The aim of the study was to analyse outcomes of AVF-RC in predialysis stage in which a clinical and radiological follow up of its maturation had been done and primary failure had been treated.
Material and methodsWe studied 127 RC-AVF in 117 predialysis patients. All cases had a preoperative map. The RC-AVF was considered mature if it had a brachial artery flow ≥500ml/min and a cephalic vein diameter of ≥4mm. Primary failure was treated radiologically or surgically depending on the type of lesion. Fifty-eight patients started dialysis at the time of the study.
ResultsIn 106 RC-AVF without thrombosis, 72 (68%) were mature and 34 (32%) were immature. A total of 97% of the immature had at least one lesion, and the most common site was the post-anastomotic vein. Lesions were found in 31% of mature RC-AVF, and 18% of patients required treatment. Radiological treatment was the most frequent for maturation failure. After 6 months, primary and secondary patency were 59% and 78%, while after 12 months they were 48% and 77%, respectively. The 80% of patients started dialysis with a distal AVF (76% RC-AVF and 4% ulnar basilic). None of the patients with treated immature RC-AVF started dialysis with CVC, while 78% of the patients started with said AVF.
ConclusionUltrasonography for monitoring maturation provides advantages over clinical monitoring. With our management of RC-AVF in predialysis, 80% of patients start dialysis with an adequate distal AVF.
El objetivo del estudio fue analizar las FAV-RC en prediálisis en las que se hizo un seguimiento clínico y ecográfico de la maduración y cuyo fracaso se trató.
Material y métodosEstudiamos 127 FAV-RC en 117 pacientes prediálisis. Todos disponían de un mapa preoperatorio. La FAV-RC era madura si tenía un flujo en la arteria humeral ≥500ml/min y un diámetro en la vena cefálica ≥4mm. Se trató el fracaso de maduración según el tipo de lesión. Un total de 58 pacientes iniciaron hemodiálisis durante el seguimiento.
ResultadosEn las 106 FAV-RC funcionantes, 72 (68%) fueron maduras y 34 (32%) inmaduras. El 97% de las inmaduras presentaron al menos una lesión y la localización más frecuente fue la vena postanastomótica. El 31% de las FAV-RC maduras tenían lesiones y en el 18% precisaron tratamiento. El tratamiento más frecuente del fracaso de maduración fue radiológico. A los 6 meses la permeabilidad primaria y secundaria fue del 59 y del 78%; a los 12 meses del 48 y del 77%, respectivamente. El 80% de los pacientes iniciaron hemodiálisis con una FAV distal (76% radio-cefálicas y 4% cubitobasílicas). Ningún paciente con una FAV-RC inmadura tratada lo hizo con CVC y un 78% lo hizo con dicha FAV.
ConclusiónLa ecografía en el seguimiento de la maduración aporta ventajas frente al seguimiento clínico. Con nuestro abordaje del AV en prediálisis conseguimos que el 80% de nuestros pacientes inicien hemodiálisis mediante una FAV distal.
A radiocephalic AVF (RC-AVF) is the vascular access (VA) of choice for haemodialysis (HD) patients, but it has a high rate of primary failure (20%–50%).1,2 Primary failure includes thrombosis and failure to mature. Maturation is a complex process that depends on the interplay of patient-dependent and surgical factors. The fundamental lesion that leads to failure to mature is stenosis wherein the pathological substrate is neointimal hyperplasia.3 In predialysis, a diagnosis of maturity tends to be made clinically, although the importance of ultrasound as a complementary method to have objective quantitative criteria is being increasingly emphasised. However, there are not many systematic ultrasound follow-up studies of maturation.4–7 There is effective treatment for failure to mature.8–12 Nonetheless, achieving a RC-AVF suitable for starting HD remains a significant challenge.
For this reason, we studied RC-AVFs placed in predialysis that had clinical and ultrasound follow-up of their maturation. The objectives of this study were to determine the characteristics of RC-AVFs by ultrasound, also the causes and frequency of failure as well as the treatment for a failure to mature and, finally, the percentage that was suitable for use at the start of HD.
