Thrombocytopenia is a hallmark of postdiarrhoeal haemolytic uraemic syndrome (D+ HUS), although it can be transient and therefore undetected. There is scarce information regarding the prevalence and the course of the disease in children with D+ HUS without thrombocytopenia.
ObjectiveTo determine the prevalence of D+ HUS without thrombocytopenia and to describe the clinical characteristics of a series of children with this condition.
Patients and methodsThe medical records of patients with D+ HUS hospitalised between 2000 and 2016 were reviewed to identify those without thrombocytopenia (>150,000mm3). Demographic, clinical and laboratory parameters of the selected cases were collected and descriptively analysed.
ResultsNine cases (5.6%) without thrombocytopenia were identified among 161 patients hospitalised during the study period. Median age at diagnosis was 17 months (7–32) and median prodromal symptom duration was 15 days (7–21). Eight patients maintained normal urine output while the remaining one required dialysis. No patient presented with severe extrarenal compromise and/or hypertension.
ConclusionsThe prevalence of non-thrombocytopenic D+ HUS was 5.6% and most cases occurred with mild forms of the disease; however, the need for dialysis in one of them indicated that normalisation of platelet count is not always an accurate marker for disease remittance. Our results also confirm that the time of onset of D+ HUS in patients without thrombocytopenia is usually delayed with respect to the initial intestinal symptoms; thus, heightened diagnostic suspicion is necessary.
La presencia de trombocitopenia es una marca distintiva del síndrome urémico hemolítico asociado a diarrea (SUH D+); sin embargo, puede ser transitoria y, por lo tanto, no ser detectada. Existe limitada información sobre la prevalencia y el curso de la enfermedad en niños con SUH D+ sin trombocitopenia.
ObjetivoDeterminar la prevalencia de SUH D+ sin trombocitopenia y describir las características clínicas de una serie de niños con esta particularidad.
Pacientes y métodosFueron revisadas las historias clínicas de los pacientes con SUH D+ internados entre 2000 y 2016 para identificar a aquellos sin trombocitopenia (>150.000mm3). De los casos seleccionados se recolectaron las variables demográficas, clínicas y de laboratorio, las cuales fueron analizadas descriptivamente.
ResultadosDe 161 pacientes internados durante el periodo de estudio se identificaron 9 sin trombocitopenia (5,6%). La mediana de la edad al diagnóstico fue de 17 meses (7-32) y la de la duración del periodo prodrómico, de 15 días (7-21). Ocho pacientes mantuvieron diuresis normal y uno requirió diálisis. Ningún paciente presentó compromiso extrarrenal severo y/o hipertensión arterial.
ConclusionesLa prevalencia de SUH D+ sin trombocitopenia fue del 5,6% y la mayoría de los casos fueron leves; sin embargo, el requerimiento de diálisis en uno de ellos señala que la normalización del recuento de plaquetas no siempre es un marcador preciso de resolución de la enfermedad. Nuestros resultados también confirman que el momento de presentación de los pacientes con SUH D+ sin trombocitopenia está usualmente alejado de los primeros síntomas intestinales, por lo que es necesario un alto índice de sospecha diagnóstica.
Diarrhoea-positive haemolytic uraemic syndrome (D+ HUS) is one of the main causes of acute kidney injury in children.1,2 It is mediated by Shiga toxin-producing Escherichia coli (STEC), which causes direct endothelial damage inducing platelet aggregation and thrombus formation that occlude the microvasculature of vital organs such as the kidneys.1,2 The diagnosis of D+ HUS is based on the presence of a prodrome of diarrhoea associated with microangiopathic haemolytic anaemia, thrombocytopenia and acute kidney injury.1–3 On rare occasions, the thrombocytopenia may be transient and therefore undetected by laboratory tests.4 Because there is scarce information on the clinical characteristics of children with D+ HUS with no thrombocytopenia, the aim of this study is to describe the clinical course of a series of patients with this particularity.
Patients and methodsWe reviewed the medical records of all hospitalised children diagnosed with D+ HUS at the Pedro de Elizalde General Children's Hospital between 2000 and 2016 to identify those without thrombocytopenia. Then, the following variables were extracted from the medical records selected: age, gender, time elapsed between the first symptom and diagnosis of D+ HUS, signs and symptoms of the prodromal phase, blood count on admission (white blood cell and platelet counts, haematocrit and haemoglobin), minimum platelet count and the percentage of decrease in relation to the initial value, maximum serum creatinine and severity of acute kidney injury, number of red blood cell transfusions received, need for and days of dialysis, presence of severe extrarenal manifestations and/or hypertension, and isolation of the aetiological agent. In addition, follow-up time after the acute stage and renal status at the time of the last check-up was recorded.
