A significant number of patients with systemic lupus erythematosus (between 20% and 60% according to different reported series) develop lupus nephritis in the course of its evolution, which directly influences their quality of life and vital prognosis. In recent years, the greater knowledge about the pathogenesis of systemic lupus and lupus nephritis has allowed relevant advances in the diagnostic approach and treatment of these patients, achieving the development of drugs specifically aimed at blocking key pathogenic pathways of the disease. Encouragingly, these immunomodulatory agents have shown in well-powered, randomized clinical trials good clinical efficacy in the medium-term, defined as proteinuria remission and preservation of kidney function, with an acceptable safety profile and good patient tolerability. All this has made it possible to reduce the use of corticosteroids and other potentially more toxic therapies, as well as to increase the use of combined therapies. The present consensus document carried out by the Glomerular Diseases Working Group of the Spanish Society of Nephrology (GLOSEN), collects in a practical and summarized, but rigorous way, the best currently available evidence about the diagnosis, treatment, and follow-up of lupus nephritis patients, including cases of special situations, with the main objective of providing updated information and well-founded clinical recommendations to treating physicians, to improve the diagnostic and therapeutic approach to our patients.
Un número importante de pacientes con lupus eritematoso sistémico (entre un 20% a 60%, según diferentes series), desarrolla nefritis lúpica en el curso de su evolución, lo que influye directamente en su calidad de vida y pronóstico vital. En años recientes, el mayor conocimiento sobre la patogénesis del lupus sistémico y de la nefritis lúpica, ha permitido avances relevantes en el abordaje diagnóstico y en el tratamiento de estos pacientes, lográndose desarrollar fármacos dirigidos específicamente a bloquear vías patogénicas claves de la enfermedad. Alentadoramente, estos agentes inmunomoduladores han demostrado en ensayos clínicos aleatorizados y bien ponderados, buena eficacia clínica a mediano plazo, definida como remisión de proteinuria y preservación de la función renal, con un aceptable perfil de seguridad y buena tolerabilidad del paciente. Todo esto ha permitido reducir el uso de corticoides y de otras terapias potencialmente más tóxicas, así como incrementar el uso de terapias combinadas. El presento documento de consenso realizado por el Grupo de Trabajo de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN), recoge de manera práctica y resumida, pero rigurosa, la mejor evidencia actual disponible acerca del diagnóstico, tratamiento y seguimiento del paciente con nefritis lúpica, incluyendo casos de situaciones especiales, con el objetivo principal de brindar información actualizada y recomendaciones clínicas bien fundamentadas a los médicos tratantes, para mejorar el enfoque diagnóstico y terapéutico a nuestro pacientes.
Systemic lupus erythematosus (SLE) is the paradigm of systemic disease and a significant number of patients with SLE develop renal involvement. These would already be sufficient reasons to justify a review and update of lupus nephritis (LN) by the GLOSEN group. But, in addition, in recent years we have witnessed the publication of studies of great importance which, have demonstrated the efficacy of new drugs and therapeutic combinations and, also, have contributed to greater precision in the definition of treatment targets and prognostic markers.
We believe that a consensus document that collects, analyzes and summarizes all this new information and offers it to the reader in an attractive and practical manner will be useful for a better management of the patient with lupus nephritis. This has been the aim of the present work, which is divided into five blocks (diagnosis, treatment objectives, therapeutic measures, treatment and special situations), all with different sections. Each section is made up of brief recommendations, followed by a synthesized justification of these recommendations. We also hope that the tables and figures will help to achieve the proposed objectives.
In the supplementary material we have included topics that are not strictly nephrological, that will undoubtedly be very useful for the reader, such as bone and ovarian protection, antibiotic prophylaxis and vaccinations, and the new problems generated by the COVID-19 pandemic. We have also included some particularly voluminous tables as supplementary material.
As is usually noted in clinical practice guidelines and consensus documents such as the present one, this type of work does not tell the clinician what he/she must do with a given patient, but rather offers updated information and a series of suggestions and recommendations to help him/her make the best diagnostic and therapeutic decisions. But, in addition, as the reader will appreciate, there are many aspects of lupus nephritis in which the scientific evidence is still poor and in which more clinical and basic studies are needed. We believe that the GLOSEN group, as it has been shown in many other glomerular diseases, should serve as an ideal space and platform for carrying out these studies and we hope that this work will serve as a stimulus in this regard.
