The mortality rate of diabetic patients on dialysis is higher than that of non-diabetic patients. Asymmetric dimethylarginine and inflammation are strong predictors of death in hemodialysis. This study aimed to evaluate asymmetric dimethylarginine and C-reactive protein interaction in predicting mortality in hemodialysis according to the presence or absence of diabetes.
MethodsAsymmetric dimethylarginine and C-reactive protein were measured in 202 patients in maintenance hemodialysis assembled from 2011 to 2012 and followed for four years. Effect modification of C-reactive protein on the relationship between asymmetric dimethylarginine and all-cause mortality was investigated dividing the population into four categories according to the median of asymmetric dimethylarginine and C-reactive protein.
ResultsAsymmetric dimethylarginine and C-reactive protein levels were similar between diabetics and non-diabetics. Asymmetric dimethylarginine – median IQR μM – (1.95 1.75–2.54 versus 1.03 0.81–1.55 P=0.000) differed in non-diabetics with or without evolution to death (HR 2379 CI 1.36–3.68 P=0.000) and was similar in diabetics without or with evolution to death. Among non-diabetics, the category with higher asymmetric dimethylarginine and C-reactive protein levels exhibited the highest mortality (69.0% P=0.000). No differences in mortality were seen in diabetics. A joint effect was found between asymmetric dimethylarginine and C-reactive protein, explaining all-cause mortality (HR 15.21 CI 3.50–66.12 P=0.000).
ConclusionsAsymmetric dimethylarginine is an independent predictor of all-cause mortality in non-diabetic patients in hemodialysis. Other risk factors may overlap asymmetric dimethylarginine in people with diabetes. Inflammation dramatically increases the risk of death associated with high plasma asymmetric dimethylarginine in hemodialysis.
La tasa de mortalidad de los pacientes diabéticos em diálisis se ha referido que es superior a la de los no diabéticos. La dimetilarginina asimétrica y la inflamación son potentes predictores de muerte en hemodiálisis. Este estudio tuvo como objetivo evaluar la interacción de dimetilarginina asimétrica y proteína C reactiva en la predicción de mortalidad en hemodiálisis según la presencia o ausencia de diabetes.
MétodosSe midieron dimetilarginina asimétrica y proteína C reactiva en 202 pacientes en hemodiálisis de mantenimiento reclutados entre 2011 a 2012 y seguidos durante cuatro años. Se investigó la modificación del efecto de la proteína C reactiva en la relación entre dimetilarginina asimétrica y la mortalidad por todas las causas dividiendo la población en cuatro categorías según la mediana de dimetilarginina asimétrica y proteína C reactiva.
ResultadosLos niveles de dimetilarginina asimética y proteína C reactiva fueron similares entre diabéticos y no diabéticos. Dimetilarginina asimétrica - mediana IQR μM - (1,95 1,75 - 2,54 versus 1,03 0,81 - 1,55 P = 0,000) difirió en los no diabéticos con o sin evolución a la muerte (OR 2379 IC 1,36 - 3,68 P = 0,000) y fue similar en los diabéticos sin o con evolución a muerte. Entre los no diabéticos, la categoría con niveles más altos de dimetilarginina asimétrica y proteína C reactiva presentó la mayor mortalidad (69,0% P = 0,000). No se observaron diferencias en la mortalidad en los diabéticos. Se encontró un efecto conjunto entre la dimetilarginina asimétrica y la proteína C reactiva, lo que explica la mortalidad por todas las causas (OR 15,21 IC 3,50-66,12 P = 0,000).
ConclusionesLa dimetilarginina asimétrica es un predictor independiente de mortalidad por todas las causas en pacientes no diabéticos en hemodiálisis. Otros factores de riesgo pueden superponerse a la dimetilarginina asimétrica en personas con diabetes. La inflamación aumenta drásticamente el riesgo de muerte asociado con niveles plasmáticos elevados de dimetilarginina asimétrica en pacientes en hemodiálisis.
