Calcific uraemic arteriolopathy (CUA), also called calciphylaxis, is a rare but potentially fatal vascular disorder that almost exclusively affects patients with chronic renal failure. The objective of this study was to analyse various risk factors for developing CUA and its subsequent clinical course according to the treatment received.
Materials and methodsA retrospective study that included patients diagnosed with CUA from December 1999 to December 2015. Various risk factors, clinical course and treatment options were analysed.
ResultsA total of 28 patients (53.6% females) with a mean age of 67.2±11.8 (38–88) years were included. At the time of diagnosis, 53.6% were on haemodialysis, 25% were kidney transplant patients and 21.4% had normal renal function. The use of steroids (100%, p=0.001) was the main risk factor in renal transplant patients. Skin lesions resolved in 60.7% (especially in those receiving multitargeted therapy). Patient survival at 12 months was 29% in transplant patients, 57% in haemodialysis patients and 100% in normal renal function patients (log-rank 6.88, p=0.032). Chronic renal failure (p=0.03) and hypoalbuminaemia (p=0.02) were the main risk factor for CUA mortality.
ConclusionsAlthough the incidence of CUA remains low, CUA mortality is very high, Special attention to its occurrence in kidney transplant patients and “non-renal” CUA forms is required. Oral anticoagulants and steroids appear to be the main risk factors, CUA is a challenge; a registry of patients and determining standard therapy are required.
La arteriolopatía urémica calcificante (CUA), también llamada calcifilaxis, es un trastorno vascular poco frecuente pero potencialmente mortal que afecta casi exclusivamente a pacientes con insuficiencia renal crónica. El objetivo de este estudio fue analizar los diferentes factores de riesgo para el desarrollo de CUA y su posterior evolución según la terapia recibida.
Material y métodosEstudio retrospectivo que recoge aquellos pacientes con diagnóstico de CUA desde diciembre de 1999 hasta diciembre de 2015. Se analizaron diferentes factores de riesgo, evolución y diferentes opciones terapéuticas.
ResultadosSe incluyeron 28 pacientes (53,6% mujeres) con una edad media de 67,2±11,8 años (38-88). En el momento del diagnóstico, el 53,6% estaba en hemodiálisis, un 25% eran pacientes con un trasplante renal y el 21,4% presentaba función renal normal. En los pacientes trasplantados, el consumo de esteroides (100%; p=0,001) fue el principal factor de riesgo. La resolución de lesiones cutáneas se produjo en el 60,7% (especialmente en los que recibieron tratamiento multitarget). La supervivencia de los pacientes a los 12meses fue de 29, 57 y 100% en los pacientes trasplantados, hemodiálisis y con función renal normal respectivamente (log-rank 6,88; p=0,032). La presencia de insuficiencia renal crónica (p=0,03) e hipoalbuminemia (p=0,02) fueron los principales factores de riesgo de mortalidad CUA.
ConclusiónAunque la incidencia de la CUA sigue siendo baja, su mortalidad es muy elevada, por lo que debe prestarse especial atención a la presentación de la CUA en los trasplantados renales y en las formas «no renales». Los anticoagulantes orales y los esteroides aparecen como principales factores de riesgo. La CUA es un reto: necesitamos un registro de nuestros pacientes y establecer una terapia estándar.
