INTRODUCTION
Collapsing glomerulonephritis (CG) is a relatively new and little known entity of glomerular disease. It was first described in the 1980s, and defined at that point as HIV-associated nephropathy. Subsequently, there have been cases of this renal disease in HIV-negative patients, and it has been renamed as CG.
It has been considered a collapsing variant of focal segmental glomerulosclerosis (FSGS). However, the podocyte lesions are different: the pathogenesis of typical FSGS is marked by podocytopoenia, while CG is characterised by podocyte proliferation. In recent classifications of podocytopathies, it is described as a primary glomerular disease, independent of FSGS.1 In CG, podocyte involvement implies phenotypic dedifferentiation, reflected in the loss of expression of markers of podocyte maturity and a “re-expression” of proliferation markers common in the immature podocyte.
We present a case of a patient with CG, with the relationship of markers of podocyte dedifferentiation as part of the study of this type of glomerulopathy.
CASE REPORT
25-year-old patient who was admitted for work-up of advanced renal failure and proteinuria in the nephrotic range.
The patient’s history was notable for mild-moderate mental retardation since infancy. The patient denied medication or illicit drug use.
The patient’s family history included a mother and sister with mental retardation, also since birth. Renal disease screening was performed (sediment, proteinuria, and renal function) on the family members, with negative results. At the time of admission, the patient was asymptomatic, without oedema or clinical uraemia.
The physical examination was unremarkable, except for high blood pressure that was controlled with low doses of angiotensin converting enzyme inhibitors (ACE-I). Relevant laboratory data included: creatinine 5.6mg/dl, urea 112mg/dl, total protein 5.2g/dl, albumin 3.30g/dl, total cholesterol 117mg/dl (HDL 29mg/dl, LDL 57mg/dl), as well as normocytic normochromic anaemia, with haemoglobin value of 9.7g/dl and haematocrit of 27.8%. On urinalysis, proteinuria in the nephrotic range was detected, with a protein to creatinine ratio of 5.3mg/mg and microhaematuria.
Viral serologies for hepatitis B and C and for HIV were negative. Other serologies performed for parvovirus B19 and cytomegalovirus (CMV), including PCR, were negative.
Blood immunoglobulins (IgG, IgM, and IgA) were within normal limits. Complement testing was also normal. Autoimmunity studies, including ANA, ANCA, and anti- DNA, were negative.
On renal ultrasound, symmetric kidneys were observed, normal in size (right kidney 11.5cm, left kidney 11.7cm), both with moderately hyperechogenic parenchyma and decreased corticomedullary differentiation.
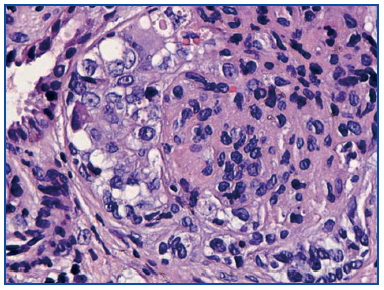
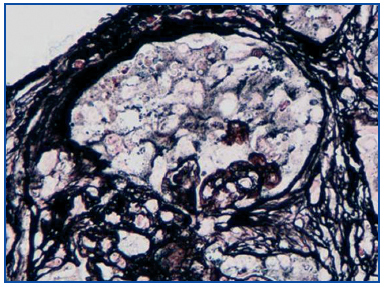
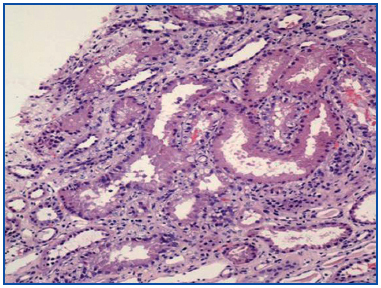
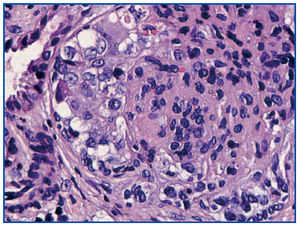
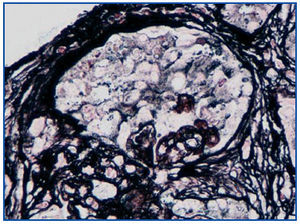
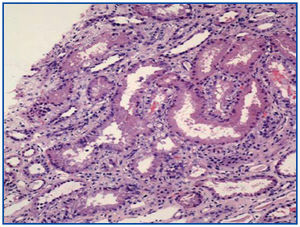
A renal biopsy was performed, showing 9 glomeruli, five of which were completely sclerosed and four with lesions of segmental sclerosis; the latter were notable for hyperplasia and hypertrophy of the visceral epithelium (pseudo-crescents) (Figure 1), at times vacuolised (PAS+), occupying Bowman’s space but with unaffected parietal epithelium. The capillaries of these glomeruli had areas of collapse and luminal occlusion (Figure 2). The interstitium was oedematous, with moderate mixed inflammatory infiltration and markedly abnormal tubules, with dilated lumens and epithelium that was at times necrotic or degenerated, with sloughed cells in its lumen (Figure 3). The vascular component showed no lesions.