Accumulation of asymmetric dimethylarginine (ADMA) contributes to hypertension, immune dysfunction, and cardiovascular diseases (CVD) in renal disease patients.1,2 ADMA, as the primary endogenous inhibitor of nitric oxide (NO), is a causal factor for endothelial dysfunction3,4 and plays a pivotal role in the process of atherosclerosis in a uremic environment.5 Indeed, it can be used as a cardiovascular and all-cause mortality biomarker in hemodialysis.6,7 ADMA is considered a full-scale uremic toxin and inflammatory inducer in end-stage renal disease (ESRD).8
On the other hand, inflammation has been recognized as a contributing factor to the pathophysiology of chronic kidney disease (CKD) and is associated with CVD and mortality.9 Higher C-reactive protein (CRP) values were strongly associated with mortality in DOPPS.10 Chronic inflammation is a major pathway leading to endothelial dysfunction in CKD.11
The interplay between ADMA and inflammation is a relevant issue. ADMA is useful in predicting cardiovascular events and enhancing CRP's predictive role in patients with diabetes mellitus (DM).12 ADMA and CRP also interact in hemodialysis (HD), functioning as an independent predictor of the progression of intima-media thickness.13 Inflammation, as assessed by CRP and interleukin 6 (IL-6), amplifies the risk of death and cardiovascular events associated with high ADMA levels in ESRD.14
DM has been associated with higher mortality in dialysis cohorts.15–17 Since DM is such a prevalent and critical comorbid condition in the ESRD population, it is essential to understand better its association with biomarkers of endothelial dysfunction and inflammation in HD. The assessment of ADMA and PCR interaction as predictors of HD mortality according to the absence or presence of DM was the study's objective.
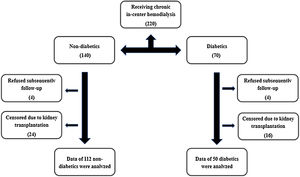
Materials and methodsStudy populationFrom April to October 2012, we enrolled two hundred and twenty patients receiving chronic in-center hemodialysis at two units or one hospital-based unit in the greater São Paulo area, using semi-synthetic or synthetic membranes (Fig. 1), for at least 3 months. Demographic, clinical, and laboratory characteristics were ascertained at the time of study enrollment. Demographic and clinical data were obtained through participant reports and review of medical charts. The race assignment was determined by patient self-classification. CVD was considered when identified in office or registry files history of stroke, peripheral vascular disease, coronary artery disease, or congestive heart failure (CHF).18 The identification of DM was made according to the American Diabetes Association Guidelines.19 After the initial assessment, patients were followed up for four years, and all-cause mortality was recorded. Eighteen patients out of two hundred and twenty were lost on follow-up. Exclusion criteria were as follows: CHF, aged under 18 years, clinical or laboratory suspicion of acute renal failure, pregnancy, and cancer.
The Ethics and Research Committee of the Federal University of São Paulo approved this study (no. 03827512.2.0000.5505). It was conducted according to the guideline of Good Clinical Practice and the principles of the Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. All patients received relevant explanations and signed the Ethics and Research Committee Informed Consent.
Laboratory measurementsEach sample was collected pre-dialysis, centrifuged, and frozen at −80°C, and it had not been previously thawed before testing. Automated enzymatic assays determined plasma concentrations of glucose, total cholesterol, and triglycerides. HDL was measured in serum by the homogeneous method. LDL was calculated according to the Friedewald equation. Creatinine was established by the Jaffé method with calibration traceable to an isotope dilution mass spectrometry reference measurement procedure.20 CRP was measured by ultrasensitive immunoturbidimetry and ADMA by HPLC.21,22
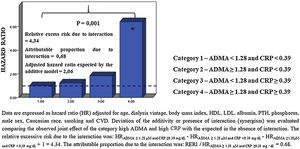
Statistical analysisResults were expressed as mean±SD, median and interquartile range (IQR), or frequency. We used the Kolmogorov–Smirnov test of a sample to test whether a variable follows the normal distribution. Comparisons among groups were made by the Student's t and Mann–Whitney tests (continuous variables) or Chi-squared test (dichotomic variables or percentage). Comparisons of frequency between groups were performed using the Pearson chi-square test. The relationship between continuous variables was investigated by the Pearson product-moment correlation coefficient (r) and P-value. The independent predictive value of ADMA and CRP for death was analyzed by multiple Cox regression analysis models adjusted for traditional risk factors and non-factors peculiar to ESRD. The effect modification of CRP on the relationship between ADMA and outcomes was investigated by dividing the study population into four categories according to the median values of ADMA and CRP.14 Synergism between CRP and ADMA was defined as a deviation from additivity23 occurring when the observed hazard ratio (HR) for study outcomes of patients with both high CRP and high ADMA was higher than that expected by summing up the hazard ratio of those with elevated CRP and low ADMA or low CRP and high ADMA minus one. In relative terms, the effect modification of inflammation biomarkers on ADMA's predictive value was investigated by multiple Cox regression analyses. The relative excess risk due to interaction (RERI) (RERI=HR++−HR+−−HR−++1) and the attributable proportion due to interaction (AP) (AP=RERI/HR++) were used to evaluate the occurrence of statistical interaction derived from Cox regression models of ADMA and CRP groups (below/above the corresponding median values).23,24 Results were reported with the separate effect of each exposure and the joint effect compared to the joint reference category to permit the evaluation of both additive and multiplicative interaction.24 In the absence of an interaction effect, RERI and AP were considered equal to 0.23 The level of nullity was fixed at 0.05 or 5% for all tests. The analysis was conducted using a standard statistical package (SPSS for Windows, version 22).