Calciphylaxis, also called calcific uraemic arteriolopathy (CUA), is an uncommon but serious condition with a high rate of mortality.1,2
CUA develops in the dermis and subcutaneous tissue, generally in areas of adiposity. It is characterised by pruritic or painful skin lesions, purplish nodules, ulcers and sores that develop into ischaemic necrosis of the skin.3 A histological examination may reveal medial calcification and intimal proliferation in the small arteries, with or without endovascular fibrosis, extravascular calcification and vascular thrombosis.4
It occurs more frequently in patients with advanced chronic kidney disease on dialysis or in renal transplant patients; however, cases of calciphylaxis have been reported in subjects with normal renal function, classified as “non-renal” CUA.5
The pathogenesis of this vascular disease is complex and not well understood, but there are several mechanisms thought to be involved in its development. Over the years, other risk factors have been added to the traditional factors of bone and mineral metabolism (hyperparathyroidism, hypercalcaemia and hyperphosphataemia) and its high prevalence in patients with low bone metabolism.6,7 These include: female gender, diabetes, obesity, local trauma, hypoalbuminaemia, hypercoagulable states and exposure to active vitamin D, calcium chelators, corticosteroids and vitamin K antagonists.8–11 This amalgam of factors has generated a new patient phenotype that shows lesions from calciphylaxis that are unlike the classic image of this condition.
Despite the poor prognosis of this condition, the use of multidisciplinary treatment in recent years (treatment of pain and of superinfections, intensification of haemodialysis, bisphosphonates, sodium thiosulfate, non-calcium chelators, cinacalcet, vitamin K and pentoxifylline) has seen survival increase in these patients.12–14
The objective of this study was to analyse the current epidemiology of calciphylaxis in the region of Hospital 12 de Octubre, the risk factors for its development and the patients’ clinical courses depending on the treatment received.
Material and methodsAn observational and retrospective study was performed on a series of patients diagnosed with calciphylaxis between December 1999 and December 2015 at Hospital Universitario 12 de Octubre in Madrid.
The following data were recorded: gender, age, Charlson comorbidity index, status at diagnosis (patients on haemodialysis, with a normally functioning renal transplant or without kidney disease), aetiology of chronic kidney disease (CKD), time to renal replacement therapy (haemodialysis and renal transplantation), cardiovascular risk factors (hypertension, diabetes mellitus, obesity), chronic ischaemic syndrome, coronary artery disease, atrial fibrillation, presence of hepatitis C virus, bone metabolism parameters (PTH, calcium, phosphorus, alkaline phosphatase), serum albumin, type of involvement (distal or proximal), usual treatment of the patients prior to the development of calciphylaxis, diagnosis of calciphylaxis (by symptoms, biopsy or both), therapeutic strategies (conventional treatment – intensification of haemodialysis, support measures or amputation of the affected limb vs. multi-target treatment – conventional treatment with sodium thiosulfate, bisphosphonates, cinacalcet, non-calcium chelators or pentoxifylline), course of the lesions, follow-up, survival, calciphylaxis-related death (to establish this connection, the cutoff point was established in the first 6 months after the development of calciphylaxis) and other causes of death.
In the statistical analysis, the quantitative variables were expressed as the mean, standard deviation and range, while the qualitative variables were expressed as the absolute number and percentage. To compare the quantitative variables, the Student's t-test was used, and the chi-squared test was used for the qualitative variables. The clinical course from diagnosis to death was described using the Kaplan–Meier method. Values of p<0.05 were considered statistically significant. The statistical analysis was performed using SPSS (version 20 for Windows).
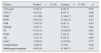
ResultsGeneral epidemiological dataWe identified 28 patients diagnosed with calciphylaxis, a 53.6% were women, and all were Caucasian. The mean age was 67.2±11.8 years (38–88). Fig. 1 shows the distribution of cases by year.
At diagnosis, 53.6% (n=15) were on replacement therapy with haemodialysis (HD); 25% (n=7) had a renal transplant with normal function (Tx); and 21.4% (n=6) had no kidney disease: they were identified as subjects with “normal renal function” (NRF).
The most common causes of CKD (in patients on HD and in those who had received a renal transplant) were nephroangiosclerosis (22.7%; n=5), diabetic nephropathy (18.2%; n=4) and glomerular disease (18.2%; n=4).
The mean time to renal replacement therapy (including HD and renal transplant) was 133.7±102.8 (10–324) months.