Patients and methodsIn this retrospective study, we analysed the 127 RC-AVFs that were placed from January 2009 to March 2013 in 117 consecutive patients with G4 and G5 chronic kidney disease (CKD) without dialysis. By protocol, all patients had recent presurgical vascular mapping that consisted of a colour-Doppler duplex ultrasound scan (Siemens Acuson 150×) and a CO2 venogram (Phillips Ayura) of the upper limb.13 All patients signed the informed consent form. None of the patients had nephrotoxicity associated with the limited quantity (3ml) of non-ionic contrast material to as complement to CO2. The radial, cubital and humeral artery were studied, as well as the superficial and deep venous system, including the central veins. The placement of a RC-AVF was not advised if any of the following conditions were present: if the vessels (radial artery and cephalic vein) were <1.6mm, if there were irreparable venous lesions or if there was central ipsilateral stenosis. The surgical procedure was performed under the same conditions in all the patients and by the same surgical team. The type of VA ultimately created by the surgeon was decided according to the indication of the presurgical mapping and the intraoperative findings.
Monitoring of the maturation of the RC-AVFs was performed in 2 ways:
- a)
A physical examination, within the first 72h post-surgery, at the time of ultrasound examination and at each follow-up visit. This included inspection, palpation and auscultation of the body and the anastomosis of the RC-AVF following the previously published protocol.14,15
- b)
Colour-Doppler duplex ultrasound scan 1–4 months post-surgery by the same vascular radiology team.
The RC-AVF was considered to be mature if the flow rate in the humeral artery was ≥500ml/min and the cephalic vein in the forearm had a diameter ≥0.4cm.4 A pseudo-delay of maturation was diagnosed when the above characteristics were present and the depth of the cephalic vein was >0.5cm from the skin surface. Stenosis was considered to be significant if it was >50% of the vascular lumen. The juxta-anastomotic segment was considered to be the segment between the anastomosis and the first 5cm of the artery or vein (post-anastomotic segment).
Maturation follow-up protocol and therapeutic approachImmature RC-AVFs were evaluated using fistulography and were treated radiologically or surgically depending on the type of lesion found. Radiological treatment consisted of a percutaneous transluminal angioplasty (PTA) associated or not associated to permeabilisation of thrombotic venous occlusion using thromboaspiration. Surgical treatment was either proximal reanastomosis or the creation of a new vascular access. They were subsequently monitored using serial ultrasound until the start of HD.
Mature RC-AVFs with associated lesions (stenosis with or without thrombotic occlusion) were followed up with ultrasound and if at any time there was a decrease in the blood flow, an increase in the resistive index or both, then the appropriate treatment, radiological or surgical, was pursued as previously described.
Mature RC-AVFs without associated lesions were monitored clinically without standardised ultrasound monitoring until the start of HD. During follow-up an imaging study was performed only if it was indicated by the clinical examination. However, in all cases an ultrasound study was performed prior to the first AVF puncture to rule out a complication that would render its use inadvisable.
The RC-AVF was considered to be suitable if it was the VA both at the start of HD and throughout the first 3 months of dialysis, without requiring another VA or a central venous catheter (CVC), even if radiological treatment was required during this time.
StatisticsQualitative variables were expressed in percentages and quantitative variables were expressed in means and standard deviations. The qualitative variables were compared using the chi-squared test, and the quantitative variables were compared using comparison of means with Student's t test for independent data. We performed a multivariate logistic regression study in which the dependent variable was an immature FC-AVF and some of the variables described in the literature were included as independent variables (sex, age, obesity, cardiovascular disease and diabetes mellitus). Kaplan–Meier survival curves were used to study the primary and secondary permeability of the 127 FC-AVFs in predialysis. Primary permeability was defined as the time elapsed from the placement of the AVF to the first therapeutic intervention or thrombosis. Secondary permeability was defined as the time elapsed from the creation of the AVF to its abandonment due to irreparable dysfunction, or to the start of HD or end of the follow-up period in patients who did not start HD during that time. Finally, we prepared a ROC curve to predict the required arterial flow rate that would determine the clinical sign of non-collapsible hyperpulsatility in immature RC-AVFs with significant post-anastomotic stenosis. A p value <0.05 was considered to be statistically significant. The data were analysed using SPSS version 20.0.
