To the Editor:
Castleman’s disease is a benign lymphoproliferative disease that develops in the form of lymphoid follicular hyperplasia in which renal involvement is uncommon. Only isolated case reports and small series (20 patients) have been reported. The two most common types of lesions reported in these cases are thrombotic microangiopathy-like (TMA-like) and AA amyloidosis.1-4
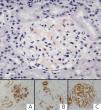
Here, we present the case of a 30-year-old male with a history of thalassemia minor and who was diagnosed with nephrotic syndrome and normal renal function. A renal biopsy revealed a mesangial deposit of an amorphous eosinophilic material prone to form nodules, which was dispersed uniformly throughout the glomeruli and in small vessel walls. This material was Congo red positive, with birefringence in polarised light and immunohistochemical expression for amyloid A. Staining with vascular endothelial growth factor (VEGF) and synaptopodin was intensely expressed in the podocytes (Figure 1). The diagnosis was made of type AA renal amyloidosis with a glomerular/vascular distribution. Serology for human immunodeficiency virus (HIV) was negative, and an immunological analysis ruled out the possibility of systemic or infectious disease.
An abdominal/pelvic computerised axial tomography revealed a solid and heterogeneous mass of 9.5cm in diameter and with calcifications that was in contact with the small intestine. The resected section of the small intestine had a fleshy mesenteric tumour with no relation to the intestinal wall. Histological analysis revealed that the tumour was an adenopathic mass with preserved structure and lymphoid follicular hyperplasia, with a heterogeneous appearance (Figure 2); the paracortical region contained a diffuse proliferation of mature plasma cells, with no atypia and abundant Russell bodies. Serology for herpesvirus 8 (HHV8) was negative.
In this case, the histopathological diagnosis was plasma cell-type Castleman’s disease. We also identified amyloid deposits in the perivascular region, the paracortex, and the submucosa of the small intestine.
The final diagnosis for our patient was systemic AA amyloidosis associated with Castleman’s disease, plasma cell type.
DISCUSSION
Castleman’s disease is an uncommon and benign lymphoproliferative disease that presents in the form of lymphoid follicular hyperplasia. In some cases it is associated with HIV or HHV8.5 It has also been correlated with malignant diseases such as Kaposi’s sarcoma and certain lymphomas. Two distinct entities have been described: localised and multi-centric, which are very different in terms of prognosis. Classically, two different histological types have also been identified: hyaline-vascular and plasma cell. The localised hyaline-vascular type is the most common (80%); it is asymptomatic and tends to have a benign prognosis. The plasma cell type is associated with a more aggressive clinical progression and the presence of constitutional syndrome. In contrast to the hyaline-vascular type, multi-centric plasma cell type may be associated with infection by HHV8, above all in patients with HIV. Renal involvement in Castleman’s disease is uncommon, but the most commonly described forms are thrombotic microangiopathic-like (TMA-like) and AA amyloidosis. The prognosis for the renal damage, once the cause is corrected, appears to be based on the extent and intensity of the glomerular deposits as well as the vascular damage produced. We are unaware of the reason why certain patients develop vascular lesions while others develop amyloidosis. Some authors have suggested that the vascular lesion is mediated by a decreasing regulation of VEGF that is produced in these lymphoproliferative diseases.1 In our patient, the conserved expression of both VEGF and synaptopodin, a crucial protein in the maintenance of the glomerular filtration barrier, could be indicators for a good prognosis, with potentially reversible proteinuria.
The cases that progress with amyloidosis AA tend to occur in patients with localised abdominal Castleman’s disease, as occurred in our case: long-term chronic inflammatory syndrome could contribute to the development of amyloidosis. It is also believed to be mediated by high levels of interleukin 6.6-7
In a young patient with AA renal amyloidosis and no other possible diagnosis, plasma cell-type Castleman’s disease may be considered the cause. A study of VEGF or synaptopodin expression can contribute to evaluating and establishing a prognosis for renal lessions.
Conflicts of interest
The authors have no conflicts of interest to declare.
Figure 1. Glomeruli with deposits of an amorphous red material. Congo red stain
Figure 2. Adenopathic mass with heterogeneous lymphoid follicular hyperplasia and preserved structure. H-E 20x