The study was approved by our institution's research and ethics committees.
DefinitionsD+ HUS is defined as the presence of diarrhoea associated with thrombocytopenia (<150,000mm3), acute microangiopathic haemolytic anaemia (haemoglobin <3rd percentile for age and gender with the presence of schistocytes in a peripheral blood smear, a negative Coombs’ test and an increase in lactate dehydrogenase) and kidney failure expressed by haematuria and proteinuria with or without elevated creatinine relative to ages.1–3 Cases with no thrombocytopenia were considered to be when no test during hospitalisation showed a platelet count<150,000mm3, but which met the rest of the criteria.5 Platelet counts in all cases were determined by automated methods and later confirmed by paediatric haematologists at our hospital who directly viewed the peripheral blood smears.
The aetiological diagnosis was based on the identification of STEC or Shiga toxin in faeces and/or positive antibodies to polysaccharides of the most common STEC serotypes.6 Given that Argentina not only has the highest global incidence of D+ HUS but also that the aetiological identification in our setting varies between 32% and 54%, cases that showed a prodrome of diarrhoea, even with no pathogen detected, were also considered STEC-mediated.6,7 Patients with familial or recurrent HUS and cases associated with specific causes, such as deregulation of the complement system, AIDS, drugs and pneumococcal infection, were excluded.8
The presence of neurological manifestations, such as decreased alertness, seizures or coma, and intestinal manifestations, such as intussusception, perforation or ischaemic colitis, were considered to be serious extrarenal involvement.9–11
Readings on the blood pressure monitor of systolic and/or diastolic blood pressure greater than the 95th percentile for age, height and gender according to the reference values were considered to be cases of hypertension.12
The indications for dialysis were anuria >24h, untreatable electrolyte disturbances and hypervolaemia.13,14
Creatinine was measured with the Jaffe reaction, and glomerular filtration rate (eGFR) was estimated by the Schwartz formula, using the maximum creatinine value.15 The severity of the acute kidney injury was stratified according to paediatric RIFLE criteria (risk decrease in eGFR>25%; injury>50%; and failure>75%), assuming a baseline eGFR of 100ml/min/1.73m2 because, as is usually the case with children with acute kidney injury, the renal function before admission was not known.16
Diuresis>1ml/kg/h17 was considered to be a normal urine output.
Haematuria was considered if there were more than 5 RBCs per field (with ×400 magnification) in centrifuged fresh urine.18
Proteinuria was determined by means of a test strip; the presence of 1+ or more on the colourimetric scale was considered a positive result.18
Statistical analysisA descriptive analysis of the variables collected was performed; continuous variables were expressed, according to the distribution type (Shapiro–Wilk test), as the median (interval) or mean (standard deviation), and categorical variables were expressed as the frequency of distribution. The statistical analysis was performed using Statistix, ver. 7 (IBM version; Analytical Software, Tallahassee, FL, United States).
ResultsFrom 2000 to 2016, 161 children with D+ HUS were admitted to our hospital, 9 of whom were identified with no thrombocytopenia (prevalence of 5.6%).
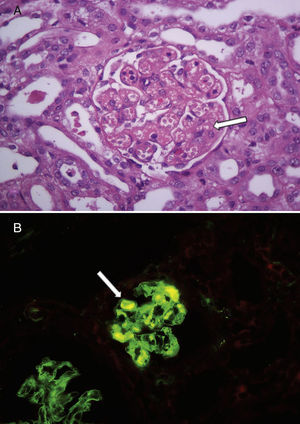
The median age at diagnosis was 17 months (7–32), and the duration of the prodrome period was 15 days (7–21). Four patients showed no decrease in platelet count compared with the value on admission, and the remaining 5 had a mean decrease of 30±17%; but all had values greater than 150,000mm3 throughout hospitalisation. The median number of red blood cell transfusions was 1 (0–2), and no patient had severe extrarenal involvement and/or hypertension. Regarding the severity of kidney failure based on the paediatric RIFLE criteria, in 3 cases there was no acute kidney injury (although haematuria and proteinuria were present as a sign of kidney disease), 2 cases fell under the risk category, 2 under injury and 2 under failure. Eight patients had normal diuresis and one young girl had anuria and severe kidney failure (creatinine 8.1mg/dl on admission) and required peritoneal dialysis. She had no history of kidney disease nor had she received drugs during the prodromal phase; her C3 (89mg/dl) and C4 (20mg/dl) levels were normal, and no bacterial or viral agents were detected in her blood. Likewise, the test for Shiga toxin and the faecal culture were negative. An ultrasound revealed normal-sized hyperechogenic kidneys. Given the absence of thrombocytopenia, together with the severity of the kidney failure and the lack of aetiological identification, a kidney biopsy was performed that confirmed the diagnosis of thrombotic microangiopathy. The main findings from the biopsy were as follows: thickening and disruption of the glomerular capillary walls; focal mesangiolysis and aneurysmal dilatation of the glomerular capillaries; patchy acute tubular necrosis; and interstitial oedema involving less than 5% of the sample. The arteries and arterioles showed no changes. Fibrin thrombi were identified by immunofluorescence (Fig. 1). She needed 7 days of dialysis and 2 red blood cell transfusions during hospitalisation, and was discharged in good clinical condition and with her kidney function improving progressively (creatinine 1.19mg/dl). During the outpatient follow-up, she had a positive result for polysaccharide antibodies to Escherichia coli O145, and her kidney function was stabilised (creatinine 0.3mg/dl). Table 1 shows the patients’ demographic and clinical characteristics, and Table 2 shows the laboratory findings during the acute phase.