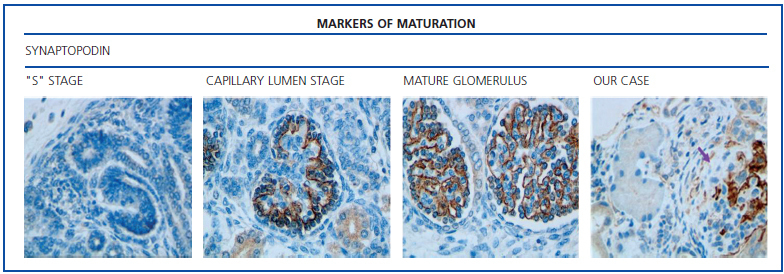
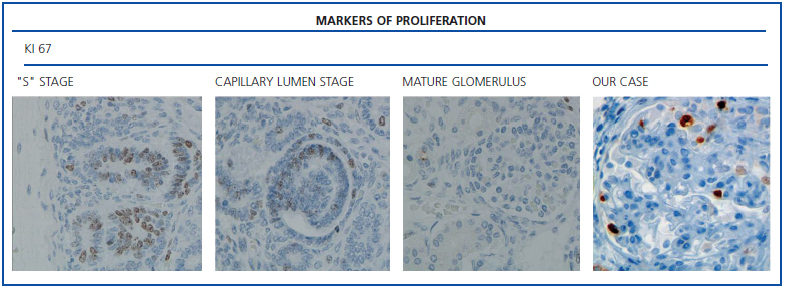
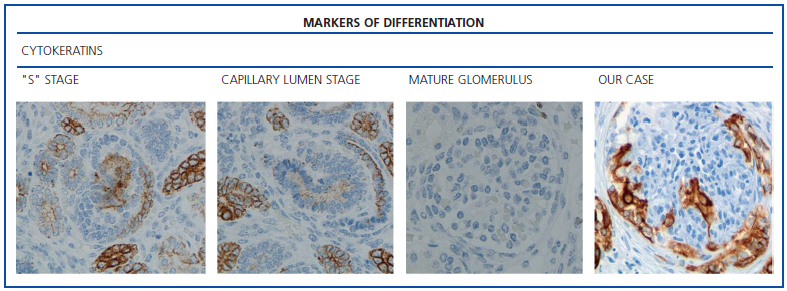
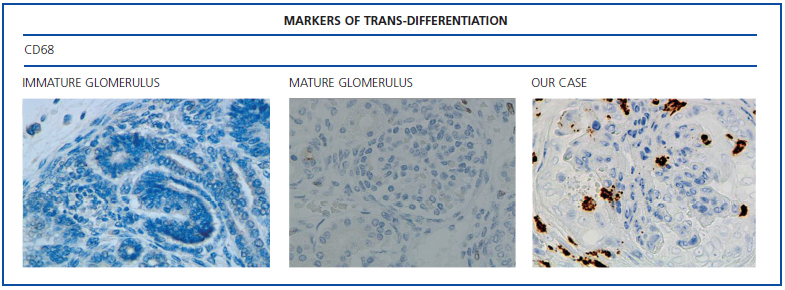
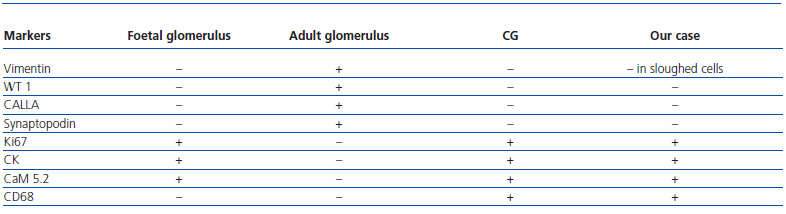
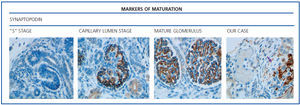
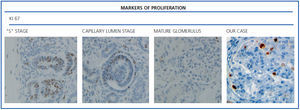
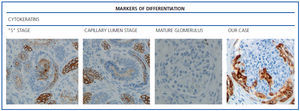
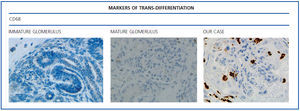
The histological examination was performed with immunohistochemical markers: the hyperplastic visceral epithelium expressed a phenotype not found in other forms of podocyte lesion (minimum change disease, membranous glomerulonephritis, etc.), only found in the foetal kidney (Table 1). In our case there is, therefore, a loss of maturation markers (vimentin, CALLA, or synaptopodin) (Figure 4) with re-expression of proliferation markers (Ki 67) (Figure 5) or dedifferentiation (cytokeratins, Cam 5.2) (Figure 6), including acquisition of a macrophagic immunophenotype (Figure 7).
The patient progressed rapidly to end-stage renal disease, and was included in a chronic haemodialysis programme.
DISCUSSION
We present here a patient with CG and expressed markers of podocyte dedifferentiation.
CG is an aggressive form of kidney disease characterised by nephrotic proteinuria, severe renal failure, and poor outcome, with minimal response to treatment leading quickly to end-stage renal disease.
This glomerulopathy is a relatively new and little known entity, so much so that it still does not appear in the most recent textbooks of Spanish nephrology as a clinically independent disease. In the first descriptions that were made in the 1970s, it was known as “malignant” focal segmental glomerulosclerosis. Subsequently, in the 1980s, it was defined as an HIV-associated nephropathy. In 1986, Weiss et al. described a similar renal lesion in HIV-negative patients that was called CG. Since then, it has been considered a variant of FSGS.2 In recent years we have witnessed significant progress in knowledge of this glomerulopathy.
