Chronic kidney disease (CKD) and atherosclerosis are 2 interrelated diseases that increase the risk of cardiovascular morbidity and mortality. The objectives of the ILERVAS project are: (1) to determine the prevalence of subclinical arterial disease and hidden kidney disease; (2) to assess the impact of early diagnosis of both diseases on cardiovascular morbidity and mortality and also on the progression of CKD; (3) to have a platform of data and biological samples.
MethodsRandomised intervention study. From 2015 to 2017, 19,800 people (9900 in the intervention group and 9900 in the control group) aged between 45 and 70 years without previous history of cardiovascular disease and with at least one cardiovascular risk factor will be randomly selected from the primary health care centres across the province of Lleida. A team of experts will travel around in a mobile unit to carry out the following baseline tests on the intervention group: Artery ultrasound, (carotid, femoral, transcranial and abdominal aorta), ankle-brachial index, spirometry, determination of advanced glycation end products, dried blood spot and urine spot tests. Additionally, blood and urine samples will be collected and stored in the biobank to identify new biomarkers using omics studies. Participants will be followed up until 2025 for identification of cardiovascular events, treatment changes and changes in lifestyle.
ConclusionsThe ILERVAS project will reveal the prevalence of subclinical vascular disease and hidden kidney disease, determine whether or not their early diagnosis brings health benefits and will also allow investigation of new risk factors.
La enfermedad renal crónica (ERC) y la ateromatosis son 2 enfermedades interrelacionadas que aumentan el riesgo de morbimortalidad cardiovascular. Los objetivos del proyecto ILERVAS son: 1) conocer la prevalencia de enfermedad ateromatosa subclínica y de enfermedad renal oculta; 2) valorar el impacto de su diagnóstico precoz sobre la morbimortalidad cardiovascular y la progresión de la ERC; 3) disponer de una plataforma de datos y muestras biológicas.
MétodosEstudio de intervención aleatorizado. Entre 2015 y 2017 se incluirá a 19.800 personas (9.900 en el grupo de intervención y 9.900 en el grupo control) entre 45 y 70 años, sin antecedentes de enfermedad cardiovascular y que presenten al menos un factor de riesgo cardiovascular, seleccionadas aleatoriamente de los centros de atención primaria (AP) de la provincia de Lérida. Un equipo técnico experto se desplazará con una unidad móvil para realizar las exploraciones basales al grupo de intervención: ecografía arterial (carótida, femoral, transcraneal y aorta abdominal), medición del índice tobillo-brazo, espirometría, detección de los productos de glicación avanzada y analítica seca de sangre y orina. Adicionalmente, se recogerán muestras de sangre y orina que serán almacenadas en el biobanco para identificar nuevos biomarcadores con biología de sistemas. Los participantes serán seguidos hasta 2025 para la identificación de eventos cardiovasculares, cambios de tratamiento y modificación de estilos de vida.
ConclusionesEl proyecto ILERVAS permitirá conocer la prevalencia de enfermedad vascular y de enfermedad renal subclínicas, evaluar si su diagnóstico precoz tiene un beneficio en la salud e investigar factores de riesgo emergentes.
