, each spanning 0.5 units, from an NLR of 0–0.5 to an NLR of >5. As shown in Fig. 1, 87.1% of the population had NLR levels between 1 and 3. Moreover, starting from an NLR value >2.5, the relative proportion of patients with an abnormal UACR clearly increased.
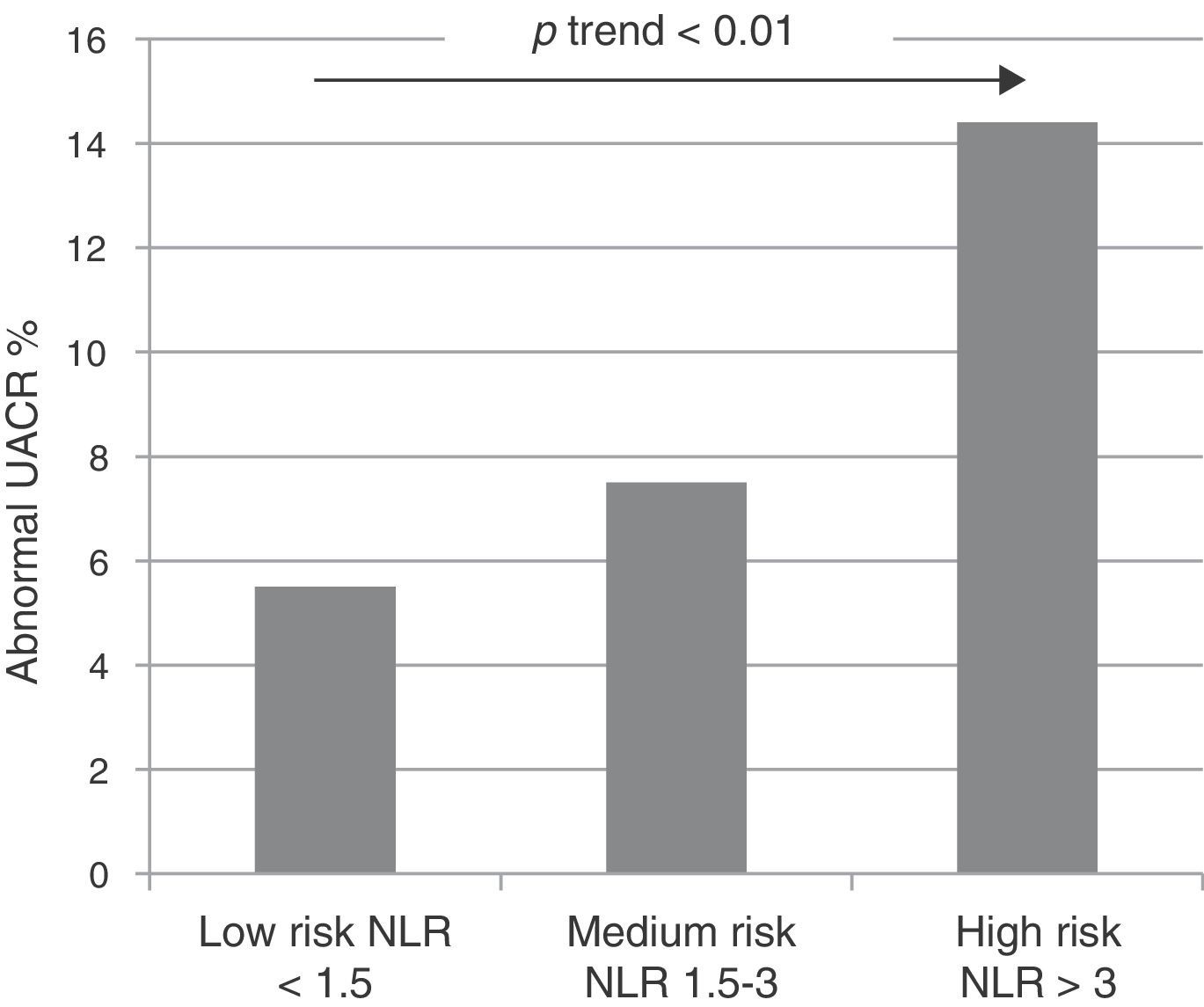
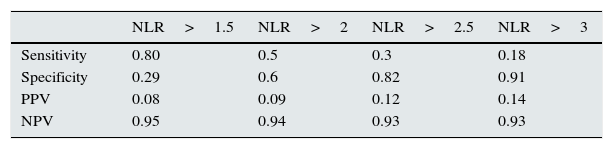
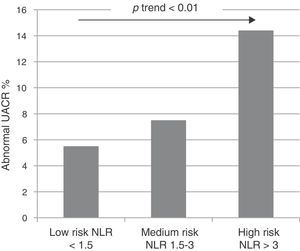
After these results were obtained, the NLR cut-off point with the greatest abnormal UACR discriminatory capacity was estimated by using a sensitivity analysis. To do this, the sensitivity, specificity, positive predictive values and negative predictive values were calculated for NLR values between 1.5 and 3. The results can be seen in Table 4. The table shows that the lowest NLR values had greater sensitivity for detecting abnormal UACRs, while higher NLR values had greater specificity. The results of this analysis defined the values of 1.5 and 3 as cut-off points for the risk subgroups, given the greater sensitivity of the former and the greater specificity of the latter. The population was divided into 3 groups of patients at risk of an abnormal UACR depending on the NLR: low risk (NLR<1.5), intermediate risk (NLR between 1.5 and 3) and high risk (NLR>3). Fig. 2 shows the distribution of abnormal UACRs in the 3 groups. It should be noted that the relative proportion of abnormal UACRs progressively and significantly increased as NLR values increased.
Analysis of sensitivity of the NLR in the prediction of an abnormal UACR.