It is considered a new clinical-pathological disease, separate from FSGS, in recent taxonomic classifications of podocytopathies. This is based on the mechanism through which the podocyte is damaged is completely different in these two diseases. While CG is associated with podocyte dedifferentiation and proliferation, podocytopoenia is the characteristic lesion in FSGS.3
Morphologically, CG is characterised by global or segmental collapse of the capillary lumens, hyperplasia, and podocyte proliferation (pseudo-crescents). In addition there is an important tubulo-interstitial lesion with microcysts, acute injury, and tubular atrophy, as in variable interstitial inflammatory infiltrates.3 The immunofluorescence study is negative or non-specific. On electron microscopy, it is typical to find podocyte fusion and hyperplasia. According to its aetiology, tubulo-reticular lesions can be seen (virus, lupus) or mitochondrial abnormalities (CoQ2 nephropathy).4
Without a doubt, the finding that is most characteristic of this disease is “deregulation” of the podocyte phenotype: markers of differentiation are lost and others of podocyte proliferation and dedifferentiation are gained.5 For example, as in our case, markers of glomerular maturation, such as vimentin, VT1, CALLA (CD10) and synaptopodin were lost (down-regulation). In contrast, markers of proliferation (Ki67) and dedifferentiation such as various cytokeratins (CK, Cam 5.2), present in the foetal glomerulus and absent in the normal adult, were expressed (up-regulation). Even the de novo expression of macrophagic markers such as CD68 in this glomerulopathy may indicate podocyte “transdedifferentiation” (Table 1).6
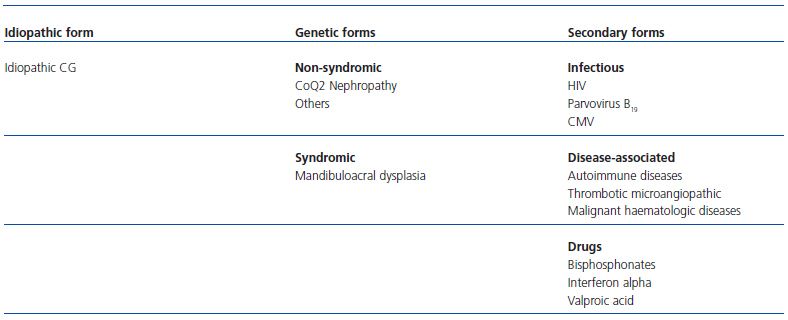
Regarding the aetiology, various forms of this disease have been described: idiopathic forms, genetic forms, and secondary forms (Table 2).
