Multiple myeloma (MM) is a haematological tumour that is characterised by uncontrolled proliferation of plasma cells and a significant volume of serum free light chains (sFLCs), which can cause acute renal failure due to intratubular precipitation, resulting in cast nephropathy.
Acute renal failure is a complication that can arise in more than 20% of patients with multiple myeloma, half of which will require dialysis.
MethodsWe report our experience with 13 patients who were treated with dialysis using high cut off filters (HCO) between July 2011 and February 2015.
A total of 6 consecutive 6-h sessions were performed using a 2.1m2 HCO filter (Theralite® by Gambro®). Afterwards, further 6-h sessions were continued on alternate days.
ResultsA total of 151 sessions were conducted, with an average of 11.6 sessions per patient (range 6–27).
The treatment proved to be effective in removing both kappa and lambda sFLCs, resulting in a 93.7% fall in sFLCs by the end of treatment. The average reduction was 57.7% per dialysis session. 10 out of the 13 cases recovered sufficient renal function to become independent of dialysis.
There were no major changes in albumin levels using an infusion protocol of 2 50-ml vials of 20% albumin at the end of the dialysis session.
ConclusionsCombination treatment with chemotherapy and long dialysis with HCO filters was effective in reducing the sFLC levels and recovering sufficient renal function in 77% of cases. With HCO filters, significant cost savings are achieved, contrary to what was previously believed.
El mieloma múltiple (MM) es una tumoración hematológica que se caracteriza por la proliferación incontrolada de células plasmáticas y la existencia de una importante cantidad de cadenas libres en sangre (CLLs) que puede ocasionar un fallo renal agudo por la precipitación intratubular de ellas, causando nefropatía por cilindros.
La insuficiencia renal aguda es una complicación que puede presentarse en más de un 20% de los pacientes con MM, y la mitad de estos precisarán diálisis.
MétodosPresentamos nuestra experiencia de 13 pacientes tratados con diálisis mediante filtros de high cut off (HCO), durante el período comprendido entre julio de 2011 y febrero de 2015.
Se realizan 6 sesiones consecutivas de 6h de duración, utilizando un filtro de HCO (Theralite® de Gambro®) de 2,1m2 de superficie. Posteriormente se continúa con sesiones a días alternos de igual duración.
ResultadosSe realizaron un total de 151 sesiones; una media de 11,6 sesiones/paciente (rango 6–27).
El tratamiento se mostró efectivo para eliminar tanto CLLs kappa como lambda. El porcentaje de disminución de CLLs desde el inicio hasta el final del tratamiento fue del 93,7%. La reducción media por sesión de diálisis fue del 57,7%. En 10 de los 13 casos se recuperó la función renal y los pacientes pudieron permanecer sin diálisis.
No hubo grandes cambios en los niveles de albúmina utilizando un protocolo de infusión de 2 viales de 50mL de albúmina al 20% al final de la sesión de diálisis.
ConclusionesEl tratamiento combinado con quimioterapia más diálisis largas con filtros de HCO resultó eficaz para reducir el nivel de CLLs y recuperar un nivel de función renal suficiente en el 77% de los casos. Con filtros de HCO se consigue un ahorro significativo, en contraposición a lo descrito previamente en la literatura.
Multiple myeloma (MM) is a neoplastic disease characterised by uncontrolled proliferation of plasma cells in the bone marrow.1 This produces an excessive release of immunoglobulins and their fragments called light chains, The formation of cast in the distal tubules as a result of the deposit of light chains along with the Tamm–Horsfall protein causes renal failure in these patients.2,3
There are two types of light chain: kappa, which is a monomeric form with a molecular weight of 22.5kDa; and lambda, which are dimers, with a molecular weight of 45kDa.
MM accounts for 0.1% of all cancers. In Spain, it represents 13% of all haematological cancers, with over 2000 new cases every year and an incidence of 5–6 cases/100,000 population.4 MM is observed in adults; only 15% of cases are under 50, and the peak incidence is between the ages of 60 and 70. It affects men more than women and black people more than white.5
Life expectancy is less than a year if the patient develops renal failure, although with treatment, it can prolonged to 5–7 years.5,6
Acute renal failure may occurs in up to 20% of MM patients and half of them may require dialysis. The acute renal failure may be aggravated by different circumstances, such as dehydration, hypercalcaemia, hyperuricaemia, hyperviscosity,7 and the use of nephrotoxic drugs.
At the beginning of the disease, clinical signs are non-specific, an this may delay the diagnosis. The first symptoms, such as bone pain, pathological fractures, anaemia, fatigue, hypercalcaemia, infections, acute renal failure and others, is usually presented late.
Acute or chronic kidney disease makes the prognosis much worse.
The causes of renal dysfunction in patients with myeloma include alteration to the proximal and distal tubules as a result of tubular cell damage caused by filtered light chains, cast nephropathy, amyloidosis, light- or heavy-chain deposition disease,8 cryoglobulinaemia, interstitial infiltration by plasma cells and, in rare cases, proliferative glomerulonephritis or interstitial nephritis.9
There are three main treatment strategies for acute renal failure:
- 1.
