Prevalence of hypertension increases as glomerular filtration rate (GFR) declines. Renalase metabolizes catecholamines and have an important role in blood pressure (BP) regulation. The purpose of the study was to evaluate the effect of kidney transplantation on renalase levels and BP in kidney donors and recipients.
Materials and methodsTwenty kidney transplant recipients and their donors were included in the study. Serum renalase levels and ambulatory BP values were measured in both donors and recipients before and after transplantation. Factor associated with change in renalase and BP levels were also evaluated.
ResultsIn donors; mean GFR and hemoglobin levels decreased while night-time systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels and serum renalase levels increased simultaneously after nephrectomy. Day-time SBP and DBP levels did not changed and the night/day ratio of mean arterial pressure (MAP) increased significantly. In recipients, mean GFR increased, while mean serum renalase levels, creatinine and BP levels decreased after transplantation. Correlation analysis revealed that changes in MAP correlated with alteration in serum renalase levels and GFR.
ConclusionsAfter transplantation, serum renalase levels increased in donors and decreased in recipients. The renalase levels are associated with change in MAP and circadian rhythm of BP in donors and recipients.
La prevalencia de la hipertensión aumenta a medida que la velocidad de filtración glomerular (VFG) disminuye. La renalasa metaboliza las catecolaminas y tiene una función importante en la regulación de la presión arterial (PA). El objetivo de este estudio fue evaluar el efecto del trasplante de riñón en los niveles de renalasa y PA en donantes y receptores de riñón.
Materiales y métodosEn este estudio se incluyeron veinte receptores de riñón y sus donantes. Se midieron los niveles séricos de renalasa y los valores de PA ambulatorios en donantes y receptores, antes y después del trasplante. También se evaluaron los factores asociados a la alteración de los niveles de renalasa y PA.
ResultadosEn donantes; la VFG media y los niveles de hemoglobina disminuyeron, mientras que la presión arterial sistólica (PAS) y diastólica (PAD) y los niveles séricos de renalasa durante la noche aumentaron simultáneamente después de la nefrectomía. Los niveles de PAS y PAD no cambiaron durante el día y el cociente noche/día de la presión arterial media (PAM) aumentó significativamente. En los receptores, la VFG media aumentó, mientras que los niveles séricos de renalasa, la creatinina y los niveles de PA disminuyeron después del trasplante. El análisis de correlación reveló que los cambios de la PAM se correlacionaron con la alteración de los niveles séricos de renalasa y la VFG.
ConclusionesTras el trasplante, los niveles séricos de renalasa aumentaron en los donantes y disminuyeron en los receptores. Los niveles de renalasa se asocian a cambios en la PAM y al ritmo circadiano de la PA en donantes y receptores.
Hypertension is the most common risk factor for cardiovascular disease and death. About 70–80% of the patients with chronic kidney disease (CKD) have hypertension, and the prevalence of hypertension increases as glomerular filtration rate (GFR) declines, such that over 80–90% patients with end stage renal disease (ESRD) are hypertensive.1
Living donor kidney transplantation becomes an important option for ESRD treatment owing to prolonged waiting times on transplant list.2 However, after donation there is an immediate 50% reduction in GFR.3 After nephrectomy up to two-thirds of donors fulfill the criteria for CKD stage 3 depending on baseline age and renal function.4 This reduction in GFR results in increased risks of ESRD and cardiovascular diseases.5 Effect of unilateral nephrectomy on blood pressure (BP) is not clear. However, a meta-analysis suggests a moderate increase of about 5mmHg in systolic BP at 10 years after donation.6 A study showed that in kidney donors; circadian rhythm of BP was disturbed as a function of GFR loss without affecting absolute levels of BP. Non-dipping of BP seemed to be the consequence of the loss of renal function.7
Renalase is a new flavoprotein secreted preferentially by the kidney (proximal tubules), which metabolizes catecholamines and may play a key role in the regulation of sympathetic tone and BP.8 Its levels are regulated by renal function, renal perfusion and plasma catecholamine levels.9 In literature, studies using the different commercial ELISA kits reported different results about the association between serum renalase levels and GFR in patients with CKD.9–12 There are several hypotheses that try to explain the discrepancy in levels of renalase when measured by western blot or different ELISA kits which were discussed in an extensive review about renalase.13 Recent studies demonstrated inverse relationship between plasma renalase and GFR in patients with CKD including renal transplant recipients.14–16 However, there are limited data about effect of the transplantation on plasma renalase and blood pressure levels in kidney donors and recipients before and after the transplantation. In this study, we hypothesize that; change in renalase levels after nephrectomy affect the blood pressure variations in donors and recipients.