ResultsThe prevalence of DM in the initial cohort was 67.3% (i.e., 136 patients out of 202). Four patients had DM type 1. Dialysis vintage was 37.2±26.9 months. The patients’ demographic, clinical, and laboratory characteristics with a statistical comparison between diabetics (DM−) non-diabetics (DM+) after censoring due to kidney transplantation are presented in Table 1. One hundred and forty were on treatment with angiotensin-converting enzyme inhibitors or angiotensin-2 receptor antagonists, with no difference between DM+ and DM-. One hundred and eight were on treatment with simvastatin, also with no difference between DM+ and DM−. DM+ were older than DM− (57.1±13.3 versus 50.2±15.1 years, P=0.002), had a higher body mass index (25.9±2.9 versus 24.9±2.4 P=0.017), and a higher prevalence of coronary disease (36.9% versus 15.0% P=0.001). There was no statistically significant difference in ADMA and CRP levels between DM + and DM-.
Demographic, clinical, and laboratory characteristics of non-diabetics and diabetics.
| TOTAL(N=162) | DM−(N=112) | DM+(N=50) | P | |
|---|---|---|---|---|
| Age (years) | 52.5±14.9 | 50.2±15.1 | 57.1±13.3 | 0.002* |
| Male | 95 (58.4%) | 63 (56.6%) | 41 (62.1%) | 0.457*** |
| Caucasian | 110 (68.3%) | 76 (67.6%) | 35(69.7%) | 0.144*** |
| BMI (kg/cm2) | 25.2±2.6 | 24.9±2.4 | 25.9±2.9 | 0.017* |
| Waist circumference (cm) | 97.8±11.0 | 96.9±11.5 | 99.4±9.5 | 0.129* |
| Hypertension | 143(88.6%) | 97 (86.8%) | 46 (92.4%) | 0.235*** |
| Cardiovascular disease | 69 (42.6%) | 44 (39.1%) | 26 (52.3%) | 0.078*** |
| Cerebrovascular disease | 17 (10.4%) | 12 (10.5%) | 05 (10.8%) | 0.958*** |
| Coronary disease | 35 (21.8%) | 17 (15.0%) | 18 (36.9%) | 0.001*** |
| Peripheral vascular disease | 37 (22.8%) | 23(20.3%) | 14 (29.2%) | 0.162*** |
| Congestive heart failure | 25 (15.3%) | 18 (15.8%) | 08 (15.4%) | 0.941*** |
| HDL-C (mg/dL) | 38.2±14.4 | 38.0±15.6 | 38.8±11.5 | 0.693* |
| LDL-C (mg/dL) | 73.0±28.7 | 73.3±29.0 | 72.3±28.3 | 0.830* |
| Triglycerides (mg/dL) | 169.4±116.1 | 169.6±106.8 | 169.2±134.2 | 0.981* |
| Albumin (mg/dL) | 3.8±0.3 | 3.8±0.3 | 3.8±0.3 | 0.571* |
| PTH (pg/mL) | 413.0±355.0 | 420.8±344.0 | 396.9±379.1 | 0.657* |
| Phosporus (mg/dL) | 5.05±1.6 | 4.86±1.5 | 5.14±1.65 | 0.249* |
| ADMA (μM) median (IQR) | 1.24 (0.86, 1.92) | 1.36 (0.88, 1.95) | 1.16 (0.82, 1.82) | 0.389** |
| CRP (mg/dL) median (IQR) | 0.45 (0.17, 1.45) | 0.49 (0.15, 1.81) | 0.39 (0.18, 0.96) | 0.342** |
Only ADMA (median IQR μM) – (1.71 1.34–2.17 versus 0.88 0.60–1.37 P=0.000) and CRP ((median IQR mg/dL) – (0.77 0.23–2.25 versus 0.38 0.15–1.18 P=0.034) differed between individuals who died (O+) or not died (O−) (Table 2). In a Cox model among individuals with DM−, only ADMA (median IQR μM) – (1.95 1.75–2.54 versus 1.03 0.81–1.55 P=0.000) showed a statistically significant difference between O+ or O− (OR 4.70 P=0.000). Among DM+ patients, ADMA and CRP showed no correlation with the death outcome.