Eliminate factors that aggravate nephrotoxicity and cast formation; this is achieved with adequate hydration (2–3l of fluids per day). Volume depletion will reduce flow through the tubules and increases the concentration of free light chains (FLC), and the formation of aggregates. Avoid furosemide (only if euvolaemic). Avoid nephrotoxic drugs, especially non-steroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists and exposure to contrast agents. Treat hyperuricaemia. Treat hypercalcaemia (if calcium is not greater than 14mg/dl, administer fluids and if hypercalcaemia persists, add bisphosphonates; if calcium>14mg/dl, administer bisphosphonates from the beginning).
- 2.
Eliminate or reduce the production of FLC by cancer cells. The usual chemotherapy regimen used in MM improves renal failure in approximately 50% of cases and tubular injury responds better than glomerular damage.10
From the point of view of chemotherapy, it should be noted that the treatment of MM has been markedely changed in recent years. For years, the standard treatment for these patients was dexamethasone plus melphalan (alkylating agent). New drugs have appeared in the last few years that have resulted in longer survival:
Bortezomib, which has been one of the major advances in the treatment of MM.
Thalidomide and lenalidomide, already being used for some years for the treatment of relapsed patients and, lately, pomalidomide has appeared.11
Bendamustine (alkylating) and melflufen, seeking a less toxic and more specific effect than other alkylating agents.12
Lastly, monoclonal antibodies, which are directed specifically against tumour cell targets. These new molecules include elotuzumab, CD38 and SLAM-F7; and others which are still being studied.13,14
All these new alternatives encourage us to be optimistic about the future of MM and the current idea is that patients are not completely cured, at least we should be able to turn MM into a chronic disease.
- 3.
Eliminate or reduce the circulating FLC. Kidney recovery is associated with an early reduction in serum FLC. Hutchison, using high cut-off (HCO) filters and long haemodialysis sessions of 6h, obtained FLC reductions of over 85%; up to 60% of patients recovered sufficient kidney function to no longer need dialysis.15
The traditional extracorporeal purification methods, such as haemodialysis or plasmapheresis, do not effectively remove FLC. In conventional dialysis, the dialysis membrane does not have the right pore size to remove FLC thus the clearance is minimal. Plasmapheresis only removes 10–20% of the FLC (from in the intravascular space), the rest are in the extravascular space, which makes it difficult to remove, added to the fact that plasmapheresis causes a significant loss of essential proteins.16,17
Another technique is haemodiafiltration with regeneration of the ultrafiltrate by adsorption in resin (SUPRA-HFR), which uses convection, adsorption and diffusion with a dual-chamber dialyser.18,19 Although it is somewhat cheaper but only few studies have been conducted with very few patients. Moreover, it only seems to be useful in kappa chains and not lambda chains.
Another type of membrane has recently been developed called high-permeability, high-pore or HCO membranes. These membranes are effective in reducing light chains in blood (up to 90%) for extended periods of 6–8h.15 Early diagnosis and treatment in reducing FLC in the first three weeks has been shown to be associated with a significant increase in survival.20 The pore size is effective for FLC removal, but it also leads to protein losses through the dialysis membrane, which means that albumin has to be administered at the end of each session. Other supplements are usually required during haemodialysis sessions, the most common being calcium, phosphorus, magnesium and potassium.
ObjectiveThe purpose of this article is to present our experience of 13 cases of treatment of acute renal failure secondary to MM with HCO filters.
MethodsWe used an Integra Hospal® monitor equipped with 1.4m2 ultrafilters, ultrapure water, bicarbonate cartridge (Bicart® from Hospal®) and closed-loop central acid-delivery system.
The circuit was primed with Prontoprime® 2000ml at a rate of 100ml/min and with ultrafiltration of 2kg/h; it is important to prime the blood circuit carefully to avoid the presence of air bubbles that causes clotting and reduce the efficacy of the dialysis.
The dialysis membrane used in all cases was a Gambro® Theralite® 2.1m2 HCO filter.
Dialysis was performed daily for a six day period, then, every other day, until FLC levels in blood fell below 500mg/l or kidney function recovered to the point that dialysis could be discontinued.
The dialysis sessions lasted 6h with low blood flow rates (250–300ml/min) and dialysate flow rate of 500ml/min. The dialysate had potassium concentration of 3mEq/l to avoid hypokalaemia, especially in the period of daily dialysis Other components were: calcium 3.5mEq/l, magnesium 0.9mEq/l, sodium 0.139mEq/l and glucose 1.5g/l.
The standard heparin regimen was: 2500IU of sodium heparin at the commencement of dialysis given as a bolus and followed by the continuous infusion of 1500IU per hour, stopping 30min before the end of the dialysis session.
Supplements were administered during the dialysis sessions; the pore size leads to the loss of protein through the dialysis membrane, so albumin has to be administered at the end of the session, two vials of 50ml of human albumin 20% (20g). Other supplements, such as calcium gluconate, magnesium sulphate and epoetin beta were necessary occasionally.