Different variables were collected in relation with comorbidity, regular treatment and type of renal replacement therapy treatment (hemodialysis (HD), renal transplantation (RT) or normal renal function (NRF)) before the calciphylaxis lesions had developed (Table 1). It is important to emphasise that there was no statistical significance between the different variables and the development of calciphylaxis, except with steroids (p=0.001). The use of anticoagulants in patients who were not on haemodialysis (renal transplant patients and patients with normal renal function) showed a trend close to statistical significance (p=0.07).
Risk factors prior to the onset of calciphylaxis.
| HD (n=15) | Tx (n=7) | NRF (n=6) | Total (n=28) | |
|---|---|---|---|---|
| HTN n (%) | 13 (87.6) | 6 (85.7) | 4 (66.7) | 23 (82.5) |
| DM n (%) | 5 (33.3) | 4 (57.1) | 4 (66.7) | 13 (46.4) |
| Obesity n (%) | 6 (40) | 4 (57.1) | 2 (33.3) | 12 (42.9) |
| CAD n (%) | 7 (46.7) | 2 (28.6) | 2 (33.3) | 11 (39.3) |
| CI n (%) | 10 (66.7) | 3 (42.9) | 3 (50) | 16 (57.1) |
| AF n (%) | 5 (33.3) | 2 (28.6) | 2 (33.3) | 9 (32.1) |
| HCV n (%) | 3 (20) | 2 (28.6) | 0 (0) | 5 (17.9) |
| Usual treatment before calciphylaxis | ||||
| Calcium carbonate n (%) | 3 (20) | 1 (14.3) | 2 (33.3) | 6 (21.4) |
| Steroids n (%) | 2 (13.3) | 7 (100)* | 1 (16.7) | 10 (35.7) |
| Active vitamin D n (%) | 9 (60) | 2 (28.6) | 2 (33.3) | 13 (46.4) |
| Iron infusion n (%) | 4 (26.7) | 0 (0) | 2 (33.3) | 6 (21.4) |
| OAC n (%) | 8 (53.3) | 6 (85.7) | 5 (83.3) | 19 (67.9) |
| Antiplatelet drugs n (%) | 6 (40) | 1 (14.3) | 2 (33.3) | 9 (32.1) |
AF: atrial fibrillation; CAD: coronary artery disease; CI: chronic ischaemia; DM: diabetes mellitus; HCV: hepatitis C virus; HD: haemodialysis; HTN: hypertension; NRF: normal renal function; OAC: oral anticoagulants; Tx: renal transplant patients.
In 82.1% of cases (n=23), the lesions showed a distal distribution, with proximal involvement in the other 17.9% (n=5).
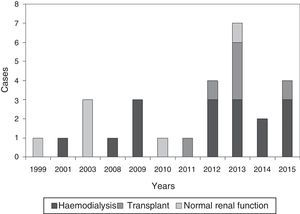
The diagnosis was based on clinical suspicion together with a biopsy in 22 patients (78.6%), while it was purely clinical in the remaining 6 (21.4%). Fig. 2 shows some representative images of the calciphylaxis lesions.
Biochemical parametersValues for bone metabolism parameters and serum albumin were collected at diagnosis, and these are shown in Table 2. It should be noted that there were no significant differences across groups, except when comparing the PTH values between patients on HD vs. patients with NRF (p=0.002) and between transplant patients vs. patients with NRF (p=0.03). As for blood albumin levels, patients with CKD also had values below the normal limit.
Biochemical parameters of bone metabolism and serum albumin.