| NLR>1.5 | NLR>2 | NLR>2.5 | NLR>3 | |
|---|---|---|---|---|
| Sensitivity | 0.80 | 0.5 | 0.3 | 0.18 |
| Specificity | 0.29 | 0.6 | 0.82 | 0.91 |
| PPV | 0.08 | 0.09 | 0.12 | 0.14 |
| NPV | 0.95 | 0.94 | 0.93 | 0.93 |
NLR, neutrophil–lymphocyte ratio; NPV, negative predictive value; PPV, positive predictive value.
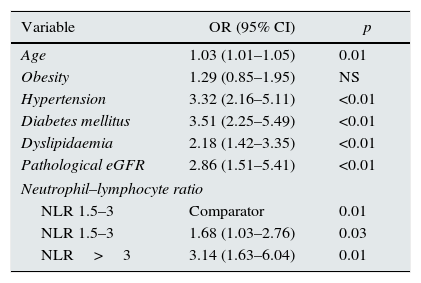
Finally, the cut-off points for the risk subgroups were included in a multivariate model that is shown in Table 5. Patients with an NLR between 1.5 and 3 had an OR of 1.68 as compared to those with an NLR<1.5. Patients with an NLR>3 had an increased risk up to an OR of 3.14 in the same comparison. This new model, which had a significant discrimination improvement rate (p<0.01), with a reclassification improvement rate of 4.05%, was subsequently analysed. This result would implicate the reclassification of 74 patients in the sample.
Multivariate analysis of vascular risk factors and different cut-off points of the NLR in relation to an abnormal UACR.
| Variable | OR (95% CI) | p |
|---|---|---|
| Age | 1.03 (1.01–1.05) | 0.01 |
| Obesity | 1.29 (0.85–1.95) | NS |
| Hypertension | 3.32 (2.16–5.11) | <0.01 |
| Diabetes mellitus | 3.51 (2.25–5.49) | <0.01 |
| Dyslipidaemia | 2.18 (1.42–3.35) | <0.01 |
| Pathological eGFR | 2.86 (1.51–5.41) | <0.01 |
| Neutrophil–lymphocyte ratio | ||
| NLR 1.5–3 | Comparator | 0.01 |
| NLR 1.5–3 | 1.68 (1.03–2.76) | 0.03 |
| NLR>3 | 3.14 (1.63–6.04) | 0.01 |
eGFR, estimated glomerular filtration rate; NLR, neutrophil–lymphocyte ratio; OR, odds ratio.