ResultsOf the 127 RC-AVFs, 21 (16%) had thrombosis in the first 72h. In the remaining 106 RC-AVFs, after the ultrasound study, 72 (68%) were mature and 34 (32%) were immature.
Mean patient age was 68±13 years, 38% were diabetic and 33% had clinical evidence of cardiovascular disease (ischaemic cardiomyopathy, ischaemic cerebrovascular accident, intermittent claudication or peripheral ischaemic lesions). clinical manifestation of cardiovascular disease was present in 47% of patients with an immature RC-AVF and 26% of patients with a mature RC-AVF (p<0.035) (Table 1). In the logistic regression study the only variable of those studied that was associated with an immature RC-AVF was cardiovascular disease with an OR of 2.6 (95% CI: 1.1–6.1; p<0.03).
Demographic and clinical characteristics of the population. Comparison of these characteristics between mature and immature RC-AVFs.
| Variable | Total population (n=117) | Immature RC-AVF (n=34) | Mature RC-AVF (n=72) | p |
|---|---|---|---|---|
| Age (mean [SD]) (years) | 68 (13) | 69 (14) | 68 (12) | NS |
| Men/women (n) | 65/52 | 18/15 | 39/31 | NS |
| Left/right RC-AVFs | 102/25 | |||
| Diabetes mellitus, n (%) | 44 (38) | 17 (50) | 23 (32) | NS |
| Cardiovascular disease n (%) | 38 (33) | 16 (47) | 19 (26) | <0.035 |
| Obesity (BMI>33), n (%) | 30 (26) | 9 (26) | 19 (26) | NS |
| CKD aetiology, % | ||||
| Diabetic | 22 | 37 | 15 | NS |
| Vascular | 21 | 15 | 24 | |
| Glomerular | 20 | 24 | 21 | |
| Polycystitis | 11 | 9 | 10 | |
| Other | 26 | 15 | 30 | |
Mean flow rate in the humeral artery was 805±479ml/min. Flow rate exceeded one litre per minute in 27% of cases and 1.5l/min in 11% of cases.
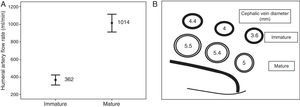
In mature RC-AVFs, mean flow rate in the humeral artery was 1014±434ml/min and internal diameters in the different thirds of the cephalic vein, from distal to proximal, were 5±1.6; 5.4±1.1 and 5.5±0.9mm, respectively (Fig. 1). We found no lesions in 69% of RC-AVFs, at least one lesion (stenosis with or without thrombotic occlusion) in 31% of them, and of these, we found more than one lesion in 18%. In 28% of cases, the lesion was post-anastomotic venous stenosis (in 10% it was significant and in 18% it was not significant), and in 8% of cases, it was located in the proximal vein (1% significant, 3% not significant and 4% occlusion). Among mature RC-AVFs, 17% had a maturation pseudo-delay. We found an aneurysm without stenosis in 6%.
In immature RC-AVFs, mean flow rate in the humeral artery was 362±164ml/min and internal diameters in the different thirds of the cephalic vein, from distal to proximal, were 3.6±1.6; 4±1.6 and 4.4±1.7mm, respectively (Fig. 1). In 97% of cases, significant stenosis was found, and of these cases, 42% had more than one lesion. It was located in the post-anastomotic vein in 76% of cases, in the proximal vein in 42% of cases (of these, half were thrombotic occlusion), in the anastomosis in 6% of cases and in the artery in 18% of cases (11% juxta-anastomotic and 7% diffuse). Only 3% of immature RC-AVFs did not have underlying lesions.
Therefore, the most common cause of failure to mature was significant stenosis in the post-anastomotic vein. This lesion often presents a non-collapsible hyperpulsatility on raising the arm. In our case, this sign was present in 60% of immature RC-AVFs with significant post-anastomotic venous stenosis. We found a significant difference (p<0.049) in the flow rate of the humeral artery between those with the sign (flow rate 443±182ml/min) and those without it (flow rate 307±114ml/min). In our population, hyperpulsatility as a sign of significant post-anastomotic venous stenosis in immature RC-AVFs was present when the flow rate was ≥385ml/min (sensitivity: 73% and specificity: 90%) (AUC: 0.76; 95% CI: 0.56–0.96; p<0.03).