Cardiovascular (CV) diseases are the leading cause of morbidity and mortality worldwide, with a major social, healthcare-related and economic impact. In 2012, in the European Union, CV disease accounted for 40% of overall mortality with a cost of €196billion, distributed as follows: 54% for healthcare spending, 24% for lost productivity and 22% for care of patients with CV disease.1 In the same year, in Spain, CV disease accounted for 30% of all recorded deaths and 15% of all hospital admissions.2 In the Spanish province of Lleida, in 2012, CV disease was the leading cause of death, with a prevalence of 26% of ischaemic heart disease of and 27%.of cerebrovascular events.3
Risk calculation tables (Framingham, Score, Regicor) based on traditional CV risk factors such as age, sex, smoking habit, blood pressure, total cholesterol and diabetes have been used to detect the population susceptible of having a CV event. However, these calculations do not take into account other factors that also have an impact. These factors include, but are not limited to a history of early CV disease in first-degree relatives (men before age 55, women before age 65), genetic factors and individual susceptibility, obesity, socioeconomic level, sleep apnoea, and chronic kidney disease (CKD). It has been indicated that more than 60% of CV events occur in individuals with a low to moderate calculated risk, and that 4 of every 10 cases of miocardial infarction or sudden death occur in people with no prior history of CV disease.4
Numerous population studies, based on large cohorts and long periods of follow-up, have demonstrated a clear association between the presence of an atheromatous plaque in the carotid arteries and the risk of having a coronary or cerebrovascular event (1.8–4.1, respectively), and have concluded that a diagnosis of subclinical atheromatosis increases the predictive value of traditional risk equations, which reclassify individuals according to degrees of risk greater than those calculated by these algorithms.5–9
Atheromatous disease is characterised by a chronic, proliferative and multifactorial inflammatory process that affects the vascular bed such that atheromatous plaques form in medium- to large-sized arteries walls (carotid, femoral, cerebral, coronary and renal).10 The clinical manifestations will depend on both the area and the number of vascular regions affected, and on the volume and vulnerability of the atheromatous plaque which, through various mechanisms, may undergo rupture, formation of a thrombus and subsequent onset of a CV event (ischaemic cerebrovascular accident, acute myocardial infarction or ischaemia of the lower limbs).11 Artery ultrasound is a non-invasive, reproducible, validated technique with high sensitivity and specificity allowing a reliable, cost-effective diagnosis of subclinical atheromatosis to be made. Indeed, there is a gradual trend towards its inclusion in current clinical guidelines for the management of CV risk.12,13
One of the diseases with high CV mortality and a elevated prevalence of atheromatosis is CKD.14,15 CKD gradually increases with age (22% in those over age 64 and 40% of those over age 80), type 2 diabetes and hypertension (35–40% in diabetic or hypertensive patients).16 Patients with CKD are asymptomatic until the disease is advanced and in the majority of cases, at the time of the diagnosis of CKD the patient requires referral to a specialised physician for replacement treatment either with dialysis or transplantation, with a high associated healthcare cost. In Spain, it is estimated that 10% of the adult population (20% in those over age 65) have hidden kidney disease (HKD) and that 40% will die of CV disease before entering a dialysis programme.17
Therefore, due to the high morbidity and mortality of subclinical atheromatous disease (SAD) and HKD it is justified to propose an epidemiological study to determine its prevalence in the asymptomatic population with at least one CV risk factor. There are no studies addressing the impact of early diagnosis of SAD and HKD on CV morbidity and mortality, CKD progression and clinical management in primary care level (PC).
In the ILERVAS study we propose performing an overall assessment of CV and renal risk with non-invasive diagnostic tools. To do this, we propose a collaborative study that involves different research groups from the Instituto de Investigación Biomédica de Lleida (IRBLleida) that will provide specific diagnostic methods from their specialty, and that will be conducted sequentially during the journey made by individuals within the mobile unit (MU) (Figs. 1 and 2).
The general aims of the ILERVAS proposal are:
- (1)
To determine the prevalence of SAD and HKD in the asymptomatic general population with at least one CV risk factor.
- (2)
To analyse the impact of the interventions performed in the MU on CV morbidity and mortality and CKD progression during a 10-year follow-up period.
- (3)
To have a platform for data encompassing baseline data (clinical, clinical-chemistry and biobank) and follow-up data allowing the association between the different known and emerging risk factors and SAD, HKD, CV morbidity and mortality, and CKD progression to be analysed. These factors include:
- a.
Biomarkers (proteomics, metabolomics, lipidomics and genetic polymorphisms).
- b.
Lung capacity and sleep abnormalities.
- c.
Advanced glycation end products (AGEs).
- d.
Diet and physical activity.
- e.
Traditional risk factors.
- a.
A randomised interventional study with 2 arms that will include 19,800 individuals with at least one CV risk factor, registered in PC in the province of Lleida, between January 2015 and December 2017, who will subsequently be followed up until January 2025. The ILERVAS study protocol, information about the population, location of the MU, informative videos and materials about CV disease, contact information and publications will be available on the following website: www.elbusdelasalut.cat/.