The usual vascular access was a temporary catheter (Shaldon) placed in the right jugular vein; catheters were sealed with 1% sodium heparin. In one patient, it was necessary to insert a permanent tunnelled Tesio® catheter. Both techniques were performed by nephrologists from our Nephrology Department.
We monitored renal function, free light chains (FLC) in blood with the Freelite Binding Site® system pre- and post-dialysis, urea, creatinine, chloride and sodium, and potassium both pre- and post-dialysis. In addition, pre-dialysis, total calcium, ionised calcium, magnesium and blood count were measured, plus albumin mid-session and, once again post-dialysis, urea, creatinine and electrolytes. We also have a protocol for determining HBV, HCV and HIV serology at the start of dialysis.
Hourly blood flow rates, venous pressure, hourly and total ultrafiltration, blood pressure and heart rate, temperature, medication administration and any incidents occurring during the dialysis session were recorded on the nursing chart.21
All patients signed one informed consent form for the insertion of vascular access and another for the haemodialysis.
ResultsOver a period of 43 months, from July 2011 to February 2015, we performed 13 treatments using haemodialysis with HCO filter in patients with acute renal failure secondary to MM. There were in fact only 12 patients, as one had a relapse and was treated a second time.
There were nine males and three females, all aged between 43 and 71, with a mean age of 60.8 years.
The number of patients with kappa chains was the same as those with lambda chains, six in each case.
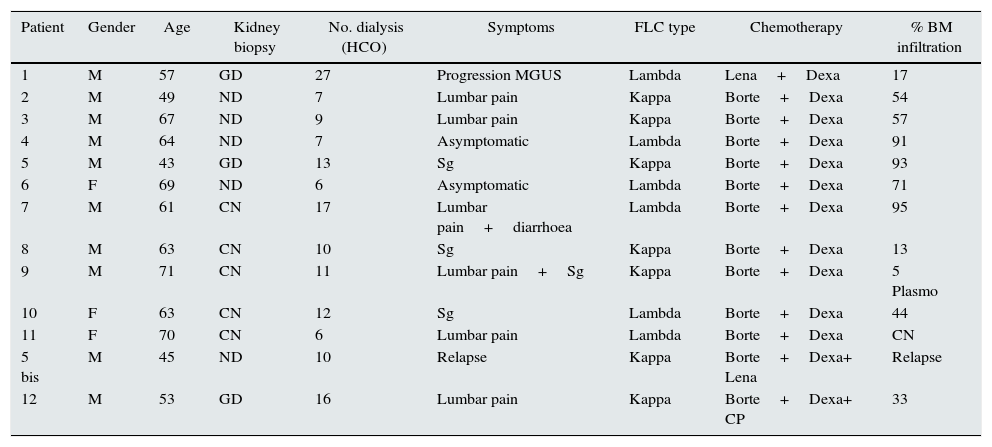
As far as bone marrow infiltration was concerned, we knew the status for 10 patients and all had elevated levels of plasma cells (and in one case, evidence of plasmacytoma) with ranges from 13% to 93%.
Eight patient had renal biopsy and the results were: five had cast nephropathy, two with glomerular injury due to deposits plus cast nephropathy and a single case of isolated glomerular injury, but with very high levels of FLC (69,000mg/l); if not treated, this patient would likely end up developing cast nephropathy (Table 1).
Personal data, kidney biopsy, number of dialysis sessions, symptoms, chain type, treatment, bone marrow infiltration.
| Patient | Gender | Age | Kidney biopsy | No. dialysis (HCO) | Symptoms | FLC type | Chemotherapy | % BM infiltration |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 57 | GD | 27 | Progression MGUS | Lambda | Lena+Dexa | 17 |
| 2 | M | 49 | ND | 7 | Lumbar pain | Kappa | Borte+Dexa | 54 |
| 3 | M | 67 | ND | 9 | Lumbar pain | Kappa | Borte+Dexa | 57 |
| 4 | M | 64 | ND | 7 | Asymptomatic | Lambda | Borte+Dexa | 91 |
| 5 | M | 43 | GD | 13 | Sg | Kappa | Borte+Dexa | 93 |
| 6 | F | 69 | ND | 6 | Asymptomatic | Lambda | Borte+Dexa | 71 |
| 7 | M | 61 | CN | 17 | Lumbar pain+diarrhoea | Lambda | Borte+Dexa | 95 |
| 8 | M | 63 | CN | 10 | Sg | Kappa | Borte+Dexa | 13 |
| 9 | M | 71 | CN | 11 | Lumbar pain+Sg | Kappa | Borte+Dexa | 5 Plasmo |
| 10 | F | 63 | CN | 12 | Sg | Lambda | Borte+Dexa | 44 |
| 11 | F | 70 | CN | 6 | Lumbar pain | Lambda | Borte+Dexa | CN |
| 5 bis | M | 45 | ND | 10 | Relapse | Kappa | Borte+Dexa+ Lena | Relapse |
| 12 | M | 53 | GD | 16 | Lumbar pain | Kappa | Borte+Dexa+ CP | 33 |
Borte: bortezomib; CP: cyclophosphamide; FLC: free light chains; Dexa: dexamethasone; GD: glomerular deposits; HCO: number of dialysis sessions with high cut-off filter; Lena: lenalidomide; BM: bone marrow; CN: cast nephropathy; ND: not done; Plasmo: plasmacytoma; Sg: general syndrome.