Treatment of radiocephalic arteriovenous fistulasOf the 127 RC-AVFs studied, 58 (46%) received some type of treatment in predialysis. The causes were: 11% post-surgical thrombosis, 24% failure to mature, 6% lesions that progressed in mature RC-AVFs and 5% a maturation pseudo-delay. The type of treatment was: in 20 (16%) a new VA (12 contralateral RC-AVFs, 4 humeral–cephalic RC-AVFs, 2 cubital–basilic RC-AVFs, one humeral–basilic RC-AVF and one humeral–axillary graft), in 24 (19%) radiological, in 8 (6%) proximal reanastomosis, and in 6 (5%) vein superficialisation.
Among mature RC-AVFs, 18% required treatment. In immature RC-AVFs, 88% require treatment, and only 12% were not treated (one case due to the absence of an underlying lesion, 2 cases due death and another case due to patient's refusal).
Table 2 shows the treatment performed in each type of AVF (mature with or without lesions, and immature with or without lesions). Among mature RC-AVFs without lesions, 10% required surgical treatment (cephalic vein superficialisation). Regarding mature RC-AVFs with associated lesions, 36% required treatment: surgical in 37.5% of cases and radiological in 62.5% of cases. Finally, in the case of immature RC-AVFs, 56% were treated radiologically, 32% were treated surgically and 12% received no treatment for the above-mentioned reasons. Therefore, the most common treatment in immature and mature RC-AVFs with lesions was radiological.
Type of treatment according to type of RC-AVF.
| Type of AVF | With lesion | Type of treatment |
|---|---|---|
| Mature AVF (n=72) | Without lesion (n=50) | 5 superficialisations |
| With lesion (n=22) | 1 superficialisation 1 reanastomosis 1 new VA 5 PTAs | |
| Immature AVF (n=34) | Without lesion (n=1) | No |
| With lesion (n=33) | 19 PTAs 5 reanastomosis 6 new VAs 3 no |
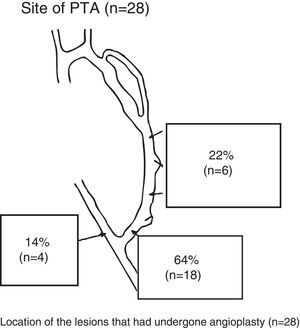
A total of 28 PTAs were performed in 24 RC-AVFs (19 immature and 5 mature with lesions that progressed). A PTA of one lesion was performed in 20 patients, and a PTA of 2 lesions was performed in the same procedure in 4 patients. In 3 cases, in addition to a PTA, the vein was repermeabilised owing to associated thrombotic occlusion. Fig. 2 shows the location of the lesions treated using a PTA.
Post-anastomotic venous stenosis was treated with a PTA in 19 cases (68%), a new VA in 5 cases (18%) and proximal reanastomosis in 4 cases (14%).
Clinical course of the arteriovenous fistulas treatedOf the patients who were treated radiologically, 17 started HD during the study, and in these patients the mean number of predialysis PTAs was 1.2±0.4. One PTA was required in 77% and 2 PTAs were required in 23% prior to the start of HD. Mean time between the first PTA and the start of HD was 6.4±8 months (1–32 months). In 94% of RC-AVFs treated radiologically, this AVF was the VA at the start of HD, and in 6%, another type of AVF had to be placed (one patient required a humeral–axillary graft since that patient had previously had a contralateral RC-AVF placed and it too had failed).
In the subgroup of patients with significant post-anastomotic venous stenosis the AVF worked at the commencement of HD in 93% of those treated with a PTA (14 of 15) and 100% (2 of 2) who underwent proximal reanastomosis.
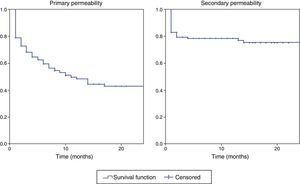
The primary and secondary permeability curves of all the RC-AVFs in predialysis are shown in Fig. 3. Primary and secondary permeability were 59% and 78% at 6 months, 48% and 77% at 12 months, and 43% and 75% at 24 months, respectively.