Material and methodsIn the present Consensus Document, have participated expert physicians in the diagnosis and treatment of patients with lupus nephritis, all members of the Glomerular Diseases Study Group of the Spanish Society of Nephrology (GLOSEN). To this end, several virtual meetings have been held with the purpose of coordination, discussion and consensus. Based on the working outline proposed by the last author, the different sections of the document were assigned to each of the authors according to the experience, knowledge or affinity with the respective sections. Each proposed recommendation was completed after an exhaustive search of the literature and, to a large extent, based on the experience and opinions of the authors. The initially proposed recommendations were reviewed and discussed by all authors, independently of the section assigned to each one, thus the final recommendations were agreed upon by all the authors.
Under each group of recommendations, a short text was developed based on the best published evidence supporting these recommendations. For this purpose, MEDLINE (with its free access search engine PubMed), EMBASE, Google Scholar and the Cochrane Library were used as bibliographic sources. As a complementary approach, the bibliographic reference lists of the individual articles selected from the databases and the abstracts of the main national and international congresses on lupus nephritis were reviewed. There was no restriction by language, geographic region or year of publication, but publications from the last 10 years were given preferrece, incorporating older references based on their clinical relevance.
Bibliographic searches were performed using search terms (keywords), mainly in English. Among other terms, the following were used individually or in combination:
- -
For diagnosis, follow-up and prognosis:
"lupus nephritis" or "lupus glomerulonephritis" or "systemic lupus erythematosus";
"diagnosis", "kidney biopsy" "serological markers" or "immunological biomarkers";
"prognosis", "cohort study" or "follow-up studys", "clinical-pathologic study"; "clinical-pathologic study".
- -
For treatment:
"antiproteinuric therapy" or "non-immunosuppressive therapy" or "supportive therapy";
"immunosuppressive therapy" or "immunosuppressive treatment" or "immumodulator treatment" or "biological therapy";
"randomized clinical trial" or "comparative clinical trial" or "interventional study";
- -
For special situations:
"chronic failure" or "chronic kidney disease", "kidney transplant" or "renal transplant";
"pregnancy", "pre-eclampsia", "pediatric lupus nephritis", "thrombotic microangiopathy", "thrombotic microangiopathy"
"thrombotic microangiopathy," "antiphospholipid syndrome," "atypical hemolytic uremic syndrome," "refractory or resistant lupus nephritis," "relapsing lupus nephritis," "flare"
- -
For supplementary material:
"ovarian failure" or "ovarian insufficiency", "infertility";
"corticosteroid-induced osteoporosis" or "secondary osteoporosis", "bone protection";
"infection", "infection prophylaxis", "vaccination", "SARS-CoV-2 infections", "COVID-19", "SARS-CoV-2 vaccination" or "SARS-CoV-2 immunization" or "COVID-19 vaccination".
In addition to the literature found and selected, special consideration was given to the clinical practice guidelines on glomerular diseases KDIGO 2021 (Kidney Disease Improved Global Outcomes) and the EULAR/ERA-EDTA 2019 guidelines on diagnosis and treatment of patients with lupus nephritis, as they are the most widely accepted and widely used international references at present.
The authors have signed this consensus document in alphabetical order, except the first two for their contribution to the manuscript and the last author as overall coordinator of the study. The document has been subjected to public review by members of the Spanish Society of Nephrology (SENEFRO). The levels of evidence of the studies consulted are mostly low: levels C and D according to the Centre for Evidence-Based Medicine (Oxford University) (http://www.cebm.net/?o=1025), although several of the most relevant and recent clinical trials on immunosuppressive treatment, are level A and B.
DiagnosisDiagnosis and classificationRecommendations1.1.1 SLE is a heterogeneous autoimmune disease with a wide range of manifestations that can affect virtually any organ. The diagnosis of SLE is based on the recognition of characteristic signs and symptoms of the disease according to the criteria established by the 2019 EULAR/ACR classification (Table S1).