Chemotherapy treatment included bortezomib plus dexamethasone on 10 occasions (1st line). Patient 1 was treated with lenalidomide plus dexamethasone (2nd line). Patient 5a (relapse) was treated with bortezomib plus dexamethasone plus lenalidomide, and patient 12 was treated with bortezomib, dexamethasone and ciclosporin.
The average number of days from onset of acute renal failure until haemodialysis with HCO filter was 27.3 days, with a range of 0–90 days. The 0 days corresponded to the patient who relapsed and the treatment was quick because he was closely monitored. The average delay was 24.5 days in those who recovered kidney function, and 34 in those who did not.
We performed a total of 151 dialysis sessions; the average number of days on dialysis with the HCO filter was 11.6, ranging from 6 to 27 days. A total of seven patients required less than 10 sessions; five required 11 to 17 sessions; and one required 27 sessions, but achieved recovery of kidney function and an FLC level less than 500mg/l (Table 1).
At present, six patients have made good progress and continue without dialysis, three patients are on conventional haemodialysis and three patients died.
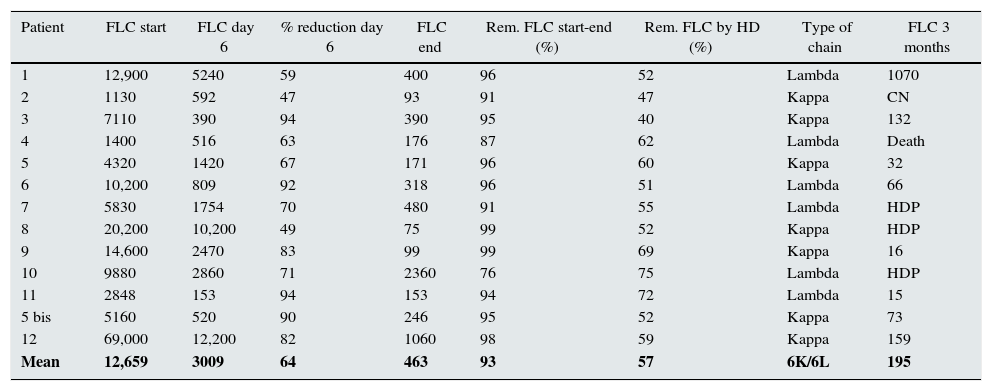
The mean FLC level at the before treatment was 11,036mg/l, with a wide range, from 69,000 to 1130mg/l. The percent decrease in FLC from the beginning to the end of treatment was 93.7%. The average reduction per dialysis session was 57.7%, ranging from 40.2% to 75.4%. In the six cases with lambda chains the mean removal was 61.3% and the seven cases with kappa chains, 54.6%. After the first six treatment sessions, the mean drop in FLC was 64.2% (Table 2).
Changes in FLC start, day 6 and end, % removal, type of light chain.
| Patient | FLC start | FLC day 6 | % reduction day 6 | FLC end | Rem. FLC start-end (%) | Rem. FLC by HD (%) | Type of chain | FLC 3 months |
|---|---|---|---|---|---|---|---|---|
| 1 | 12,900 | 5240 | 59 | 400 | 96 | 52 | Lambda | 1070 |
| 2 | 1130 | 592 | 47 | 93 | 91 | 47 | Kappa | CN |
| 3 | 7110 | 390 | 94 | 390 | 95 | 40 | Kappa | 132 |
| 4 | 1400 | 516 | 63 | 176 | 87 | 62 | Lambda | Death |
| 5 | 4320 | 1420 | 67 | 171 | 96 | 60 | Kappa | 32 |
| 6 | 10,200 | 809 | 92 | 318 | 96 | 51 | Lambda | 66 |
| 7 | 5830 | 1754 | 70 | 480 | 91 | 55 | Lambda | HDP |
| 8 | 20,200 | 10,200 | 49 | 75 | 99 | 52 | Kappa | HDP |
| 9 | 14,600 | 2470 | 83 | 99 | 99 | 69 | Kappa | 16 |
| 10 | 9880 | 2860 | 71 | 2360 | 76 | 75 | Lambda | HDP |
| 11 | 2848 | 153 | 94 | 153 | 94 | 72 | Lambda | 15 |
| 5 bis | 5160 | 520 | 90 | 246 | 95 | 52 | Kappa | 73 |
| 12 | 69,000 | 12,200 | 82 | 1060 | 98 | 59 | Kappa | 159 |
| Mean | 12,659 | 3009 | 64 | 463 | 93 | 57 | 6K/6L | 195 |
FLCs: changes in free light chains in blood.
Units: mg/l.