Source of information and instruments for data collectionSociodemographic variables (age, sex and race), medical history of comorbidities and medical treatments will be collected from the electronic PC medical history (e-CAP).
The other variables will be collected in the MU, which will travel throughout the province of Lleida. This MU is has the equipment to perform vascular diagnostic tests, spirometry and collection of anthropometric and clinical-chemistry data. In addition, variables related to physical exercise will be collected from the International Physical Activity Questionnaire (IPAQ),18 variables related to diet will be collected from the diet survey adapted from the PREDIMED study19 and variables related to daytime somnolence will be collected from the Berlin questionnaire20 and the Epworth somnolence scale.21
Each week, all ILERVAS study data entered in the e-CAP will be extracted for quality control and to be exported to a general database designed for this purpose.
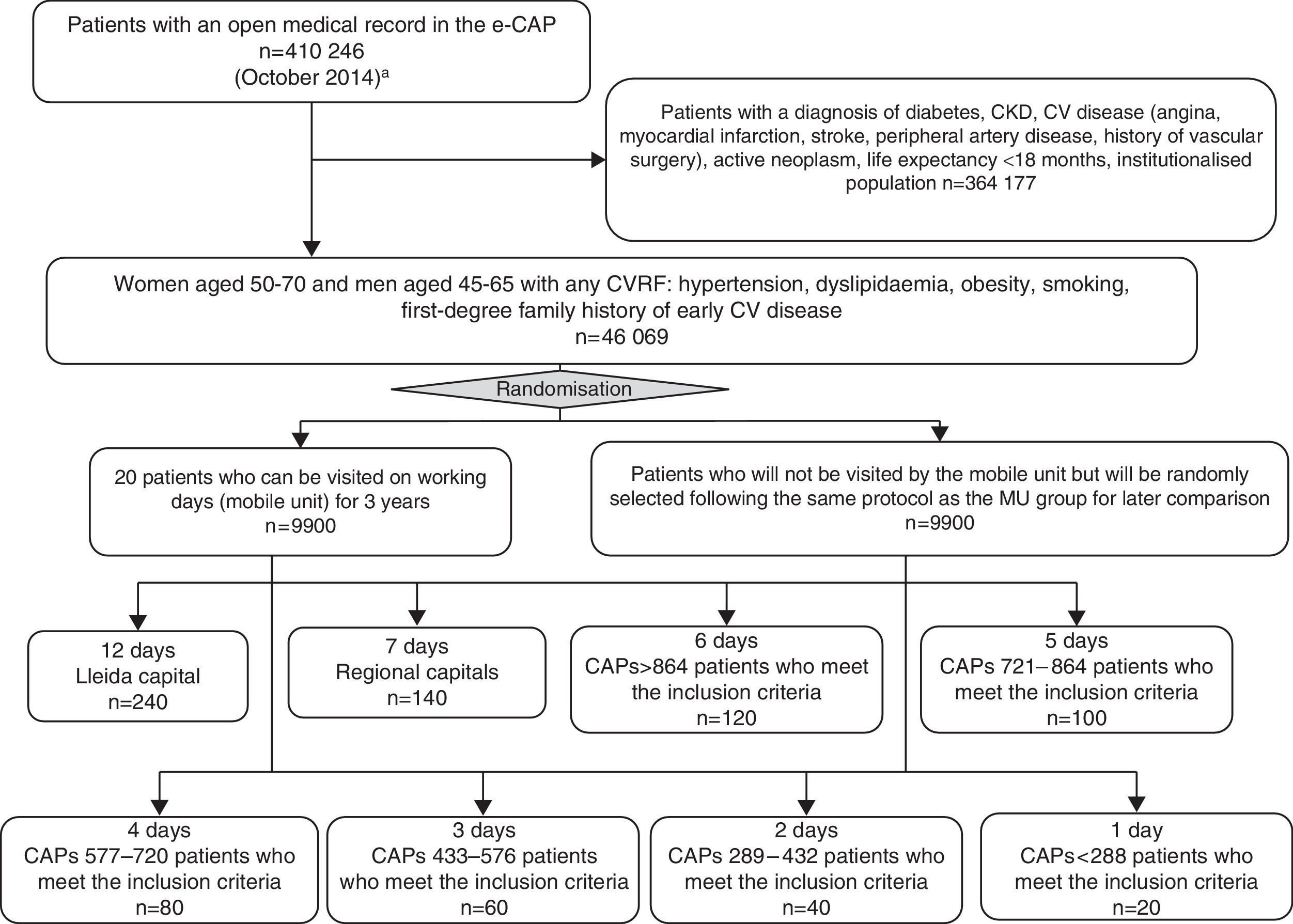
Participant selectionThe participant selection process is shown in Fig. 3. From a total of 410,246 people with an open history in the e-CAP (on October 24, 2014), the study population that meets the inclusion criteria will be identified. The inclusion criteria are: women between the ages of 50 and 70 and men between the ages of 45 and 65 with at least one CV risk factor (hypertension, dyslipidaemia, obesity, smoking habit, first-degree family history of early CV disease), no prior medical history of CV disease, diabetes, CKD or active neoplasm, and a life expectancy no shorter than 18 months (n=46,069). Of these, a total of 19,800 people (9900 in the intervention group and 9900 in the control group) will be selected through simple randomisation without replacement, within clusters defined by basic healthcare area, PC centre or doctor's office, in accordance with the total number of people who may be visited in the MU during f one year period (20 patients per working day). Each day 10 people will be added to cover a potential non-response rate of up to 50%. Finally, 3300 people per year will be studied in the MU, following selection of a total of 4950 candidates each year. From the second year of the study, sample selection will be performed every six months. People who have participated in previous years will be excluded. To identify the population with no electronic medical history in the e-CAP (Val d’Aran, Solsonès and Cerdanya) the ILERVAS team will request information from the responsible of each healthcare area.