Inflammation plays a fundamental role in the pathophysiology of CVDs.6 Among the various markers of inflammation, the NLR has been previously assessed as a predictor of mortality in patients with acute coronary syndrome,25 congestive heart failure26 and diabetes mellitus27 and patients having undergone a coronary catheterisation.28 In addition, it has recently been evaluated as a risk factor in a study of asymptomatic patients in the cohort from Framingham's study, which confirmed to be a powerful predictor of cardiovascular mortality. This study refers NLR as a parameter with a potential cardiovascular risk reclassification capacity that is greater than other markers such as ultrasensitive CRP, the N-terminal fragment of the B-type natriuretic peptide, glycated haemoglobin, cystatin and homocysteine.29
Previous publications have reported an association between NLR values and urinary albumin excretion, in both diabetic patients30 and patients with symptomatic chronic kidney disease.31 The importance of detecting the mechanisms of endothelial damage to prevent CVD justifies the use of an abnormal UACR as an early marker of both cardiovascular risk and systemic endothelial dysfunction.5,32–35 In this study's cohort, a clinically asymptomatic patient population, the NLR was also significantly correlated with an abnormal UACR. This association was present regardless of other risk factors associated with an abnormal UACR.
Several studies have associated inflammation with greater insulin resistance.36 Recently, Lou et al.19 presented a significant correlation between an increase in the NLR and the HOMA-IR (OR: 7.23 p<0.01). The association between insulin resistance and the NLR was confirmed in our sample. The anti-inflammatory effect of HMG-CoA reductase inhibitors could also have an influence on the NLR.23,24 However, an article by Gungoren et al.37 demonstrated no statistically significant differences in NLR among patients treated with statins and patients not treated with statins. In our analysis, patients on statins had a low NLR without statistical differences between the groups. Our results may justify the study of this association in cohorts with a larger number of patients. Finally, our results showed that as compared with CRP, the NLR has a greater capacity to detect an abnormal UACR. In fact CRP did no show statistically significance in this context.
A useful risk marker requires to know what are the levels with clinically significance These levels can be properly selected using a sensitivity and specificity analysis of different cut-off points of the marker in question. This methodology has been extensively studied and used in selecting cut-off points in other clinical situations.22 Noteworthy in our study was the limited sensitivity that all the levels offered, except the cut-off point of 1.5, which had a sensitivity of 0.80. Sensitivity is the result of the ratio between the true positives in a test and the sick patients in the population. A low sensitivity is related to an increase in the false negatives in the test. As the classic risk factors for an abnormal UACR were not found to be associated with the NLR in our sample, many patients would have been false negatives if had only the NLR had been considered. However, statistically significant differences between the NLR groups in the multivariate analysis validated the usefulness of the cut-off points selected using this method.
The main limitation of our study was its cross-sectional design, which prevented the establishment of a causal relationship between the NLR and an abnormal UACR. Moreover, although the population recruited from a single centre limited extrapolation of the results, the sample size and the fact that the patients were from different regions of Spain increased the study's external validity. In any case, the association between the NLR and an abnormal UACR deserves to be studied in greater depth to determine its role not only as an inexpensive, quick and non-invasive marker of cardiovascular risk but also as a potential therapeutic target.
This study demonstrated a significant correlation between, clinically significant NLR values and cardiovascular risk and systemic endothelial dysfunction associated to microvascular disease in a cohort of asymptomatic patients. Given the limitations set forth above, interventional, multi-centre studies with a greater number of outcomes are required to assess other potential clinical implications of this finding.
ConclusionThe NLR may represent a new tool for the assessment of cardiovascular risk and the risk of systemic endothelial dysfunction associated with microvascular disease in asymptomatic patients.
FundingThe study required no external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martínez-Urbistondo D, Beltrán A, Beloqui O, Huerta A. El índice neutrófilo/linfocito como marcador de disfunción sistémica endotelial en sujetos asintomáticos. Nefrologia. 2016;36:397–403.