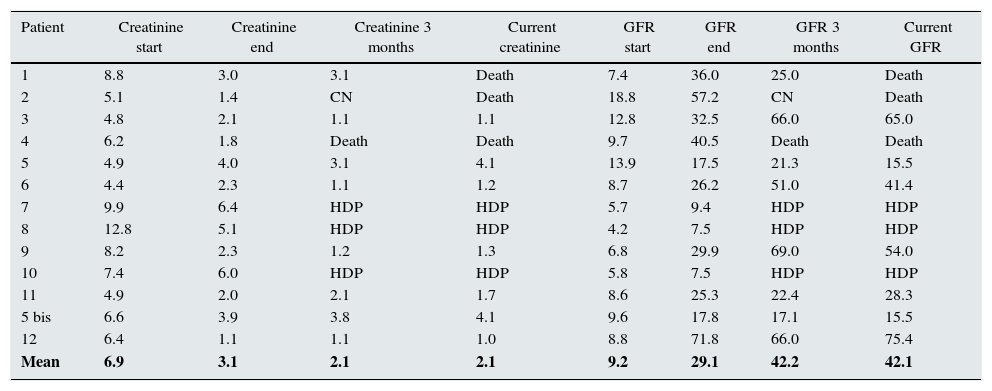
Ten out of the 13 cases, achieved a partial recovery of kidney function, and they not to require dialysis. The mean serum creatinine before treatment was 6.95mg/dl (12.8–4.4); at the end, it was 3.18mg/dl (6.0–1.1). For the eight patients who remained off dialysis, the mean creatinine at three months was 2.2mg/dl (3.8–1.1) and in the last analysis performed the mean creatinine is 2.1mg/dl (4.1–1.0). Out intention is to follow the clinical course of these patients for as long as we can (Table 3).
Changes in creatinine and GFR in patients with ARF secondary to MM treated with HCO filters.
| Patient | Creatinine start | Creatinine end | Creatinine 3 months | Current creatinine | GFR start | GFR end | GFR 3 months | Current GFR |
|---|---|---|---|---|---|---|---|---|
| 1 | 8.8 | 3.0 | 3.1 | Death | 7.4 | 36.0 | 25.0 | Death |
| 2 | 5.1 | 1.4 | CN | Death | 18.8 | 57.2 | CN | Death |
| 3 | 4.8 | 2.1 | 1.1 | 1.1 | 12.8 | 32.5 | 66.0 | 65.0 |
| 4 | 6.2 | 1.8 | Death | Death | 9.7 | 40.5 | Death | Death |
| 5 | 4.9 | 4.0 | 3.1 | 4.1 | 13.9 | 17.5 | 21.3 | 15.5 |
| 6 | 4.4 | 2.3 | 1.1 | 1.2 | 8.7 | 26.2 | 51.0 | 41.4 |
| 7 | 9.9 | 6.4 | HDP | HDP | 5.7 | 9.4 | HDP | HDP |
| 8 | 12.8 | 5.1 | HDP | HDP | 4.2 | 7.5 | HDP | HDP |
| 9 | 8.2 | 2.3 | 1.2 | 1.3 | 6.8 | 29.9 | 69.0 | 54.0 |
| 10 | 7.4 | 6.0 | HDP | HDP | 5.8 | 7.5 | HDP | HDP |
| 11 | 4.9 | 2.0 | 2.1 | 1.7 | 8.6 | 25.3 | 22.4 | 28.3 |
| 5 bis | 6.6 | 3.9 | 3.8 | 4.1 | 9.6 | 17.8 | 17.1 | 15.5 |
| 12 | 6.4 | 1.1 | 1.1 | 1.0 | 8.8 | 71.8 | 66.0 | 75.4 |
| Mean | 6.9 | 3.1 | 2.1 | 2.1 | 9.2 | 29.1 | 42.2 | 42.1 |
Periodic haemodialysis and glomerular filtration rate in ml/min; creatinine in mg/dl.
GFR: glomerular filtration rate; HCO: high cut-off filters; HDP: periodic haemodialysis; MM: multiple myeloma.
We also monitored kidney function using the MDRD-4 equation; we are aware that this is not the optimal method in cases of acute renal failure. Moreover, this was the method used in the, as yet, unpublished multicentre study, EuLITE, led by Hutchison.
Before haemodialysis treatment with HCO, the mean estimated glomerular filtration rate (GFR) was 9.29ml/min (18.8–4.2). After treatment, the mean GFR was 29.16ml/min (71.8–7.5). If we consider the mean GFR of the 10 patients who responded to the combination treatment of chemotherapy plus HCO-filter dialysis, this GFR increased to 35.4ml/min. Meanwhile, the mean for the three patients who did not respond to treatment remained at 8.12ml/min.
The estimated glomerular filtration rate (eGFR) of the eight patients who remained off treatment at three months was 39.8ml/min (69–17.1) and now, the seven patients who made good progress and continue not to require dialysis have a mean GFR of 41.44ml/min (75.4–15.5) (Table 3).