Findings from the renal histology of one child with diarrhoea-positive haemolytic uraemic syndrome with no thrombocytopenia. (A) Renal glomeruli with capillary dilatation and mesangiolysis (white arrow). Haematoxylin and eosin, 400×. (B) Immunofluorescence showed fibrin thrombi in some glomeruli (white arrow), 400×.
Demographic and clinical characteristics of children with diarrhoea-positive haemolytic uraemic syndrome with no thrombocytopenia.
| Case | Age (months) | Gender (F/M) | Duration of prodromal phase (days) | Symptoms of prodromal phase | RBCT (number) | Dialysis (days) | Serious extrarenal involvement | Maximum BP (mmHg) | Aetiology |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | F | 15 | Bloody diarrhoea Pallor | 0 | – | – | 95/50 | Escherichia coli O157:H7 and Stx2 in stools |
| 2 | 26 | F | 15 | Bloody diarrhoea Fever Pallor | 1 | – | – | 90/60 | – |
| 3 | 7 | M | 15 | Bloody diarrhoea Fever Pallor | 1 | – | – | 85/60 | – |
| 4 | 7 | M | 8 | Diarrhoea with no blood Pallor | 1 | – | – | 90/60 | – |
| 5 | 26 | M | 7 | Bloody diarrhoea Pallor | 2 | – | – | 85/50 | – |
| 6 | 17 | M | 15 | Diarrhoea with no blood Vomiting | 1 | – | – | 95/50 | Escherichia coli O157:H7 and Stx2 in stools |
| 7 | 32 | M | 21 | Abdominal pain Diarrhoea with no blood Fever Pallor | 0 | – | – | 90/60 | Stx2 in stools |
| 8 | 24 | F | 15 | Diarrhoea with no blood Pallor | 2 | 7 | – | 90/50 | Polysaccharide antibodies to Escherichia coli O145 |
| 9 | 8 | M | 10 | Bloody diarrhoea | 1 | – | – | 80/50 | – |
F: female; M: male; BP: blood pressure; RBCT: red blood cell transfusion; Stx: Shiga toxin.
Laboratory parameters during the acute period in children with diarrhoea-positive haemolytic uraemic syndrome with no thrombocytopenia.
| Case | Proteinuria | Red blood cells in urine | WBC on admission (mm3) | Ht (%) on admission | Hb (g/dl) on admission | Platelets on admission (mm3) | Minimum platelet count (m3) | Maximum LDH (IU/l) | Maximum creatinine (mg/dl) | Minimum eGFR (ml/min/1.73m2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | +++ | >15 | 6900 | 19 | 5.9 | 599,000 | 599,000 | 1340 | 0.3 | 109 |
| 2 | +++ | 10–15 | 13,700 | 28 | 9.6 | 309,000 | 309,000 | 1200 | 0.9 | 56 |
| 3 | ++ | 8–10 | 27,200 | 18 | 6 | 350,000 | 151,000 | 1560 | 1.1 | 26 |
| 4 | ++ | 10–15 | 12,000 | 19 | 7.1 | 374,000 | 374,000 | 1180 | 0.34 | 90 |
| 5 | +++ | 25 | 15,800 | 16.5 | 5 | 199,000 | 153,000 | 1845 | 2.2 | 23 |
| 6 | + | 6–8 | 8500 | 23 | 7.7 | 435,000 | 388,000 | 1500 | 0.66 | 65 |
| 7 | +++ | 15 | 11,200 | 21 | 6.9 | 374,000 | 278,000 | 1460 | 0.6 | 81 |
| 8 | +++ | 15–20 | 13,300 | 18 | 6.3 | 570,000 | 360,000 | 1494 | 8.1 | 6 |
| 9 | + | 6 | 14,800 | 22 | 7.1 | 250,000 | 250,000 | 1230 | 1 | 37 |
eGFR: estimated glomerular filtration rate; WBC: white blood cells; Hb: haemoglobin; Ht: haematocrit; LDH: lactate dehydrogenase.