The participants selected will be informed of the study objectives and the diagnostic tests to be performed by means of a personalised letter with an appointment day and time. Prior to the inclussion in the study they must sign the informed consent form. The study protocol has been approved by the Hospital Universitario Arnau de Vilanova (Lleida) Ethics Committee.
One month before the MU reaches each population, 2 investigators responsible for the project will travel to hold meetings on information and updates about subclinical atheromatous arterial disease, HKD and abdominal aortic aneurysm; to explain the project to healthcare workers and administrators of the different healthcare basic areas; and to help with the interpretation of the results of the examinations and recommendations that will be included in the ILERVAS final report in the electronic medical history of each patient which are based on clinical practice guidelines10,22–25 (Appendix B: Supplementary material).
Follow-up periodAs this is an asymptomatic population with a low to moderate CV risk, a minimum 10-year follow-up period has been established to observe the onset of CV events.26,27 Initially the cohort will be followed up from January 2015 to January 2025. The follow-up period may be lengthened, given that all data will be recorded in the e-CAP. The population selected will be monitored every six months to collect changes in medical history related to the study objectives.
Cardiovascular events and CKD progressionThe onset of a CV event will be recorded according to the tenth version of the International Statistical Classification of Diseases (ICD-10), which includes: angina pectoris, myocardial infarction, transient ischaemic attack, cerebrovascular accident, heart failure, arrhythmia, peripheral artery disease, aortic aneurysm, revascularisation and angioplasty of any artery region. Likewise, the patient's cause of death, whether of CV origin (myocardial infarction, arrhythmia, heart failure, cerebrovascular accident, aortic aneurysm, mesenteric infarction or sudden death) or of non-CV origin (infections, cancers, accident or kidney disease) will be recorded.
The information sources used to identify CV events will be: review of medical records (through the e-CAP) and consultation of the Catalonia mortality registry.
CKD progression is defined as a doubling of creatinine or entry into renal replacement therapy, and will also be collected through the e-CAP.