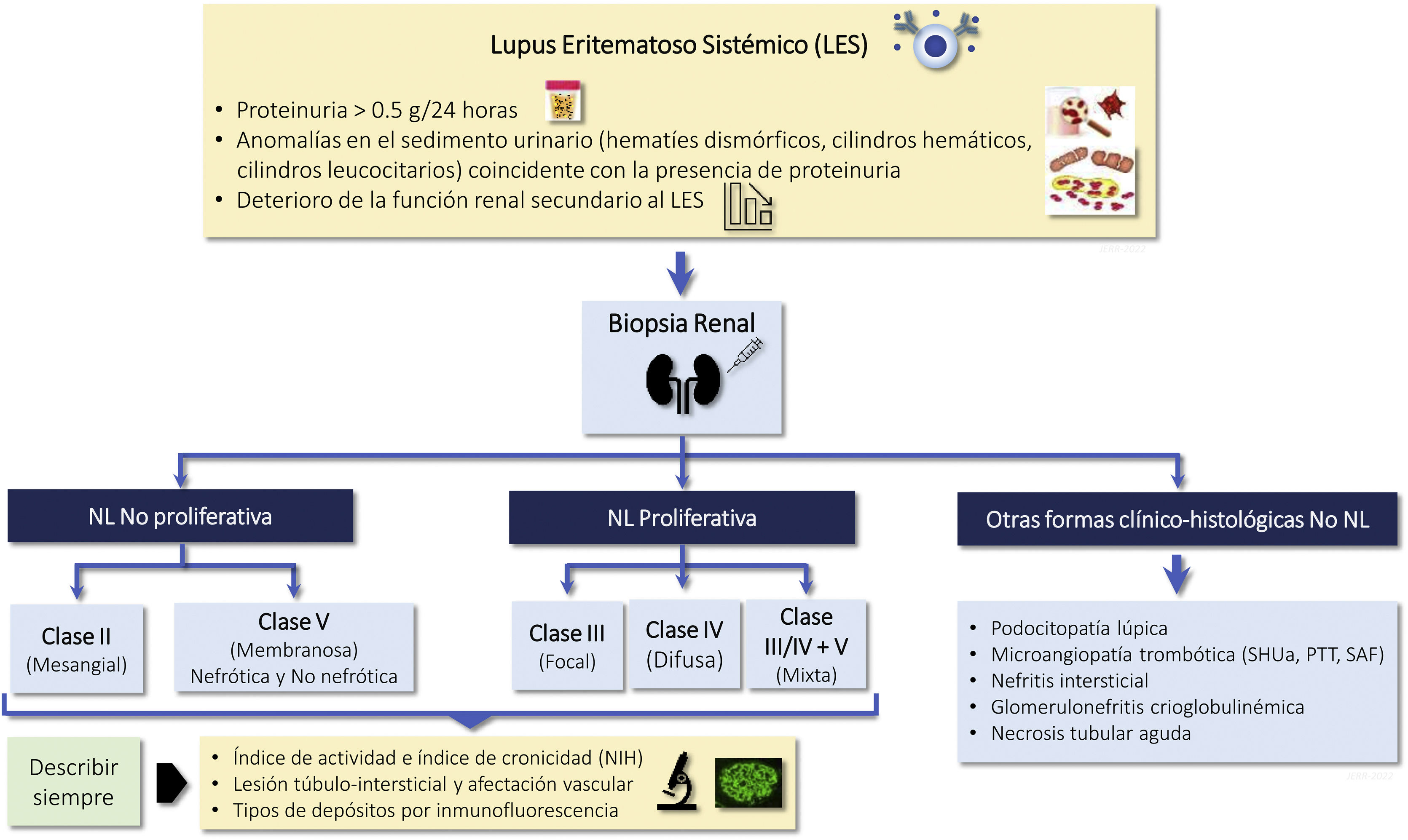
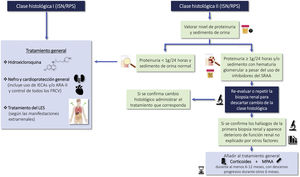
1.1.2 LN is a nephropathy secondary to immunocomplex deposition in a patient diagnosed with SLE. Renal biopsy is necessary for its diagnosis and classification and should be assessed by expert nephropathologists. The classification of LN according to the latest revision of the International Society of Nephrology/Renal Pathology Society (ISN/RPS) are summarized in Table 1. The activity and chronicity indices are shown in Table S2.