After discharge, the patients were followed for 2 years (1.5–4), and all had a normal eGFR, no proteinuria or haematuria and normal blood pressure.
DiscussionThe presence of thrombocytopenia is a distinctive sign of D+ HUS; however, it may be transient and therefore go undetected.4 There is limited information on the prevalence of D+ HUS with no thrombocytopenia, and the course of the disease in these patients has not been described in detail. In a cohort of 102 patients with D+ HUS, Schifferli et al.19 found that 7 (6.8%) had no thrombocytopenia, a prevalence similar to that seen by Siegler et al.20 (6%) in 157 cases (89% with prodrome of diarrhoea). Similarly, in our series of 161 children, the prevalence of lack of thrombocytopenia was 5.6%. These results differed from those seen by Giménez Llort et al.,21 who noted a lower prevalence (one case in 51 children, or 2%), and from that reported by Ardissino et al.,22 who found this condition in 11% of their cases; although it should be clarified here that in the latter study, this percentage corresponds to patients with platelet counts>150,000mm3 on admission, but this does not specify whether they later declined during the course of the disease. Finally, in Argentina, in 254 children with bloody diarrhoea, López et al.5 found that 6 developed HUS and 14 had “incomplete forms” (they did not meet 3 diagnostic criteria), with 3 showing no thrombocytopenia, i.e. the missing criterion. The case reported by Meier et al. is interesting; it concerns a child with diarrhoea due to STEC, who developed microangiopathic anaemia with no kidney impairment or thrombocytopenia. The authors speculated that Shiga toxin may cause direct damage to the erythrocyte membrane as a possible explanation for such a case.23
To the best of our knowledge, this is the first case-series to specifically describe the disease's course in patients with D+ HUS with no thrombocytopenia. The median age of the group studied (17 months) is consistent with that of children who meet all the diagnostic criteria.6 It should be noted that the median time between the first symptom and the diagnosis (15 days) was longer than usual (the median is approximately 7 days).24 Late diagnosis may explain the absence of thrombocytopenia; it is possible that the children studied here presented with transient thrombocytopenia, but that at the time of the laboratory tests the rebound of the platelet count was detected.24 Moreover, no patients showed a decrease of less than 150,000mm3, nor were there sudden reductions in platelet counts compared with the baseline values (the mean decrease was 30%). Late diagnosis may also have been responsible for the low rate of isolation of STEC in stools in our patients,3 which was only possible in 3 of 9 cases, although the isolation rate is within the norm in our setting.6,7
In D+ HUS, platelet counts are commonly normalised before kidney function is recovered; in fact, the time to reaching a count>150,000mm3 was proposed as an indirect marker that the microangiopathic process has resolved.8 Accordingly, it has been postulated that these late cases with no thrombocytopenia are usually mild.24 Our results confirm this assumption: no patient presented with serious extrarenal involvement, and, although the severity of acute kidney injury varied, 8 of 9 cases maintained adequate diuresis with no need for dialysis, i.e. a lower rate than previously reported in children with D+ HUS.13,14,25 In addition, no patient had any signs of renal sequelae at the last check-up.
In conclusion, the prevalence of D+ HUS with no thrombocytopenia was 5.6% and most cases were mild; however, since one patient required dialysis, it is worth noting that the normalisation of platelet counts is not always an accurate marker that the disease has resolved. Additionally, our results show that the time when D+ HUS cases present with normal platelet counts is usually long after the first intestinal symptoms, and a high rate of diagnostic suspicion is therefore necessary.
Conflicts of interestThe authors declare that they have no potential conflicts of interest related to the contents of this article.
Please cite this article as: Balestracci A, Toledo I, Meni Battaglia L, de Lillo L, More N, Cao G, et al. Síndrome urémico hemolítico asociado a diarrea sin trombocitopenia. Nefrologia. 2017;37:508–514.