In our case, possible secondary causes involved with this glomerulopathy were examined: there was no history of taking drugs such as pamidronate or valproic acid, nor were viral infections such as HIV, CMV, or parvovirus B19 detected. There were no signs or symptoms of autoimmune diseases, which are occasionally associated with this disease.6 Our patient, as is characteristic in CG, progressed rapidly to end-stage renal disease and became dependent on dialysis.
The clinical presentation (nephrotic syndrome and rapid progression to renal failure), the histological findings (capillary collapse, pseudo-crescents with podocyte hypertrophy and tubulo-interstitial involvement) and the markers of podocyte dedifferentiation (CALLA, Ki67, CD68, CAM5.2, and others) were consistent with the diagnosis of CG.
The family and personal history of mental retardation in our patient keeps us from ruling out, for the moment, a genetic disease based primarily on a CoQ2 mitochondrial dysfunction, as a cause of this disease.7 As a result, our current diagnosis is idiopathic CG. We therefore believe that, in addition to the clinical and histological features, these markers of podocyte phenotype dysregulation are a useful and valuable tool for the study of CG.
Questions and responses
- Dr. Eduardo Vázquez-Martull (La Coruña). Congratulations on the magnificently presented case. I do not have personal experience with this, but I recognise the excellent differential diagnosis and the techniques that were used to reach the diagnosis.
- Response: In daily practice, it is not uncommon to find, as in this case, lesions that are quite welldeveloped at the time of biopsy evaluation. For that reason, it may be useful to determine certain differential markers.
- Dr. Pilar Arrizabalaga (Barcelona). Collapsing glomerulonephritis, in contrast to other types of glomerulonephritis, is characterised by the irreversible loss of podocytes, the loss of differentiation antigens, and, paradoxically, podocyte proliferation, demonstrated by the strength of Ki67. The mature podocyte and the neuron share a cellular differentiation antigen in synaptopodin. Given that this patient and two of the patient’s family members had mental retardation, do you believe that there may be some kind of relationship?
- R: In this patient, physical examination and neurophysiological studies were normal, but mental retardation was evident both in the patient as well as in the patient’s family members (mother and sister). Regarding the relationship between mental retardation (or neurological disease) and collapsing nephropathy, at the moment we can only speculate. On the one hand, we are waiting for a muscle biopsy and the respiratory chain study in order to rule-out mitochondrial disease; in some patients with CoQ2 nephropathy, neurological disorders have been described (encephalopathy). On the other hand, one could also speculate about the role of synaptopodin in mental retardation. As you rightly mentioned, synaptopodin is a cellular marker that is expressed exclusively in the mature podocyte and in the dendritic spines of telencephalic synapses. In our case (as with all collapsing nephropathies) there is a loss of this podocyte marker. Recently,8 a very close relationship has been shown between synaptopodin (loss of expression) and proteinuria. It would be interesting if the neurological changes were caused by a loss of this neuronal marker. Nevertheless, as we said, this is all speculation.
- Dr. Pilar Arrizabalaga (Barcelona). This case makes the point of stressing the utility of checking synaptopodin in patients with idiopathic collapsing glomerulonephritis, especially if it presents in patients with mental retardation.
- Dr. Víctor Gutiérrez-Millet (Madrid). The visceral epithelial hyperplasia is the cause of the appearance of the shapes called pseudo-crescents. It is a shame that this concept does not continue to be used to differentiate these abnormalities from the true crescents in other extracapillary types of glomerulonephritis.