2018 Review of the Classification of Lupus Nephritis according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS).
| Histological class | Histopathological findings | ESRD risk |
|---|---|---|
| Deposits of immune complexes in the mesangium | Very low |
| Deposits of immune complexes in the mesangium and mesangial hypercellularity | Very low |
| Hypercellularity endo or extracapillary, subendothelial deposits of immune complex
| 25% |
| GBM thickening, presence of spikes, subepithelial deposits of immune complex | <10% |
ERCT, End-stage chronic kidney disease; GBM, glomerular basement membrane.
The clinical and analytical parameters used in routine clinical practice do not allow predicting histological findings in a high percentage of cases, so the renal biopsy provides essential information to: a) identify the class of LN; b) establish a prognosis, and c) plan the treatment. Histological study requires optical microscopy and immunofluorescence techniques, and electron microscopy is recommended, so it must be interpreted by expert nephropathologists.1Table 1 summarizes the 5 histologic classes proposed in the latest revision of the ISN/RPS classification published in 20182 and the associated risk of developing in end-stage chronic kidney disease (CKD) after 5 years. A key aspect to consider is that the histologic lesions of LN are dynamic and there may be transitions between the different classes, either spontaneously or after treatment. In addition, there may be overlap between classes II, III and IV and membranous class (V) at any time during evolution and this finding may imply changes in treatment. In the biopsy the degree of endocapillary hypercellularity, karyorrhexis, fibrinoid necrosis, hyaline deposits, cellular crescents and interstitial inflammation should be assessed to calculate the activity index between 0 and 24 and the degree of glomerulosclerosis, fibrous crescents, tubular atrophy and interstitial fibrosis to calculate the chronicity index between 0 and 12 (Table S2). Although the classification is based primarily on glomerular abnormalities, it is also important to highlight the prognostic importance of the tubulointerstitial and vascular lesions described in the classification. Finally, patients with SLE may present other lesions in the renal biopsy not being considered in the ISN classification that can also have therapeutic implications. These histological findings include: podocytopathy, thrombotic microangiopathy (TMA) vasculitis and acute tubulointerstitial nephritis.3–5
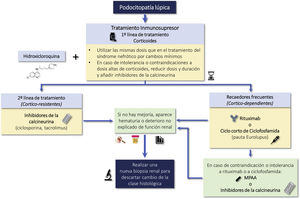
Lupus podocytopathy (LP) is an infrequent manifestation (<1% of cases) of SLE that is not included in the classification of LN, the clinical manifestation is nephrotic syndrome and is characterized by a renal biopsy showing on light microscopy normal glomeruli or lesions of focal and segmental glomerulosclerosis, with or without mesangial proliferation; the absence of subepithelial or subendothelial deposits on immunofluorescence and the presence of diffuse effacement of podocyte foot processes on electron microscopy.6
The clinical course of LP is similar to minimal change disease and primary focal segmental glomerulosclerosis and is clearly distinct from other forms of LN and involves a mechanism independent of typical immunocomplex deposition.