Characteristics of the vascular access at the start of haemodialysisOf the 58 patients who started HD at the time of the study, 76% did so with a RC-AVF, 10% with another AVF (5% humeral–cephalic, 4% cubital–basilic and 1% humeral–axillary graft) and 14% with a CVC. Mean time between surgery and the start of HD was 12.7±10.7 months.
Of the 44 RC-AVFs that were suitable at the start of HD, 43% of them were treated in predialysis (36% radiologically and 7% surgically).
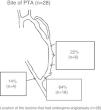
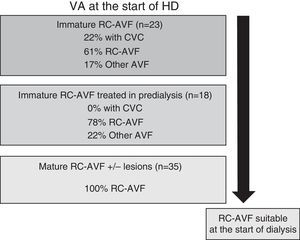
Fig. 4 schematically represents the type of VA used to start HD according to RC-AVF, whether it was immature, immature specifically treated in predialysis or mature. Of the 58 patients who started HD, 23 had an immature RC-AVF and 35 had a mature AVF with or without lesions. Of the 23 patients with an immature RC-AVF, 5 (22%) required a CVC since the start of HD was unexpected and, as a result, there was not time enough to perform a treatment in predialysis. One out of the 5 patients with immature RC-AVF was not treated owing to the patient's refusal and the AVF was irreparable at the time of the start of dialysis. The 4 remaining patients received a treatment during the first week of dialysis session: one case with a PTA, 2 cases with proximal reanastomosis and one case with a contralateral RC-AVF. In patients with an immature RC-AVF specifically treated in predialysis (18 patients), none of them required a CVC and 78% did so with this RC-AVF. Finally, all the patients with a mature RC-AVF with or without associated lesions started HD using their RC-AVF.
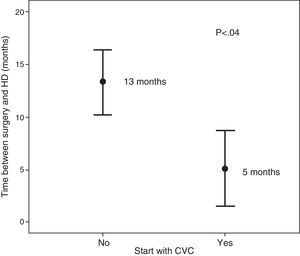
The patients who started HD using a CVC (n=8) and those who did so with an AVF (n=50), had no significant differences in relation to age, sex, diabetes mellitus, cardiovascular disease, obesity or CKD aetiology. The only difference observed was the time elapsed between surgery and the start of HD (5 months versus 13 months, p<0.04) (Fig. 5).
DiscussionConsistent with the literature, 43% of our RC-AVFs had a primary failure that required a treatment in predialysis to avoid the use of CVC at the start of HD.1,2 In 16%, thrombosis occurred in the first 72h, and 32% of non-thrombosed RC-AVFs had a failure to mature.
Maturation is a very complex process that makes a RC-AVF becomes suitable for HD. It starts at when the anastomosis is created and is followed by vascular remodelling that includes an increase in the diameter and arterial and venous blood flow, as well as a thickening of the venous wall that will allow repeated venipuncture and a sufficient flow rate for HD.16,17 The complexity of this process reflects the fact that it is a result of the interplay of multiple factors. Some are patient-dependent factors that will determine the capacity for vascular remodelling. Others are surgical factors that will create the intra-FAV shear pressure required for vascular remodelling.18
Presurgical factors reported to be associated with a failure to mature include female sex, obesity, diabetes, age and cardiovascular disease.12,18,19 In our case, the only one of the above variables associated with a failure to mature was clinically manifestation of cardiovascular disease. The many traditional and non-traditional factors (oxidative stress, inflammation, calcium and phosphorus metabolism, nutrition, and endothelial dysfunction) associated with CKD that determine a greater cardiovascular risk in these patients may contribute to a VA's failure to mature.20 CKD is known to accelerate venous neointimal hyperplasia in animal models.21 Moreover, 83% of patients with advanced CKD have venous neointimal hyperplasia at the time of surgery.3 Finally, the potential contribution to a failure to mature of the abnormal capacity for arterial and venous vasodilatation in patients with CKD should be taken into consideration.22,23
Currently, we do not have vascular anatomical and functional able to define the risk for a failure to mature of a fistula7; however presurgical mapping in all patients, as well as a good surgical technique, may contribute to obviate the negative effect of the above-mentioned factors on a failure to mature.24–27 Multiple studies have demonstrated similar results in these populations at risk when preoperative ultrasound is used.28–33
It is known that systematic preoperative ultrasound evaluation increases the percentage of RC-AVFs performed, although it also increases the number of immature RC-AVFs.33 However, if a protocol is applied for early and effective treatment of a failure to mature, the final result will be an increase in the number RC-AVFs that ready to be use at the start of HD. Our protocol for approaching a VA in predialysis is an example of this concept, such that 78% of the patients with an immature RC-AVF that is treated in predialysis will start HD with this AVF and none with a CVC.