Vascular ultrasoundThe examination and reading will be performed by 2 nurses specialised in vascular imaging diagnostics, following a standardised protocol with the patient in supine decubitus.28 The VIVID I version BT12 ultrasound system (GE Healthcare) will be used. The system has a 12L-RS/4–13MHz linear probe (ultrasound of the carotid and femoral arteries), a 4C-RS/1.5–6MHz convex probe (ultrasound of the abdominal aorta) and a 3S-RS/1.5–2.5MHz sector probe (transcranial ultrasound). It also has a module for measuring intima-media thickness and pulsed Doppler ultrasound to assess haemodynamic abnormalities in the case that atheromatous plaques are present, and also to analyse the intracranial circulation. The DICOM network connectivity system will be used to record the results and ultrasound images online in the e-CAP.
Carotid arteriesA total of 8 vascular regions will be analysed (common carotid, bifurcation or bulb, internal carotid and external carotid), with the patient in supine decubitus and the head turned 45° towards the opposite side in the examination. The transversal examination will be started with a 2D longitudinal slice of the common carotid up to the bifurcation. At this level colour Doppler will be used to distinguish the external carotid from the internal carotid and then examine both carotids simultaneously.
The presence of an atheromatous plaque will be identified as an intima-media thickness with a height greater than 1.5mm.28 In the event that a plaque is demonstrated, the degree of stenosis (<50%, 50–70% or 70–99%) will be quantified by colour and pulsed Doppler and the peak systolic velocity (PSV in cm/s), peak diastolic velocity (PDVcm/s) and ratio (between common carotid and the location of the stenosis) will be determined to assess the degree of haemodynamic abnormality.
Femoral arteriesWith the patient in supine decubitus, the presence of a plaque will be examined in the common femoral artery (1cm proximal to the bifurcation) and the superficial femoral artery on both sides. The same criterion will be followed for definition of an atheromatous plaque.
Abdominal aortaThe aim is to measure the diameter of the abdominal aorta for early diagnosis of aortic aneurysm in men age 60 and older. With the patient in supine decubitus, the abdominal aorta will be examined in the midline of the abdomen from the base of the sternum until the bifurcation the iliac arteries is visualised. In an axial view, at the points where a greater diameter is observed, 2 images of the aorta will be captured, and 2 measurements will be made (anterior–posterior and lateral–lateral). An aortic aneurysm will be considered to be present when the diameter is greater than 3cm.
Transcranial ultrasoundThe arteries of the circle of Willis and their branches will be insonated through the transtemporal and transforaminal acoustic windows. The Doppler spectrum of each intracranial artery will be determined using the colour-coded signal. Flow direction, peak systolic velocity, mean flow velocity and diastolic flow velocity will be established. The intracranial carotid artery, the medial cerebral artery in the M1 and M2 segments, the anterior cerebral artery (segment A1) and the posterior cerebral artery in segments P1 and P2 will be studied through the transtemporal acoustic window.
The V4 segment of the vertebral arteries and the basilar artery will be studied through the transforaminal acoustic window. In each patient the number, location and severity of stenoses will be recorded. Baumgartner's criteria will be used to establish the severity of the stenosis based on peak systolic wave velocity (moderate to serious stenosis, if it is ≥155/≥220cm/s for the medial cerebral artery, ≤120/≥155cm/s for the anterior cerebral artery, ≥100/≥145cm/s for the posterior cerebral artery and the basilar artery, and ≥90/≥120cm/s for the vertebral artery).29
Ankle–brachial indexA continuous Doppler (Hadecco ES100X MiniDop), sphygmomanometer and blood pressure cuffs (Riester minimus III) will be used. Systolic blood pressure will be measured in the brachial artery, posterior tibial artery and dorsalis pedis artery in both limbs. The ratios between tibial and pedal systolic blood pressure in each leg and the higher brachial blood pressure will be calculated. The final value for each limb will be the lower value of those obtained between tibial and pedal blood pressure.30 An ankle–brachial index value <0.9 will be considered to be suggestive of stenosis; a value <0.7 will be considered to be stenosis and a value ≥1.4 will be considered to be suggestive of rigidity.