Materials and methodsPatientsTwenty kidney donors that underwent unilateral nephrectomy for living related renal transplantation and twenty kidney recipients with ESRD were selected from living related transplantation pairs for the study according to exclusion criteria which were delayed graft function and acute kidney rejection. Kidney donors had no medication use except for that related to surgery and ate a relatively low-sodium diet containing (approximately 6g/day of NaCl) throughout the hospitalization.
After transplantation, all kidney recipients were under triple immunosuppression treatment including corticosteroid, calcineurin inhibitor (tacrolimus at doses to maintain blood through levels of 6–9ng/mL) and mychophenolate mophetil 750–1000mg twice per day.
Demographic characteristics, including recipient age, gender, primary renal disease, co-morbidities, and baseline laboratory values of the study population were recorded at the initiation of study. The demographic and laboratory, pre-transplant and transplant characteristics of donors and recipients are summarized in Table 1. Serum creatinine of the healthy donors and patients with ESRD were obtained from medical records. Collected 24-h urine volume, urinary creatinine, serum creatinine was used for calculation of creatinine clearance.17
Demographic and laboratory characteristics of the study groups.
| Parameters | Kidney donors (n=20) | Kidney recipients (n=20) | p value |
|---|---|---|---|
| Age (y) | 46.2±8.5 | 50.5±12.6 | 0.21 |
| Sex (male) (%) | 45 | 55 | 0.44 |
| Pre-transplant renal replacement therapy (n,%) | |||
| Hemodialysis | 14 (70.0%) | ||
| Preemptive | 6 (30.0%) | ||
| Primary renal disease (n) | |||
| Glomerulonephritis | 6 | ||
| Polycystic kidney disease | 4 | ||
| Urologic disorders | 2 | ||
| Diabetes | 3 | ||
| Amiloidosis | 2 | ||
| Unknown | 3 | ||
| Comorbidities (%) | |||
| Hypertension | 5% | 50% | 0.0029 |
| Use of antihypertensives (number of patients) | 1 | 10 | 0.001 |
| BMI (kg/m2) | 28.0±4.4 | 26.0±5.1 | 0.47 |
| Serum renalase (mcg/mL) | 125.2±35.0 | 242.4±147.0 | 0.001 |
| Serum hemoglobin (g/dL) | 13.4±0.9 | 10.5±1.2 | 0.0006 |
| Serum creatinine (mg/dL) | 0.80±0.17 | 7.45±2.30 | 0.00019 |
| GFR (mL/min) | 115.0±9.4 | 7.0±2.9 | 0.0006 |
| Proteinuria (mg/24h) | 78±23 | 1328±1198 | 0.0003 |
| SBP (mmHg) | |||
| Day | 126.4±5.4 | 130.6±10.2 | 0.001 |
| Night | 113.4±6.3 | 125.1±9.0 | 0.0003 |
| 24-h | 120.2±6.8 | 127.4±9.4 | 0.009 |
| DBP (mmHg) | |||
| Day | 75.8±4.9 | 82.3±6.5 | 0.001 |
| Night | 70.3±4.9 | 79.2±6.0 | 0.0001 |
| 24-h | 72.8±5.2 | 80.5±6.4 | 0.0002 |
| MAP | 88.6±5.0 | 96.1±7.1 | 0.001 |
| Night/day ratio of MAP | 0.91±0.02 | 0.96±0.01 | 0.0002 |
n: number, GFR: glomerular filtration rate, SBP: systolic blood pressure, DBP: diastolic blood pressure, N/D: night-to-day, BP: blood pressure.