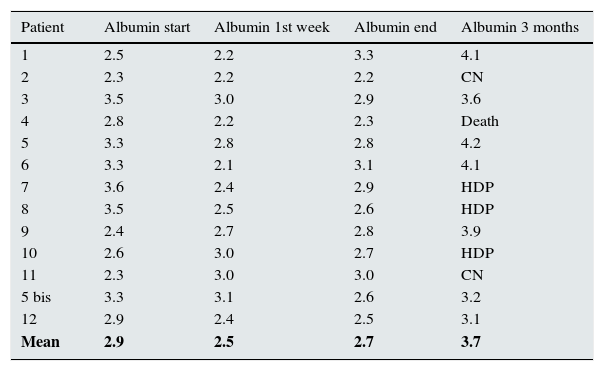
The loss of proteins during the sessions is a main concern. Mean serum albumin concentration before starting the treatment was 2.95g/dl (2.3–3.6). After the first week of treatment, albumin values had fallen to 2.58g/dl (3.2–2.1), despite infusion of two vials of 50ml of human albumin 20% (20g). However, at the end of the dialysis treatment, albumin had increased to 2.75g/dl (3.3–2.2) and, three months later, in the patients who had made good progress and remained off dialysis, serum albumin was within normal limits at 3.85g/dl (4.2–3.2, Table 4).
Changes in albumin in patients with ARF secondary to MM treated with HCO filters.
| Patient | Albumin start | Albumin 1st week | Albumin end | Albumin 3 months |
|---|---|---|---|---|
| 1 | 2.5 | 2.2 | 3.3 | 4.1 |
| 2 | 2.3 | 2.2 | 2.2 | CN |
| 3 | 3.5 | 3.0 | 2.9 | 3.6 |
| 4 | 2.8 | 2.2 | 2.3 | Death |
| 5 | 3.3 | 2.8 | 2.8 | 4.2 |
| 6 | 3.3 | 2.1 | 3.1 | 4.1 |
| 7 | 3.6 | 2.4 | 2.9 | HDP |
| 8 | 3.5 | 2.5 | 2.6 | HDP |
| 9 | 2.4 | 2.7 | 2.8 | 3.9 |
| 10 | 2.6 | 3.0 | 2.7 | HDP |
| 11 | 2.3 | 3.0 | 3.0 | CN |
| 5 bis | 3.3 | 3.1 | 2.6 | 3.2 |
| 12 | 2.9 | 2.4 | 2.5 | 3.1 |
| Mean | 2.9 | 2.5 | 2.7 | 3.7 |
Albumin [g/dl].
ARF: acute renal failure; HCO: high cut-off filters; HDP: periodic haemodialysis; MM: multiple myeloma.
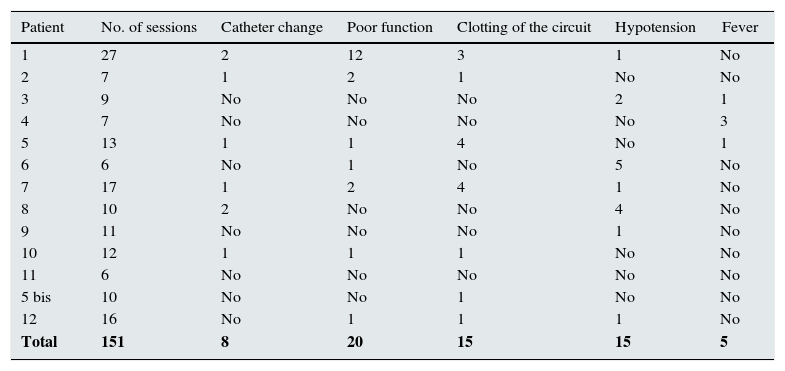
All patients had a temporary jugular catheter. Catheters were changed eight times in six patients; in patient 1, a permanent Tesio® catheter was placed due to the long period of treatment (27 sessions).
Mean flow rates were 250–300ml/min, as prescribed, but in 20 sessions, affecting seven patients, either flow problems or obstruction were recorded, with flow rates under 200ml/min.
In 15 sessions, affecting seven patients, clotting of the circuit, chambers or the dialysis membrane occurred, requiring them to be changed for new ones.
Episodes of hypotension with systolic blood pressure below 100mm/Hg or symptomatic hypotension occurred in seven patients during 15 sessions; these episodes were resolved by infusing varying amounts of 0.9% saline solution.
There were 13 febrile episodes affecting six patients with pyrexia above 37.5°C. Blood cultures were negative in all except in four patients. The following germs were found: Escherichia coli (treated with amoxicillin+clavulanic acid), Staphylococcus aureus (treated with daptomycin), Staphylococcus epidermidis (treated with meropenem) and Klebsiella pneumoniae (treated with meropenem); these were the same four patients whose catheters had to be changed (Table 5).