| HD (n=15) | Tx (n=7) | NRF (n=6) | Total (n=28) | |
|---|---|---|---|---|
| PTH (pg/ml) | 433.5±627.4 (12–2613)* | 269.1±235.6 (3–617) | 34.8±15 (21–50.8) | 339.6±552 (3–2613) |
| Calcium (mg/dl) | 9.2±0.5 (8–10.2) | 8.6±1.4 (6.3–9.9) | 9.4±1.1 (8–11.1) | 9.1±0.9 (6.3–11.1) |
| Phosphorus (mg/dl) | 4.3±1.5 (2.2–6.5) | 4.2±1 (2.6–5.7)** | 3.1±0.2 (2.9–3.4) | 4.0±1.3 (2.2–6.5) |
| Alkaline phosphatase (IU/l) | 156.8±107.4 (45–423) | 102.5±48.9 (64–205) | 103±59.2 (63–171) | 130.2±91.4 (45–423) |
| Product Ca×P | 39.1±12.1 (21.3–58.5) | 36.1±10.9 (24.4–56.4) | 29.8±5.1 (23.2–37.7) | 36.3±10.9 (21.3–58.5) |
| Serum albumin (g/dl) | 3.2±0.7 (1.7–4.5) | 3.2±0.7 (2.1–4.4) | 3.8±0.7 (2.5–4.3) | 3.3±0.7 (1.7–4.5) |
NRF: normal renal function; HD: haemodialysis; Tx: renal transplant patients.
In terms of treatment options, 71.4% (n=20) received a combined treatment (multi-target), while the remaining 28.6% (n=8) received conventional treatment.
Focusing on the combined treatment, Table 3 shows all the therapeutic options that were used (by group and in total). It should be noted that with respect to multi-target treatment, there were significant differences across the different groups. Haemodialysis sessions were performed with low calcium baths; the bisphosphonates used were ibandronate or alendronate; and the non-calcium chelators were sevelamer or lanthanum.
Treatment options used.
| HD (n=15) | Tx (n=7) | NRF (n=6) | Total (n=28) | |
|---|---|---|---|---|
| Intensification of HD n (%) | 13 (86.7)* | 4 (57.1)** | 0 (0) | 17 (60.7) |
| Sodium thiosulfate n (%) | 10 (66.7)* | 6 (85.7)** | 1 (16.6) | 17 (60.7) |
| Bisphosphonates n (%) | 11 (73.3)* | 5 (71.4)** | 1 (16.6) | 17 (66.7) |
| Cinacalcet n (%) | 9 (60)* | 3 (42.9)** | 0 (0) | 12 (42.9) |
| Non-calcium chelators n (%) | 8 (53.3)* | 1 (14.3) | 0 (0) | 9 (32.1) |
| Vitamin K n (%) | 5 (33.3) | 0 (0) | 1 (16.6) | 6 (21.4) |
| Pentoxifylline n (%) | 5 (33.3) | 1 (14.3) | 0 (0) | 6 (21.4) |
| Amputation n (%) | 1 (7.1) | 1 (14.3) | 0 (0) | 2 (7.4) |
NRF: normal renal function; HD: haemodialysis; Tx: renal transplant patients.
Patients were followed for an average of 26.29 months (1–192).
The lesions resolved in 60.7% of the patients (n=17), while in the remaining 39.3% (n=11) they did not disappear. With respect to the rate of mortality, 39.3% (n=11) died due to the calciphylaxis itself: 40% of the patients on HD (n=6/15) and 71.4% of the transplant patients (n=5/7).
The main cause of death was infection (64%), followed by cardiovascular disease (27.2%).
The different risk factors regarding the calciphylaxis-related mortality rate (Table 4) were analysed and the presence of CKD (p=0.03) and central distribution of the lesions (p=0.04) were statistically significant.
Influence of different factors on calciphylaxis-related mortality.