Blood samples were obtained from kidney recipients under hemodialysis at the initiation of the hemodialysis session. After collection, blood samples were centrifuged at 1000-g for 10min and stored at −80°C until analysis. Serum renalase levels were measured using an ELISA kit for renalase (Human Renalase ELISA Kit, Eastbiopharm Co Ltd, Hangzhou, China). For each patient renalase levels were measured twice and the average of the two values was recorded. The intraassay coefficient of variation (CV) was 5–10%, and inter-assay CV was 5–10%. The detection range of the assay was 50–480mcg/mL. Change in renalase levels (ΔRenalase) before and after nephrectomy was calculated as ΔRenalase=[(Serum renalase before transplantation−serum renalase after transplantation)/Serum renalase before transplantation]×100. Collected 24h urine was used to calculate creatinine clearance and measurement of daily urinary protein excretion. Creatinine clearance formulation was used as a marker of GFR. The percentage decrease and increase in GFR (ΔGFR) was calculated as ΔGFR=[(GFR before nephrectomy−GFR after nephrectomy)/GFR before nephrectomy]×100. Urinary protein levels were measured with nephelometry (Immage 800; Beckman Coulter, Brea, CA) and an automated turbidimetric method using benzalkonium chloride.
Ambulatory BP was measured over a 24-h period by the oscillometric method using an automatic noninvasive recorder (Spacelab Inc., Redmond, WA, USA). The monitor was programmed to measure BP at 15min intervals between 8:00a.m. and 10:00p.m. and at 30min intervals between 10:00p.m. and 8:00a.m. During measurements, patients continued to perform their usual regular daily activities. Measurements were only included if more than 85% of the readings were successful. MAP was calculated as diastolic BP (DBP) plus one third of the pulse BP. Change in MAP (ΔMAP) was calculated as ΔMAP=[(MAP before transplantation−MAP after transplantation]/MBP before transplantation)×100. The change in the night/day ratio of MAP was obtained as the difference between the night/day ratio of MAP after nephrectomy and the night/day ratio of MAP before nephrectomy. These examinations were performed one day before transplantation, and then were repeated at the end of first month after transplantation when serum creatinine levels were stabilized.
EthicsThe study is approved by the local institutional research committee of Hacettepe University Medical Faculty (GO-13/42-30) and conducted şn accordance with the 1964 Helsinki declaration and its later amendments and declaration of Istanbul.
Statistical AnalysisData were expressed as mean±standard deviation. Kolmogorov–Smirnov test and Levene test were used to determine distribution characteristics and homogeneity of variances. Categorical variables were compared with chi square test. Continuous variables were compared with independent samples T test or Mann–Whitney U test where appropriate. The significance of differences in continuous variables before and after nephrectomy was determined by paired samples test or two related samples test. Pearson correlation coefficient was used for continuous variables with normal distribution, and Spearman correlation coefficient was used for continuous variables that are not normally distributed. A p value of <0.05 was considered statistically significant.
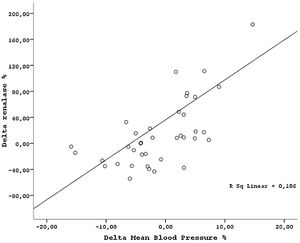
ResultsThe demographic and laboratory data of the study population are presented in Table 1. There were no significant differences in demographic characteristics between kidney donors and recipients. Mean serum creatinine, renalase levels, 24-h proteinuria and BP measurements were lower in healthy donors than patients with ESRD. Hemoglobin and GFR levels were higher in kidney donors than patients with ESRD. After transplantation, while the mean GFR and hemoglobin levels decreased, serum renalase levels increased in donors (Table 2). GFR was reduced by 34.9±5.7% in average from the baseline value of 115.0±9.4ml/min. Proteinuria did not change by nephrectomy. In addition, MAP levels slightly increased in donors after nephrectomy but remained within normal limits defined as SBP<140mmHg, and DBP<90mmHg. However, the night-time SBP and DBP levels increased, day-time SBP and DBP levels did not changed and the night/day ratio of MAP increased significantly by nephrectomy (Table 2). The rise in MAP after nephrectomy seemed related with night time BP elevations. In transplant recipients, while the mean GFR increased, the mean serum creatinine, serum renalase levels, and BP levels decreased after transplantation (Table 3). The night/day ratio of MAP slightly reduced in transplant recipients after transplantation (Table 3). However, we could not find any association between pre and post transplantation renalase levels and MAP levels (r=0.38, p=0.13 and r=−0.11; p=0.49 respectively), correlation analysis in whole study population showed that changes in MAP (ΔMAP) correlated with alteration in serum renalase levels (ΔRenalase) (r=0.31, p=0.0004) (Fig. 1) and change in GFR (ΔGFR) (r=−0.27, p=0.012). Similarly, we found a strong association between change in night/day ratio of MAP and ΔRenalase (r=0.35, p=0.001). ΔRenalase was also found to be inversely correlated with change in GFR (ΔGFR) (r=0.50, p=0.00057).