Complications during dialysis treatment with HCO.
| Patient | No. of sessions | Catheter change | Poor function | Clotting of the circuit | Hypotension | Fever |
|---|---|---|---|---|---|---|
| 1 | 27 | 2 | 12 | 3 | 1 | No |
| 2 | 7 | 1 | 2 | 1 | No | No |
| 3 | 9 | No | No | No | 2 | 1 |
| 4 | 7 | No | No | No | No | 3 |
| 5 | 13 | 1 | 1 | 4 | No | 1 |
| 6 | 6 | No | 1 | No | 5 | No |
| 7 | 17 | 1 | 2 | 4 | 1 | No |
| 8 | 10 | 2 | No | No | 4 | No |
| 9 | 11 | No | No | No | 1 | No |
| 10 | 12 | 1 | 1 | 1 | No | No |
| 11 | 6 | No | No | No | No | No |
| 5 bis | 10 | No | No | 1 | No | No |
| 12 | 16 | No | 1 | 1 | 1 | No |
| Total | 151 | 8 | 20 | 15 | 15 | 5 |
Complications: number of dialysis sessions; catheter changes; poor function; clotting of the circuit; hypotension; and fever.
We have presented our experience of 13 treatments in 12 patients who had acute renal failure secondary to MM, some with histopathological diagnosis of cast nephropathy and others with glomerular deposits.
The combined treatment of chemotherapy, usually bortezomib plus dexamethasone, and long-term haemodialysis with HCO filter was effective and achieved a recovery rate of 77%, which allowed patients to live without haemodialysis. These results are superior to those obtained in other series; Hutchison reported 60%,15 while Martín Reyes stated 50%.22
We do not know yet for how long patients will survive, but some of them have already exceeded three years. Several authors report a very significant increase in survival of 8–42 months.
Some authors argue that kappa-chain-producing myelomas respond better because they have a lower molecular weight and are therefore easier to remove through the HCO filter,12 while others have found the efficacy is similar.23
Among our patients, six were kappa-chain producers, of whom five recovered kidney function, while six were lambda-chain producers, four of whom improved (remembering that one patient was treated twice due to relapse). We therefore draw the conclusion that the treatment is effective for both kappa-chain- and lambda-chain-producing myelomas.
In terms of the need for kidney biopsies, the different authors were more selective about indicating treatment with HCO filters and required proof of cast nephropathy by kidney biopsy before starting treatment.15 As time goes on, the criteria in this regard is becoming less strict. In their more recent publications, some of these authors, like Hutchison,24 reported that biopsies were performed in less than 60% of patients.
At the beginning we did not consider that the renal biopsies was necessary to start treatment. From patient 7 on, we became much more demanding and biopsies were performed on all patients and as soon as possible, since we believe it provides important information both for diagnosis and prognosis.25
Until now, treatment with HCO filters was only indicated in cases of cast nephropathy. We used dialysis on three patients whose primary lesions were glomerular deposits and all three recovered kidney function. We therefore ask ourself two questions. Should we treat patients with glomerular deposits, given the good response achieved in our short experience? And how many of the patients treated and not biopsied (over 40%) and who had dialysis, with good results, might actually have had glomerular deposits?
There are three methods that, up to now, have been used to remove FLC:
- 1.
The first attempts to remove FLC used plasmapheresis, but this technique proved ineffective for several reasons: poor removal of FLC due to the fact that 80% is extravascular; the short duration of the sessions; albumin loss due to the large pore-size of the plasmafilter26; there was little benefit from exchange of 3.5l of plasma27, etc. Treatments for two or more months would be needed and still may no be sufficient to significantly reduce the level of FLC.19
The study with the largest number of patients treated with plasmapheresis was conducted by Clark (97 patients), and plasmapheresis was not found to add benefit in the parameters of GFR, dialysis dependence or death.16
- 2.
Another method used is haemofiltration with regeneration of the ultrafiltrate (HFR); this technique combines the three properties of the dialysis membranes (diffusion, convection and adsorption).
A dual-chamber dialyser is used, the first high-permeability, allowing the passage of the FLC, especially kappa chains, which are the smaller of the two. Ultrafiltration then takes place, and the resulting ultrafiltrate passes through a cartridge of adsorptive resin, where FLC adhere, but not albumin. The albumin is reinfused between the two dialysers18; the second dialyser is low permeability and is where the diffusion takes place.
There are no reports in the literature to demonstrate the utility of this method. It has the advantage that albumin is not lost and it has lower cost, but it does not remove enough lambda chains, so it can be used only in kappa-myeloma. Moreover, the few articles found in the literature talk about three sessions a week with an average of 52 sessions, which would mean more than 3 months of treatment.19 Some authors estimate the cost to be eight to nine times lower than dialysis with HCO filter, but do not take into account costs of staff and other aspects such as the difference between being on treatment for three weeks (HCO) or three months (HFR).
- 3.
Dialysis with Theralite Gambro HCO filters has been proved to be highly effective in removing both kappa and lambda FLC. We have already explained above how this technique works.