Demographic, clinical, and laboratory characteristics according to progression (O+) or not to death (O−).
| O+(N=121) | O−(N=41) | P | |
|---|---|---|---|
| Age (years) | 53.4±15.4 | 56.8±13.4 | 0.214* |
| Male | 71 (58.7%) | 23 (56.1%) | 0.772*** |
| Caucasian | 79 (65.3%) | 33 (80.5%) | 0.187*** |
| BMI (kg/cm2) | 25.3±2.6 | 25.8±2.8 | 0.270* |
| Waist circumference (cm) | 98.2±11.1 | 98.5±9.8 | 0.873* |
| Diabetes mellitus | 40 (33.1%) | 12 (29.3%) | 0.653*** |
| Cardiovascular disease | 53 (44.2%) | 20 (51.3%) | 0.439*** |
| Cerebrovascular disease | 11 (11.2%) | 07 (12.0%) | 0.133*** |
| Coronary disease | 24 (20.0%) | 12 (30.8%) | 0.163*** |
| Peripheral vascular disease | 28(23.3%) | 09 (23.1%) | 0.974*** |
| Congestive heart failure | 23 (19.2%) | 05 (12.8%) | 0.366*** |
| HDL-C (mg/dL) | 38.7±15.6 | 37.7±13.4 | 0.693* |
| LDL-C (mg/dL) | 74.3±30.9 | 75.4±26.9 | 0.841* |
| Triglycerides (mg/dL) | 168.4±125.5 | 156.8±75.1 | 0.579* |
| Albumin (mg/dL) | 3.8±0.3 | 3.8±0.3 | 0.741* |
| PTH (pg/mL) | 410.0±349.3 | 403.9±373.1 | 0.924* |
| ADMA (μM) – median (IQR) | 0.88 (0.60, 1.37) | 1.71 (1.34, 2.17) | 0.000** |
| CRP (mg/dL) – median (IQR) | 0.38 (0.15, 1.18) | 0.77 (0.23, 2.25) | 0.034** |
There were 41 (25.3%) deaths over the follow-up time – 29 deaths in DM+ and 12 deaths in DM− (26.4% versus 23.1% P=0.653). DM + and DM− differed significantly in the time of evolution for the death outcome (24.07±13.60 versus 13.08±6.93 months P=0.012).
ADMA and CRP levelsPlasma ADMA levels did not significantly correlate with plasma CRP levels (r=−0.045, P=0.570) in the whole cohort or subgroup.
Effect modification of CRP on the ADMA-outcome relationshipIn the whole cohort, the relationship between ADMA and the incidence rate of mortality was closely dependent on CRP categories, with the incidence rate of mortality being maximal in patients with high ADMA (≥1.28μM) and high CRP (≥0.39mg/dL). On the other hand, the interplay was minimal in patients with low ADMA (<1.28μM) and low CRP (<0.39mg/dL) (55.8%×7.3% P=0.000). In DM− patients, this relationship was also identified (69.0%×0.0% P=0.000); whereas in DM+ patients an effect modification23 by CRP categories about ADMA was not observed in the incidence of mortality (33.3%×25.0% P=0.060) (Fig. 2).