Laboratory characteristics of the kidney donors before and after transplantation.
| Parameters | Before (n=20) | After (n=20) | p value |
|---|---|---|---|
| Serum hemoglobin (g/dL) | 13.4±0.9 | 12.5±0.6 | 0.0063 |
| Serum creatinine (mg/dL) | 0.80±0.17 | 1.2±0.20 | 0.0003 |
| GFR (mL/min) | 115.0±9.4 | 75.0±7.7 | 0.0004 |
| Proteinuria (mg/24h) | 78.4±23.8 | 88.4±28.1 | 0.11 |
| Serum renalase (mcg/mL) | 125.2±35.0 | 140.2±73.6 | 0.0004 |
| SBP (mmHg) | |||
| Day | 126.4±5.4 | 126.5±6.0 | 0.88 |
| Night | 113.4±6.3 | 118.9±5.7 | 0.0003 |
| 24-h | 120.2±6.8 | 123.9±6.2 | 0.001 |
| DBP (mmHg) | |||
| Day | 75.8±4.9 | 76.9±4.9 | 0.27 |
| Night | 70.3±4.9 | 73.5±4.2 | 0.002 |
| 24-h | 72.8±5.2 | 75.8±4.5 | 0.003 |
| MAP (mmHg) | 88.6±5.0 | 91.8±3.7 | 0.001 |
| Day MAP (mmHg) | 92.6±3.9 | 93.4±3.6 | 0.32 |
| Night MAP (mmHg) | 84.7±4.7 | 88.6±3.5 | 0.0047 |
| Night/day ratio of MAP | 0.91±0.02 | 0.94±0.02 | 0.0003 |
GFR: glomerular filtration rate, SBP: systolic blood pressure, DBP: diastolic blood pressure, N/D: night-to-day, BP: blood pressure.
Laboratory characteristics of the kidney recipients before and after transplantation.
| Parameters | Before (n=20) | After (n=20) | p value |
|---|---|---|---|
| Serum hemoglobin levels (g/dL) | 10.5±1.2 | 10.2±0.8 | 0.2 |
| Serum creatinine (mg/dL) | 7.45±2.30 | 1.20±0.20 | 0.00017 |
| GFR (mL/min) | 7.0±2.9 | 67.9±10.2 | 0.00063 |
| Proteinuria (mg/24h) | 1328±1198 | 230±130 | 0.001 |
| Serum renalase levels (mcg/mL) | 242.4±147.0 | 162.3±53.0 | 0.003 |
| SBP (mmHg) | |||
| Day | 130.6±10.2 | 124.7±4.8 | 0.007 |
| Night | 125.1±9.0 | 119.2±4.9 | 0.0003 |
| 24h | 127.4±9.4 | 121.2±5.0 | 0.001 |
| DBP (mmHg) | |||
| Day | 82.3±6.5 | 76.8±6.0 | 0.0003 |
| Night | 79.2±6.0 | 74.0±5.8 | 0.0009 |
| 24h | 80.5±6.4 | 75.1±5.7 | 0.00041 |
| MAP (mmHg) | 96.1±7.1 | 90.5±4.9 | 0.00029 |
| Day MAP (mmHg) | 98.4±7.2 | 92.7±5.0 | 0.002 |
| Night MAP (mmHg) | 94.5±6.7 | 89.0±4.9 | 0.00026 |
| Night/day ratio of MAP | 0.96±0.01 | 0.94±0.09 | 0.041 |
GFR: glomerular filtration rate, SBP: systolic blood pressure, DBP: diastolic blood pressure, N/D: night-to-day, BP: blood pressure.
This study showed that kidney transplantation causes a shift in renalase levels, MAP levels and circadian pattern of BP in kidney donors and recipients. Changes in MAP and pattern of BP were associated with changes in renalase levels.