| Factors | Death n=11 (%) | Living n=17 (%) | p |
|---|---|---|---|
| >70 years | 4 (36.4) | 8 (47.1) | 0.57 |
| CCI >7 | 9 (81.8) | 8 (47.1) | 0.06 |
| CKD | 11 (100) | 11 (64.7) | 0.03 |
| HTN | 9 (81.8) | 14 (82.4) | 0.97 |
| DM | 5 (45.5) | 8 (47.1) | 0.93 |
| Obesity | 5 (45.5) | 7 (41.2) | 0.82 |
| CI | 6 (54.5) | 10 (58.8) | 0.81 |
| CAD | 4 (36.4) | 7 (41.2) | 0.81 |
| AF | 3 (27.3) | 6 (35.3) | 0.65 |
| OAC | 7 (63.6) | 12 (70.6) | 0.77 |
| Central lesion | 4 (36.4) | 1 (5.9) | 0.04 |
| Multi-target treatment | 9 (81.8) | 11 (64.7) | 0.31 |
AF: atrial fibrillation; CAD: coronary artery disease; CCI: Charlson comorbidity index; CI: chronic ischaemia; CKD: chronic kidney disease; DM: diabetes mellitus; HTN: hypertension; OAC: oral anticoagulation.
The differences of the main variables of bone and mineral metabolism were analysed according to the presence of CKD and calciphylaxis-related death (Table 5). Statistical significance was found between the latter, hyperparathyroidism (p=0.02) and elevated blood phosphate (p=0.05).
Different variables related to the presence or absence of CKD and death due to calciphylaxis.
| CKD (n=18) | NRF (n=6) | p | Death due to CUA Yes (n=11) | Death due to CUA No (n=17) | p | |
|---|---|---|---|---|---|---|
| PTH (pg/ml) | 381.2±553.3 (3–2613) | 34.9±15 (21–50.8) | 0.02 | 281.3±202.1 (3–617) | 385.4±688.9 (21–2613) | 0.62 |
| Calcium (mg/dl) | 9±0.9 (6.3–10.1) | 9.4±1.1 (8–11.1) | 0.31 | 8.9±1.1 (6.3–9.9) | 9.3±0.8 (8–11.1) | 0.31 |
| Phosphorus (mg/dl) | 4.3±1.3 (2.2–6.5) | 3.1±0.2 (2.9–3.4) | 0.05 | 4.3±1.4 (2.2–6.5) | 3.8±1.2 (2.6–6.4) | 0.27 |
| Alkaline phosphatase (IU/l) | 139.6±95.1 (45–423) | 103±59.2 (63–171) | 0.52 | 147.2±112.7 (45–423) | 125.8±73.6 (47–187) | 0.57 |
| Serum albumin (g/dl) | 3.3±0.7 (1.7–4.4) | 3.8±0.7 (2.5–4.3) | 0.16 | 2.9±0.6 (1.7–3.7) | 3.6±0.6 (2.2–4.4) | 0.02 |
CKD: chronic kidney disease; CUA: calcific uraemic arteriolopathy; NRF: normal renal function; PTH: parathyroid hormone.
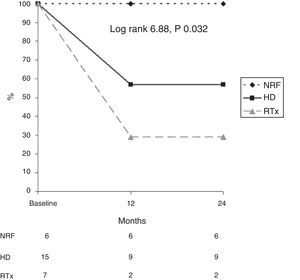
In addition, survival as related to death due to calciphylaxis was analysed in each diagnostic group (Fig. 3). After 12 months, 56% of patients on HD, 23% of renal transplant patients and 100% of patients with NRF were alive. After 24 months, 56% of patients on HD, 100% of patients with NRF and 23% of renal transplant patients were alive (log rank 6.88; p=0.032).
DiscussionCalciphylaxis is a rare condition that is usually associated with CKD, with a prevalence between 1% and 4% in dialysis patients.1,15 In our series, 28 patients were diagnosed over a period of 16 years, which suggests that, despite the low incidence, calciphylaxis should be taken into account in any differential diagnosis.
Contrary to what might be expected, the patient profile has changed: calciphylaxis does not exclusively affect subjects with CKD on dialysis, but it also occurs in renal transplant patients with normal graft function and even in patients with no kidney disease16,17 (in our study, 21.4% had normal renal function).