A joint effect was found between ADMA and CRP for explaining all-cause mortality in the whole cohort through multiple Cox regression analysis (Fig. 3). Indeed, the adjusted hazard ratios for the death of patients with increased ADMA and CRP were higher than those expected in the absence of interaction under the additive model and significantly higher (P=0.001) than those in patients with only one biomarker increased. Remarkably, the proportion of mortality related to interaction among persons with both high ADMA and high CRP was 0.68. Because the HR of one or more groups in DM− and DM+ was below 1 (protective), it was not possible to interpret the analysis (data not shown).
The Cox model analysis confirmed the increased risk for all-cause mortality associated with ADMA≥1.28μM and CRP≥0.39mg/dL (Table 3).
Cox model of the association between category 4 (ADMA≥1.28μM and CRP≥0.39mg/dL) and death.
| HR (95% CI)P | |
|---|---|
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL | 13.89 (3.69–52.34)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL and age | 14.07 (3.71–53.39)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age and dialysis vintage | 13.92 (3.67–52.92)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage and BMI | 13.81 (3.62–52.68)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage, BMI and HDL | 14.75 (3.77–57.78)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage, BMI, HDL and LDL | 16.39 (1.47–3.40)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage, BMI, HDL, LDL albumin | 16.41 (4.05–66.44)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage, BMI, HDL, LDL albumin and phosporus | 16.50 (4.07–66.90)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage, BMI, HDL, LDL albumin, phosporus and PTH | 15.87 (3.90–64.53)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage, BMI, HDL, LDL albumin, phosporus, PTH and male | 16.69 (4.04–68.95)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage, BMI, HDL, LDL albumin, phosporus, PTH, male and Caucasian | 14.84 (3.53–62.43)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage, BMI, HDL, LDL albumin, phosporus, PTH, male, Caucasian and tabagism | 14.88 (3.53–62.67)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage, BMI, HDL, LDL albumin, phosphorus, PTH, male, Caucasian, tabagism and hypertension | 14.55 (3.43–61.73)0.000 |
| Adjusted for ADMA≥1.28μM and CRP≥0.39mg/dL, age, dialysis vintage, BMI, HDL, LDL albumin, phosphorus, PTH, male, Caucasian, tabagism, hypertension and CVD | 15.21 (3.50–66.12)0.000 |
Our data evidenced that, independently of traditional and nontraditional risk factors, high levels of ADMA are associated with an elevated risk of death in prevalent HD patients.4,5,14 It also reports that this association only occurs within individuals without DM. On the other hand, CRP alone was not informative of mortality risk.11,25,26 Nevertheless, CRP, and so inflammation, augments the risk of death associated with high plasmatic ADMA in dialysis patients.14 Individuals with high ADMA and CRP levels exhibited an increased risk of dying than those with only one elevated biomarker. Such an excess risk exceeded what one would expect by adding the individual risks of these factors (synergism).23
According to our results, ADMA can be used as a biomarker of hemodialysis mortality only in patients without DM. DM is associated with a significant vascular burden and mortality among hemodialysis patients. Hence, the importance of assessing the likelihood of asymmetric dimethylarginine to be used as a biomarker of death in the diabetic hemodialysis population is warranted. Even although ADMA had been tested already for explaining death and fatal and nonfatal cardiovascular events, the authors did not have the opportunity to evaluate DM patients separately.4,5,14
The literature cites one example of a hemodialysis population where ADMA values are not associated with all-cause mortality. Drew et al.27 showed lower levels of ADMA in African American versus non-African American patients on hemodialysis. ADMA level was associated with all-cause mortality, a finding exclusive to non-African American patients on hemodialysis. They also confirmed a lower hazard for mortality in African American patients versus non-African American patients and this difference was not explained by ADMA levels. There is evidence that, in addition to influencing ADMA levels, DDAH may also directly affect vascular health, independent of ADMA.28 We can hypothesize that DDAH polymorphisms may be responsible for the lack of association between ADMA and mortality in diabetic patients on hemodialysis.