In immature RC-AVFs, we found an underlying lesion in almost 100% of cases. As in other series, the most common location of the lesion was the post-anastomotic vein.8 There were different reasons to explain this, but essentially, the configuration of the AVF itself is contributing factor due the abnormal stress generated shear pressure exerted on the endothelium and also the potential injury due to surgical manipulation. Both of these factors would stimulate neointimal hyperplasia and vasoconstriction resulting in stenosis.16,34–38 In our case, this lesion was treated radiologically in the majority of cases with a good outcome and required re-interventiont in a relatively low percentage of cases (20%). Currently, the superiority of surgical vs radiological treatment in this type of lesion has not been established.39 Availability at each centre will determine the type of treatment used. In our department, the most common treatment is radiological; surgical treatment is reserved for certain lesions that would not benefit from PTA (long or multiple lesions). Therefore, in our case, we cannot compare the outcomes of the two treatments. Another fact of interest supporting ultrasound follow-up in immature RC-AVFs is that only 60% of cases with significant post-anastomotic stenosis had non-collapsible hyperpulsatility, and so a clinical examination would have a limitation their diagnosis.14
It should be noted that mature RC-AVFs had lesions in 31% of cases and that 18% of cases will progress and will require treatment in predialysis. This highlight the fact that ultrasound follow-up of the maturation offers advantages over exclusively clinical follow-up; this had already been published in other series but with a lower number of RC-AVFs.5,6 Moreover, ultrasound was essential for the diagnosis of a maturation pseudo-delay (which affected 17% of our mature AVFs). Finally, it should be noted that a high percentage of our AVFs had high flow rates, which alert about the potential deleterious cardiological effect in the mid- to long term.40,41
In our patients the most common type of treatment of failure to mature was radiological. The difference between the primary and secondary permeability curves, which is very similar to those previously published,8,12 illustrate the efficacy of the treatment of failure to mature in our patients. Overall, 94% of immature RC-AVFs treated radiologically will be suitable at the start of HD.
Finally, the most determining factor for avoiding a CVC was placement of RC-AVF with enough time in advance sp the diagnose and treatment of primary failure before can be made before commencenment of HD. The time to place a VA in predialysis is very difficult to determine. In our case, it seems that VA should be placed at least 6–12 months prior to the start of HD, which would be in line with the recommendations of the different guidelines.42–44
In summary, ultrasound was very useful in the study of maturation of VA in predialysis since it allowed us: A) To determine the patient's vascular status regardless of his or her potential comorbidities. B) To diagnose an immature AVF and its potential causes and guide its treatment. C) To diagnose, in mature AVFs, potential associated lesions, which may initially have no functional repercussions but may progress and require treatment. D) To diagnose a maturation pseudo-delay. E) To quantify the vascular flow rate. F) To diagnose in some cases lesions that did not have clinical expressivity. Therefore, all the above contingencies show that ultrasound in the follow-up of maturation offers advantages over exclusively clinical follow-up, such that they should complement each other.
In conclusion, with our approach to the VA in predialysis we have achieved that 80% of our patients were able to start HD using a distal AVF (76% radiocephalic and 4% cubital–basilic), potential complications of more proximal VA have been prevented and therefore we have saved venous resources that may be vital for our patients’ future.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Muray Cases S, García Medina J, Pérez Abad JM, Andreu Muñoz AJ, Ramos Carrasco F, Pérez Pérez A, et al. Importancia del seguimiento y tratamiento del fracaso de maduración en la fístula arteriovenosa radio-cefálica en prediálisis. Papel de la ecografía. Nefrologia. 2016;36:410–417.