Renalase immunostaining in biopsy specimens from patients with ESRD was found clearly decreased in renal cortex.18 However, our results demonstrated that patients with ESRD have higher plasma renalase levels than healthy donors. This finding thought that high level of plasma renalase in ESRD patients may be sourced from increased extra-renal renalase production. Li G et al. showed that plasma norepinephrine levels were measured markedly higher before transplant in ESRD patients in comparison with donors.12 This finding suggested that sympathetic system was overactive in patients with ESRD. Over activated sympathetic system may have also possible role in the enhanced secretion of renalase from extra-renal sources. Renalase overproduction may be a compensate mechanism to suppress the sympathetic over activity in patients with CKD. On the other hand, transplant donors lost some glomerular function while plasma renalase levels increased after transplantation. Our findings showed that after donation, there was an immediate approximately 35% reduction in GFR in the remaining kidney. The decline in renalase clearance due to the low GFR may contribute the rise in plasma renalase levels. These findings were similar with the results of the study reported by Santaos et al. in which they showed that recovery of the renal function in transplant recipients was paralleled by a progressive decrease in plasma renalase levels and decline in glomerular function in donors was associated with increase in plasma renalase levels after transplantation.15
BP generally varies according to a circadian rhythm characterized by a reduction during sleep and an increase during wakefulness. The circadian fall in BP during sleep does not always occur in all individuals.19 Some individuals display a fall in BP during sleep (dippers) and others do not (non-dippers). Abnormal blood pressure diurnal rhythm (non-dipping) is more prevalent in patients with CKD. The underlying mechanism, however, is not clear, growing evidence indicates that sympathetic over activity may have role in development of hypertension and abnormal circadian BP variation in patients with CKD.20 In agreement with previous data,21,22 the study showed that patients with ESRD have higher MAP levels and night time BP levels compared with healthy donors. After transplantation, MAP and night time BP levels decreased in transplant recipients. There was a direct correlation between renalase decrease and decline in MAP in day and night time. This association suggested that beside the well-known factors, decreased sympathetic over activity and renalase levels due to improvement of the GFR may have role in the decline MAP and abnormal diurnal BP rhythm in patients with ESRD after transplantation. However, a study performed in heart transplant recipients could not demonstrate a link between renalase levels and sympathetic nervous system activity.23
In contrast to recipients, slightly increased MAP was measured in donors after nephrectomy. This MAP elevation mostly depends on the night time BP levels. In an earlier study Goto et al. suggested that unilateral nephrectomy disturbs the circadian rhythm of BP as a function of renal dysfunction in kidney donors.7 Our results seem parallel with the findings of Goto's study. Our results also showed that rise in night-time MAP was associated positively with increase in renalase levels in transplant donors. Both the rise in night-time MAP and plasma renalase levels in the donors after transplantation may be consequence of loss of GFR. The association between change in the plasma renalase levels and night-time MAP suggested that sympathetic over activity due to GFR decline may have role in rise in night-time MAP of donors after nephrectomy.
To the best of our knowledge, the study is the first study evaluating renalase levels and alteration in MAP and circadian rhythm of BP in transplant donors and recipients before and after transplantation. Renalase was suggested to play role in the pathogenesis of hypertension and kidney damage in general population; therefore, this connection may be established in kidney donors and recipients. However, we could not find the direct association renalase and BP levels. Our findings suggested that renalase may not have direct role on the blood pressure rise in transplant recipient and donors. However, its role may be suppressing the sympathetic system by degradation of catecholamines (noradrenalin, adrenaline and dopamine). Therefore; high plasma renalase levels may contribute blood pressure regulation indirectly due to balance the pressor effect of the catecholamines.24
Small numbers of the study population, lack of the measurement of catecholamines or sympathetic activity and lack of evaluation of renal clearance of renalase are the limitation factors of our study. The cross-sectional nature of the study also limits the ability to conclude any causal relationship between renalase, BP levels, circadian rhythm of BP and GFR.
In summary, this study showed that renalase levels are associated with change in MAP and circadian rhythm of BP in kidney transplant donors and recipients. Additionally, high levels of serum renalase were correlated with BP elevation and renal injury, presented by low levels of GFR in kidney donors. This observation is highlighted by understanding the possible pathophysiological significance of serum renalase in the development of hypertension in kidney donors. However, further prospective large-scale studies are needed to better define these observations.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.