At the same time, ADMA may have less relative importance for the significant all-cause mortality of diabetic patients on hemodialysis due to several other inflammatory factors. Diabetic patients on hemodialysis have a different response to alleged interventions to their higher inflammatory state. Sattar et al. demonstrated an increased risk of mortality over time associated with DM in prevalent hemodialysis patients, which may relate to the accumulation of end-organ damage or mediators of inflammation and oxidative stress.14 In a post hoc analysis of the AURORA study, the treatment of diabetic individuals with Rosuvastatin significantly reduced the occurrence of coronary atherosclerotic events.29 In the MPO study, patients with DM treated with high-flow membranes, had a higher survival rate than those treated with low-flow membranas.30
Since the late 1990s, the relationship between inflammation and worse outcomes in ESRD has been recognized. Traditional and nontraditional risk factors peculiar to ESRD may promote inflammation by synthesizing and releasing several pro-inflammatory cytokines. In the DOPPS, CRP was informative regarding mortality risk in hemodialysis beyond that provided by other inflammatory and nutritional markers, with significantly higher risk seen at CRP>0.3mg/dL.11 Our cohort had ninety-six (59.2%) ESRD patients with circulating levels CRP above this value. In a study developed in Brazil among prevalent patients in hemodialysis, clinically significant inflammation was defined as CRP>5.1mg/L, based on the receiver operating characteristics curve for CRP as a predictor of death.31 Thus, we can suggest that our cohort was not representative of CRP's relation to mortality. Plasma concentrations of CRP in our group were low, but the literature values are diverse. Median plasma concentrations of CRP ranged from 0.1mg/dL in Japan to 0.6mg/dL in Europe and New Zealand in a study by Bazeley et al.32
The absence of a positive correlation between plasma concentrations of ADMA and CRP suggests that endothelial dysfunction and inflammation were not parallel processes in the patients evaluated. The relationship between high ADMA and CRP is context-dependent. In the acute phase of bacterial infections, plasma concentrations of CRP are elevated, and those of ADMA are similar to those of healthy individuals.33 In vitro, ADMA induces TNF-α production via reactive oxygen species/NF-κB-dependent pathway.34 On the other hand, the generation of reactive oxygen species, a crucial initial event in inflammation, inhibits the enzyme that degrades ADMA (dimethylarginine dimethylaminohydrolase), facilitating local and systemic ADMA accumulation.35 ADMA, on the other hand, increases the generation of the downstream pro-inflammatory cytokines TNFα and IL-8 and activates the NF-κB pathway and the binding of monocytes to endothelial cells.35 At the same time, there is no change in plasma concentrations of ADMA after prolonged periods on HD.36 The correlation of plasma concentrations of ADMA and CRP may have been influenced by a healthier cohort than in other studies.37,38
ADMA is a marker of cardiovascular risk in patients with DM and is associated with the development and progression of diabetic nephropathy.39 Diabetic-specific risk factors may abolish ADMA's performance as a marker of mortality risk in patients with diabetes in HD.
Following the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement,23 the effect modification of CRP on the relationship between ADMA and outcomes was investigated by dividing the study population into four categories according to the median values of ADMA and CRP. Such variables with four possible exposure categories give enough information to evaluate both additive and multiplicative interaction.23 A joint effect was recognized between ADMA and CRP for explaining all-cause mortality in the group with increased ADMA and CRP, even with CRP levels considered beneath clinically significant inflammation. As the risk ratio of one or more groups with and without DM was less than 1 (protective), it was not feasible to interpret the joint effect analysis.
This study has some limitations. ADMA and CRP were measured at a single time point, and the concentration may change over time.31 It is descriptive, and the mechanisms underlying those associations cannot be inferred here. It would have been interesting to analyze the association of ADMA levels with cardiovascular mortality as well. It was impossible to recognize specific causes of mortality, such as cardiovascular due to the transfer of several patients to other hemodialysis centers and lack of medical records with reliable information. Given the modest sample size, these exploratory findings should be replicated in larger cohorts of diabetic patients on hemodialysis.
ConclusionADMA can be used as a risk marker for all-cause mortality among non-diabetic prevalent patients in HD. Other risk factors may overlap ADMA among patients with DM. Besides, we observed a synergistic effect between inflammation, represented by CRP, and endothelial dysfunction, which stood for by ADMA to explain all-cause mortality in HD.
Conflict of interestThe authors have no conflicts of interest to declare.