SpirometrySpirometry will be used to assess lung capacity. It will be performed by the same nurse who will measure forced vital capacity (FVC); forced expiratory volume in one second (FEV1); the ratio between FEV1 and FVC and the lower limit of normality, as a percentage.
Determination of advanced glycation end productsThese will be measured using skin autofluorescence (SAF) in the forearm with the AGE Reader® system (Diagnoptics, the Netherlands). SAF is measured using spectrophotometry which is calculated as the relationship of the intensity of reflected light compared to refracted light. The result obtained will be divided into 4CV risk groups, taking into account age and sex.
Clinical and clinical-chemistry dataThe following variables will be recorded:
- -
Weight, height, waist circumference and neck circumference. Self-calculable body mass index in kg/m2.
- -
Systolic blood pressure, diastolic blood pressure and pulse pressure (mmHg). These will be measured 3 times (Omron 6) at 2-min intervals and the mean of the last 2 will be collected.
- -
Hours of fasting.
- -
Dried capillary blood testing (fingertip puncture): creatinine (mg/dl), uric acid (mg/dl) and total cholesterol (mg/dl), using the Reflotrons® Plus system (Roche). Determination of the complete lipid profile in cases in which total cholesterol is greater than 200mg/dl: HDL cholesterol (mg/dl), LDL cholesterol (mg/dl) and triglycerides (mg/dl) (Cobas B 101® system [Roche]). Calculation of non-HDL cholesterol levels (total cholesterol – HDL cholesterol [mg/dl]). Glycated haemoglobin (%) will be analysed with the Cobas B 101 system (Roche).
- -
From the creatinine value, taking into account race and sex, the CKD-EPI glomerular filtration rate will be determined.31
- -
Urine sample laboratory testing: using Clinitek Microalbumin 2 Reagent Strips and a Siemens Clinitek Status® analyser from a urine sample from spontaneous micturition collected in the bus itself (which has a bathroom): albuminuria (mg/l) and albumin/creatinine ratio (mg/g).
- -
Drawing of blood samples from a peripheral vein of the hand or forearm to obtain serum, plasma, DNA and RNA. These samples will be prepared in aliquots following a standardised protocol, and sent frozen (dry ice) to a centralised biobank (RedinRen at Universidad de Alcalá) for processing and storage for subsequent studies of CV, inflammatory and mineral-metabolism biomarkers and genetic polymorphisms.
- -
Urine samples frozen and stored in a biobank for subsequent study of biomarkers.
Descriptive analysis will include absolute and relative frequencies for the qualitative variables, means and standard deviations for the continuous variables that follow a normal distribution, and median and interquartile range in the cases that do not follow a normal distribution. Their distribution will be analysed with the chi-squared test, in the case of qualitative variables, and Student's t test or ANOVA for quantitative variables with a normal distribution, or, failing this, non-parametric Mann–Whitney U or Kruskal–Wallis tests will be used for those without a normal distribution. The correlation between quantitative variables will be analysed using Pearson's or Spearman's test, depending on the distribution. Variables with outliers will be processed for their standardisation and subsequent analysis. The existence of collinearity between 2 variables will be analysed to enter the variable that better predicts the result variable in the multivariate model.
Multivariate analysis will be performed using logistic regression if the dependent variable is qualitative, or linear regression if it is quantitative. Beta regression coefficients, odds ratios (ORs) and their respective 95% confidence intervals will be estimated as measures of association. In the longitudinal study, the incidence of CV morbidity and mortality and mortality due to any cause will be analysed, based on the diagnostic tests used in the MU and the biomarkers studied using Cox regression. Statistical significance will be set at a p value<0.05. The SPSS version 21 (SPSS Inc. Chicago, United States) software program will be used for data analysis.
The results will be weighted by the inverse of the probability of selection which requires the selection strategy applied in the sampling phase. Results will be provided for both the entire province and each predefined territory within the province, applying the corresponding post-stratification weights in each case.