In general, it is more prevalent in obese women,2 as our case-series shows. The majority of the patients showed cardiovascular risk factors (hypertension, diabetes mellitus, obesity), coronary disease and chronic ischaemia, which causes tissue hypoperfusion.8,10,18 These factors are replacing the classic protagonists of calciphylaxis, such as those attributed to bone and mineral metabolism.19,20 One example of this can be found in our series, where hyperparathyroidism, elevated blood phosphate levels and normal calcium were observed only in patients with CKD. However, other risk factors, such as steroids (in the group of renal transplant patients, 100% took these drugs) are increasingly prominent as the causes behind the onset of this condition. This finding is of great importance and clinical interest for reconsidering indicating these treatments for high-risk patients. With all these findings we can describe a new phenotype of patients with calciphylaxis to which we must pay more attention: a subject with renal failure (patient on dialysis or renal transplant patient), elevated comorbidity and a pronounced cardiovascular profile (hypertensive, diabetic and with peripheral vascular disease), treated with oral anticoagulants and with hypoalbuminaemia due to chronic inflammation or malnutrition.3,8
In all cases, the skin lesions showed a poor clinical course and were highly suggestive of calciphylaxis, which was confirmed with a biopsy in 78.6% of the cases; in some patients, X-rays were performed which showed the calcification of the vessels.
A fundamental and increasingly important aspect of calciphylaxis is the use of combined or multi-target treatment. There is strong evidence showing that skin lesions improve and survival increases when combined or multi-target treatment is used.13,21,22 Although there is no standardised therapy, it is recommended to discontinue calcium chelators, oral anticoagulants (using low molecular weight heparin instead) and vitamin D; the frequency of dialysis should also be increased (if necessary) to 5–6 weekly sessions with a low calcium bath.23 In addition, drugs such as non-calcium chelators24–26 (e.g. sevelamer, which inhibits ectopic calcifications and reduces cholesterol), cinacalcet27,28 (which reduces PTH, calcium and blood phosphates in patients on dialysis with secondary hyperparathyroidism), sodium thiosulfate29 (an antioxidant and anti-inflammatory that mobilises the calcium deposits in the vessels) and bisphosphonates30,31 (inhibitors of ectopic calcification formation, with an anti-inflammatory effect) should be used. Vitamin K administration should be considered in cases of deficiency.32 The use of hyperbaric oxygen therapy33 (it could be beneficial for distal lesions) and parathyroidectomy in cases of severe hyperparathyroidism is a subject of much debate, because they do not seem to improve the prognosis in these patients.2
The prognosis of this disease is very poor, as it shows a mortality rate of up to 60–80%, with calciphylaxis-related sepsis as the most common cause of death,2,4 as occurred in 64% of our patients. Specifically, proximal lesions have a higher mortality rate than distal lesions (p=0.04).9 In this series, the main risk factors for calciphylaxis-related death were the presence of CKD (p=0.03) and hypoalbuminaemia (p=0.02).
In addition, it is important to note that patients with normal renal function have an excellent prognosis with regard to calciphylaxis (none of them died due to this reason), compared with patients with CKD (especially renal transplant patients), who have a very high probability of dying within a few months (p=0.032).
This study has some important limitations: it is retrospective and single-centre, includes a small number of cases, and there was no comparative analysis with a control group of subjects without calciphylaxis. However, this study does reflect the current reality at the different nephrology centres.
In conclusion, although calciphylaxis is a rare condition, it has a high mortality rate. The patient profile has changed: it is not constrained to uraemic situations, but also occurs in patients with normal functioning renal transplants or in subjects with no kidney disease. Cardiovascular risk factors (diabetes, obesity, peripheral vascular disease, anticoagulation with vitamin K antagonists, steroids) are displacing the classic factors of bone and mineral metabolism. A “risk score” should be established for an early diagnosis, as well as standard treatment. Multicenter studies that enable the treatment of this condition to be standardised are needed.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fernández M, Morales E, Gutierrez E, Polanco N, Hernández E, Mérida E, et al. Calcifilaxis: más allá de CKD-MBD. Nefrologia. 2017;37:501–507.