Cutaneous cryptococcosis is a very uncommon disease. It was initially described in 1928 by Buscke,1 although the first to describe its impacts on the central nervous system (CNS) was Zenker in 1861.2 This disease occurs most frequently in immunocompromised patients as an opportunistic infection,3 with primary cutaneous infection in the absence of systemic involvement a very rare form of the disease.4
Cryptococcus neoformans is the most commonly found encapsulated fungus in humans. It can produce pulmonary, meningeal, or cutaneous infection.5 Three different species have been identified: C. neoformansgatti, C. neoformans grubii, C. neoformans neoformans, and 5 serotypes.6 The gatti is the most commonly found in immunocompromised patients, and neoformans is the most commonly observed in immunodepressed patients.7 The natural habitat of these fungi is in the faeces of pigeons and other birds, as well as in the floor contaminated by them.8
The infection is usually acquired by inhalation, direct inoculation, or through infection of a cutaneous lesion/wound.9
Here we describe the cutaneous lesions produced by Cryptococcus neoformans in an immunocompromised patient with chronic kidney disease and on periodic haemodialysis.
Case report
Our patient was a retired 53-year-old male who lived in a rural area. He was diagnosed 10 years prior with inflammatory bowel disease, which was under treatment with high doses of steroids. Two years earlier, he had been admitted to an intensive care unit due to intestinal sepsis with multi-organ failure; since then, the patient had been on periodic haemodialysis and had not recovered renal function. A renal biopsy revealed tubulo-interstitial nephropathy.
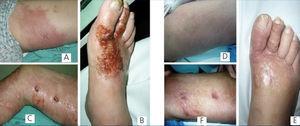
Since 1 month prior to hospitalisation, the patient had suffered cutaneous erythematous lesions in indurated, pruritic and painful areas: the dorsal surface of the right foot and the internal surface of the thighs, with a poor response to antibiotic treatment (started empirically due to suspicion of cellulitis) and that continued to evolve to desquamation and ulceration (Figure 1). We performed a cutaneous biopsy that revealed the presence of Cryptococcus (Figure 2). A direct examination with Indian ink and Gram stain revealed abundant large spherical yeasts that were encapsulated and had substantial budding. A yeast cell culture was urease positive, compatible with Cryptococcus neoformans, neoformans variety. Serum cryptolatex was also positive (1/2048). We ruled out pulmonary and neurological involvement by computerised axial tomography and lumbar puncture.
We started the patient on treatment with voriconazole at 200mg/12 hours, which produced a notable change in liver test results after 10 days of treatment (total bilirubin: 9.59mg/dl; direct bilirubin: 9.41mg/dl; glutamic oxaloacetic transaminase [GOT]: 176U/l; glutamic pyruvic transaminase [GPT]: 226U/l), requiring replacement with amphotericin B at 100mg/day. In addition, we decreased the dose of prednisone. Under this treatment regimen, the patient experienced a significant improvement in the cutaneous lesions after 2 weeks, with an almost complete disappearance of ulcerations (Figure 1) and normalised liver test results.
Discussion
Since the initial description provided by Buschke, very few cases have been described of cutaneous cryptococcosis.3 This infection is more common in males and in the elderly. It is also frequently associated with organ transplants, tumours, human immunodeficiency virus, and prolonged steroid use.10-12 Vogelaers described in 1997 a case of primary lesions in Belgium in a male pigeon breeder who was on chronic steroid therapy due to chronic obstructive pulmonary disease.13 This was similar to the case of our patient, who suffered inflammatory bowel disease that required high doses of prednisone, and who lived in a rural area in close proximity to fowls (hens).
The clinical spectrum of this infection varies widely; it may produce papules, nodules, ulcers, abscesses, or pustules,14 which can lead to confusion with other possible causal entities, such as the suspicion of cellulitis in our patient. Our differential diagnosis also considered pemphigus and pyoderma gangrenosum3 due to their association with inflammatory bowel disease. The diagnosis is made by identifying the fungus through direct microscopic evaluation, a histological analysis, and microbiological culture.14
Azoles provide the treatment of choice for patients with pulmonary affectation and immunocompetent patients without CNS involvement. Amphotericin B together with flucytosine is used to treat the most severe forms of meningeal infection as a primary induction therapy, followed by fluconazole as a consolidation therapy, according to the IDSA guidelines.15 The use of voriconazole to treat this infection produces hepatic toxicity within a few days, which is why we switched the treatment of our patient to amphotericin B, which produced a notable improvement in 2-3 weeks.
To conclude, our patient had several risk factors for cryptococcosis: chronic renal failure, inflammatory bowel disease, high doses of steroids, and the epidemiological risk of living in a rural area. The lesions resembled bacterial cellulitis, necessitating a histological and microbiological analysis of tissue samples in order to confirm the diagnosis.
Specific treatment must be maintained for a prolonged period, no less than 6 months.15
Conflicts of interest
The authors have no conflicts of interest to declare.
Figure 1. Evolution of lesions
Figure 2. Cutaneous biopsy