An initial general data analysis in the first 3 months of each year (January–March 2016, 2017 and 2018) is planned.
The first publication will make reference to the overall results of the study (prevalence of atheromatous disease and HKD). Subsequently potential differences in prevalence between regions and the effects of diet, exercise, changes in habits, changes in medication and other factors will be analysed.
In a second phase, the relationship between CV events (major and minor) and CKD progression for the group that has been included in the MU compared to the group with the same inclusion criteria, but that has not been studied in the MU, will be analysed.
DiscussionThe ILERVAS project is the first study that will make a diagnosis of the prevalence of SAD and HKD in an asymptomatic population with at least one CV risk factor and link both diseases with CV morbidity and mortality over time (10 years). In addition, it will determine whether early diagnosis of both diseases results in a health benefit. It will also contribute to the knowledge on the impact of respiratory diseases and biomarkers on CV risk and will show the prevalence of unknown cases of diabetes, dyslipidaemia and hyperuricaemia. One notable consideration is that this will allow inequalities in health linked to region, diet and socioeconomic level to be detected.
The use of imaging diagnostic tools will allow early identification of atheromatous disease and therefore early identification of patients with a high risk of having a CV event in the future. In addition, this project has an added value in terms of primary prevention. In this regard, healthcare education that will emphasise modifiable risk factors common to all the diseases studied, such as obesity, smoking habit, sedentary lifestyle and hypertension, will be carried out in the MU and at the PC centres.
Working with a shared medical history will enrich the project, allow long-term follow-up of patients and reveal the impact of early diagnosis of atheromatosis (artery ultrasound) in CV prediction, management and prevention, as well as HKD detection, on the incidence of dialysis, compared to those patients with the same risk factors who have not visited the MU and who are managed according to conventional CV risk calculation population tables. In addition, assessment of new biomarkers and genetic polymorphisms will add predictive value to the known risk factors for both diseases. Knowledge of factors may set the basis for the development of a more precise and personalised care that should help to reduce the incidence of these diseases.
An added value is that this database will be fed without interruption, as it will be shared with the electronic history used daily in PC, which will allow access to clinical and clinical-chemistry parameters and CV events throughout time. All this, together with the parameters of proteomics, metabolomics, genetic polymorphisms and other biomarkers, will contribute to the creation of a large-scale database. Its analysis (computation and modelling) will involve the collaboration of experts in management of big data from the Scientific and Technical Department at IRBLleida.
Moreover, it is not known what are the challenges that the future may bring us regarding potential biomarkers that could allow the design of a medicine personalised with respect to risk factors and treatments. Therefore, plasma, serum, RNA, DNA and urine samples will be collected; these will be available for external investigators, after presentation of projects that will be evaluated by a scientific committee, which will take into account both the quality of the project and the assurance of having the resources required to complete it.
In conclusion, the ILERVAS project aims to achieve a paradigm shift in the diagnosis of CKD and vascular disease, through improvement in accessibility of part of the population with risk factors to cost-effective interventions that allow early prevention of the onset and progression of these diseases. This will result in a reduction in the socioeconomic and health impact (reduction in hospitalisations and social and healthcare costs); which in large part are associated to a late diagnosis.
FundingProject funded by the Lleida Provincial Council in collaboration with the Fundación Renal Jaume Arnó.
Conflicts of interestThe authors declare that there are no conflicts of interest.
We are grateful to the entire UDETMA diagnostic imaging team; the Primary Care management, physicians and nurses; the Regional Health Management of the province of Lleida; and RedInRed, Roche Diagnostics and Amgen SA for their collaboration in the project.
Jose Manuel Valdivielso and Elvira Fernández share senior authorship.
Please cite this article as: Betriu A, Farràs C, Abajo M, Martinez-Alonso M, Arroyo D, Barbé F. Estudio de intervención aleatorizado para evaluar la prevalencia de enfermedad ateromatosa y renal ocultas y su impacto en la morbimortalidad: Proyecto ILERVAS. Nefrologia. 2016;36:389–396.