In our experience, in 10 out of the 13 cases (77%), we achieved an improvement in kidney function sufficient to allow the patients to live without being dependent on dialysis. It was as effective for kappa chains as lambda chains, with a rate of removal by dialysis of 58%, which is similar to that obtained by other authors who report loss of 53–57% and a final reduction in light chains of 93%.21
Martín Reyes reported a 60% reduction in FLC at 3 weeks and stated that this brought with it an 80% likelihood of recovery. After 6 days of treatment (6 sessions), we already had an FLC removal rate of 71%; the patients who recovered had a higher rate (74%) than those who did not recover (63%).15
In terms of loss of albumin, we found that infusion of two vials of human albumin 20% towards the end of dialysis helped maintain acceptable levels, with a mean of 2.58g/dl after six treatment sessions compared to 2.94g/dl at the start. By the end of the treatment, albumin levels had improved, with a mean of 2.75g/dl. Three months after finishing treatment, patients had normal levels of 3.85g/dl; only one of the six patients with data available had albumin for below 3.5g/dl.
With regard to the complications that may occur during dialysis sessions, the studies we found hardly mention any complications. Several articles in the literature do not report any complications at all, either because they were not recorded in that particular study28 or because there were none.29–31
In the Martín Reyes article,6 with six patients and 60 sessions, the most common problem reported was clotting of the system, representing half of all complications, followed by poor functioning of the catheter.
In the Muñoz study,32 there were two episodes of clotting in 9 patients and in the Mallol study33 the dialysis circuit clotted in nearly all the sessions with one patient.
In our sessions, the most common complications were problems with the flow rate (in 20 sessions), clotting of the circuit, and episodes of hypotension; the incidence of complications seems to be low and never was serious.
Discussion about the cost of treatmentHCO filters have been used for the treatment of acute renal failure secondary to MM in 2007, first with a Gambro HCO 1100 1.1m2 filter, then it was changed to the Theralite Gambro 2.1m2.
In 2011, Grima et al. published a study based on a cost-effectiveness model comparing treatment with HCO filters vs conventional dialysis.34 This study was carried out in patients from United Kingdom and the costs were valued in pounds sterling, taking into account the cost of the HCO filter, the cost of albumin and the extra nursing time (2h per dialysis session).
The cost savings were based on avoiding long-term haemodialysis and the model predicted survival of 20 months for patients on standard dialysis, compared to the 34 months obtained from treatment with the HCO filter. The longer survival was also accompanied by better quality of life, since the patients no longer required dialysis. Grima found the cost savings to be 6000 pounds, about 8000 euros.34
We used a similar model to assess the cost, but without including the cost of 2 extra nursing hours; these patients were considered as just another acute renal failure and the number of staff remained the same. Our patients’ survival needs to be evaluated at long term. Certainly, we are able to affirm that three patients have survived for more than three years without dialysis. To compare the outcomes, we chose to accept Grima's estimates for survival. Thus, we based our economic evaluation primarily on the cost of the HCO filter and albumin, as we consider the cost of the rest of the material to be very similar to conventional haemodialysis.
The cost of the Theralite Gambro HCO filter in our hospital is 825 euros. The cost of each 50ml vial of human albumin 20% is 17.68 euros. The cost of a dialysis session with HCO filter is therefore 860.36 euros. We also added up the total cost of the sessions for all 13 treatments (151 sessions) and, with a mean of 11.53 sessions per patient, this resulted to p to be 9919.95 euros.
The cost per dialysis session in our hospital, adding the pharmacy costs and supplies (dialysis equipment, lines, liquid, dialysers, ultrafilters, catheters, disinfectants, gloves, gauze dressings, etc.), is 85 euros on average per session; this data can be obtained from the departmental financial reports. A patient usually requires three sessions per week, meaning 156 per year, the total cost of which we estimate to be 13,260 euros.
If we apply the results from the Grima study, mean survival of patients with MM on dialysis is 20 months, representing a cost of 85 euros×260 sessions, which equals 22,100 euros in total; we would therefore be saving 12,181 euros. If we consider the cost of staff, transportation and other expenses, the savings would be much greater.
We also wanted to make a comparison with HFR; this technique is more expensive than conventional haemodialysis, but cheaper than haemodialysis with HCO filters. The report published on three patients by the Cordoba group19 showed that they do an average of 52 dialysis sessions compared to the 11.53 that we needed in our study. In other words, the treatment lasted almost three months longer and the number of sessions was five times the number used for our patients treated with HCO filter. We are therefore of the opinion that this dilutes any cost savings that might be obtained with HFR. However, studies with larger numbers of treated patients are needed to be able to draw conclusions in terms of both efficacy and cost. What has been demonstrated is the lower loss of albumin during dialysis with HFR.
Conclusions- 1.
The combined treatment of chemotherapy plus long dialysis sessions with HCO filters was effective in reducing the level of FLC and recovering a sufficient degree of kidney function in 77% of cases.
- 2.
There are significant savings with the use of HCO filters. In contrast to what has previously been reported in the literature, the saving is over 12,000 euros per patient, even without taking into account essential factors such as the cost of staffing and transport.
The authors have no conflicts of interest to declare.
Please cite this article as: Berni Wennekers A, Martín Azara MP, Dourdil Sahun V, Bergasa Liberal B, Ruiz Laiglesia JE, Vernet Perna P, et al. Trece tratamientos de la insuficiencia renal aguda secundaria a mieloma múltiple con filtros de high cut off. Nefrologia. 2016;36:418–426.