- Dr. Montserrat Díaz (Barcelona). The fact of Ki67 being positive does not allow for differentiation between a pseudo-crescent and an epithelial crescent since it is a marker of proliferation and not a difference between epithelial cell and podocyte.
- R: In fact, Ki67 only indicates proliferative activity of the epithelium; it is the location of this nuclear staining, combined with the rest of the podocyte markers, which determines that this proliferation is linked, in our case, to the visceral epithelium and not to the parietal epithelium.
- Dr. Miguel Ángel Frutos (Málaga). Podocyte apoptosis or the loss of anchoring proteins to the GBM is the cause of podocyturia in some types of glomerulonephritis and usually in the collapsing forms. What current utility is there in the evaluation of podocyturia in the urine in the diagnosis or follow-up of certain types of glomerulonephritis?
- R: In fact, several studies have shown podocyturia in a large variety of glomerular diseases in humans and in experimental nephropathies.9,10 This loss of podocytes in the urine with the resulting baring of the GBM is probably the cause of synechiae and, in short, of glomerulosclerosis. We believe that it could be very useful in the follow-up of glomerular diseases, although we have not studied that aspect. Regarding your question, some authors11 have shown that it is a marker, more specific than proteinuria, of glomerular damage. For years it has been thought that it was a highly differentiated terminal cell and that it did not proliferate. Today, we know that it can enter the cell cycle and that it can have a potential regenerative role. Therefore, theoretically, the normal number of podocytes could be restored in diseases with progressive loss of podocytes, thus preventing the progression of chronic renal failure.
- Dr. Pedro Aljama (Córdoba). Under normal conditions, a considerable number of podocytes can be isolated from the urine, and their quantification may have diagnostic value in certain glomerular processes. When 60% of the podocytes are lost is when glomerular sclerosis/hyalinosis first appears. There is growing experimental evidence that the podocyte can regenerate from renal stem cells located selectively at the vascular pole of the glomerulus. These renal stem cells progress via the parietal layer of Bowman’s capsule to the visceral layer and when they arrive at the capillary tuft they are already “adult” or differentiated podocytes that can replace those lost in urine or those destroyed by pathologic processes (inflammation, apoptosis, etc.).
- Dr. Eduardo Vázquez-Martull (La Coruña). I think I remember that in congenital nephrotic syndrome (NS), the hyperplastic podocytes show hyperplasia that is reminiscent of the foetal glomerulus. Is that so?
- R: There are some cases relating to this dedifferentiation in atypical focal and segmental forms and, as Dr. Vázquez Martull mentions, in congenital NS and in diffuse mesangial sclerosis, the podocyte phenotype may resemble early glomerular forms from nephrogenesis. But, in addition to dedifferentiation, the proliferative nature of the podocytes in collapsing glomerulopathy must be added, which is different from the established mechanisms of podocyte lesion in minimal change disease and in other forms of idiopathic focal and segmental glomerulosclerosis, with the exception of the cellular type.
- Dr. Eduardo Vázquez-Martull (La Coruña). It matters little whether it is mesenchymal or epithelial cellularity. The later transformation is what is responsible for the cell becoming laden with actin and starting its transformation into a fibroblast.
- R: In fact, glomerulosclerosis is thus a predictable result.
Acknowledgements To Novartis Pharma for the facilities for presentation and discussion of this case at the meeting of the Nephropathology Club and GLOSEN.
Figure 1. Pseudo-crescents: hypertrophy and hyperplasia of the visceral epithelium. PAS 40x.
Figure 2. Collapse of glomerular capillaries. Silver Methenamine 40x.
Figure 3. Necrosis and tubular dilation. HE 20x.
Figure 4. Markers of maturation: synaptopodin.
Figure 5. Markers of proliferation: Ki67.
Figure 6. Markers of differentiation: cytokeratins (AE1/AE3).
Figure 7. Markers of trans-differentiation: CD68.
Table 1. Podocyte phenotype in collapsing glomerulonephritis (CG)
Table 2. Classification of collapsing glomerulonephritis (CG)