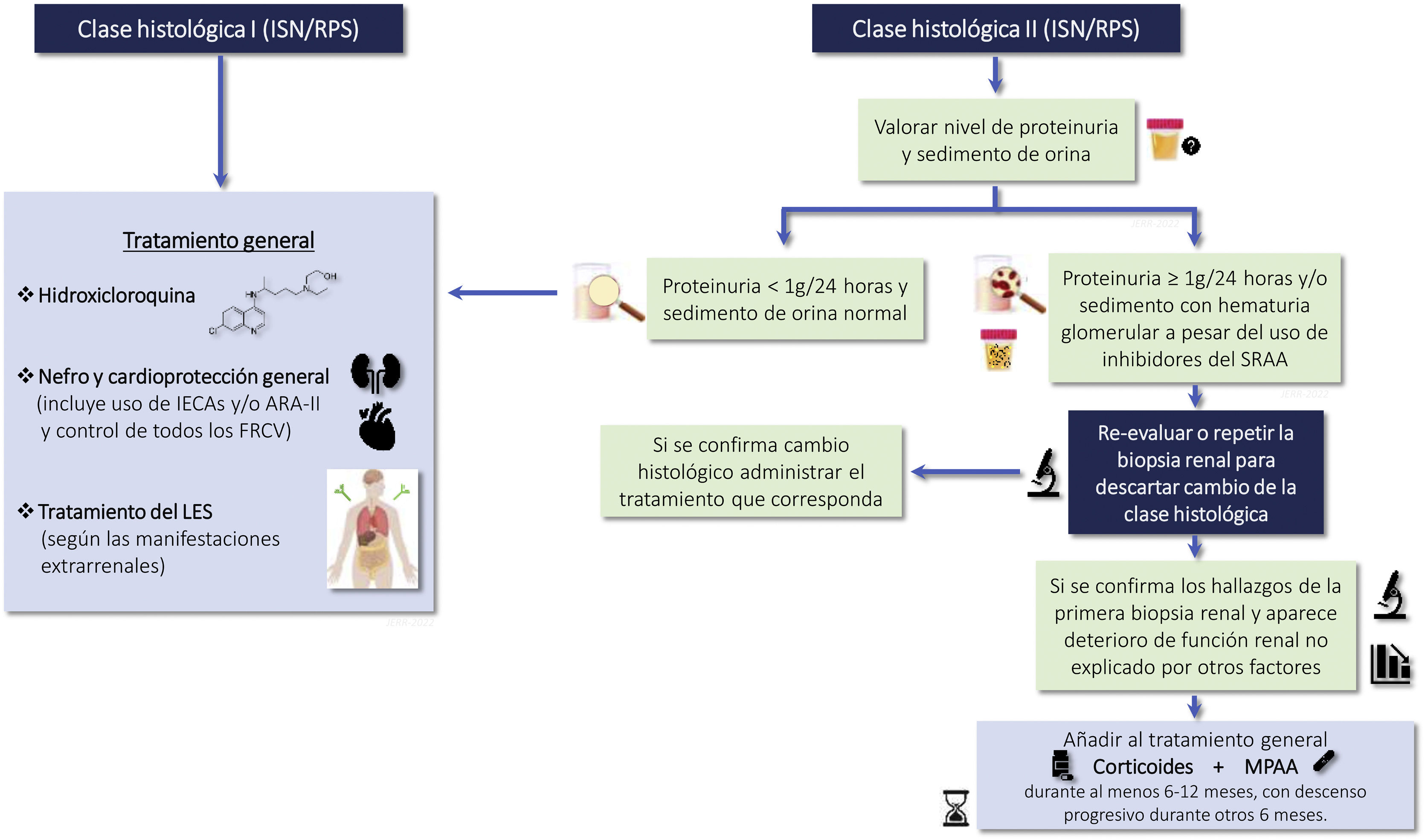
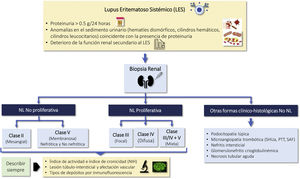
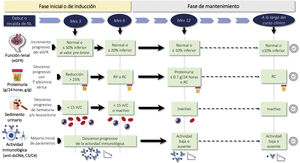
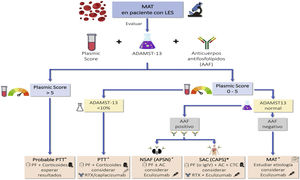
Indications for renal biopsy and rebiopsyRecommendations1.2.1 Renal biopsy is recommended in patients with SLE and a proteinuria >0.5 g/24 h (or a urine protein/creatinine ratio >0.5 g/g). The presence of an active sediment (hematuria, leukocyturia or cylindruria) and/or unexplained deterioration of renal function coupled with the detection of proteinuria further strengthens the indication for biopsy. The indication for renal biopsy in patients without proteinuria but who present active sediment and/or unexplained deterioration of renal function requires a careful previous study to rule out causes other than SLE (Fig. 1).
Diagnosis of kidney injury in SLE.
Figure adapted from: Parikh SV, Almaani S, Brodsky S, Rovin BH. Update in lupus nephritis: Core Curriculum 2020. Am J Kidney Dis. 2020;76:265−81.
SLE: systemic lupus erythematosus; NIH: National Institute of Health, LN: nephritis lupus; PTT: purple thrombotic thrombocytopenic; APS: Antiphospholipid syndrome; aHUS: Hemolytic-uremic Syndrome atypical.
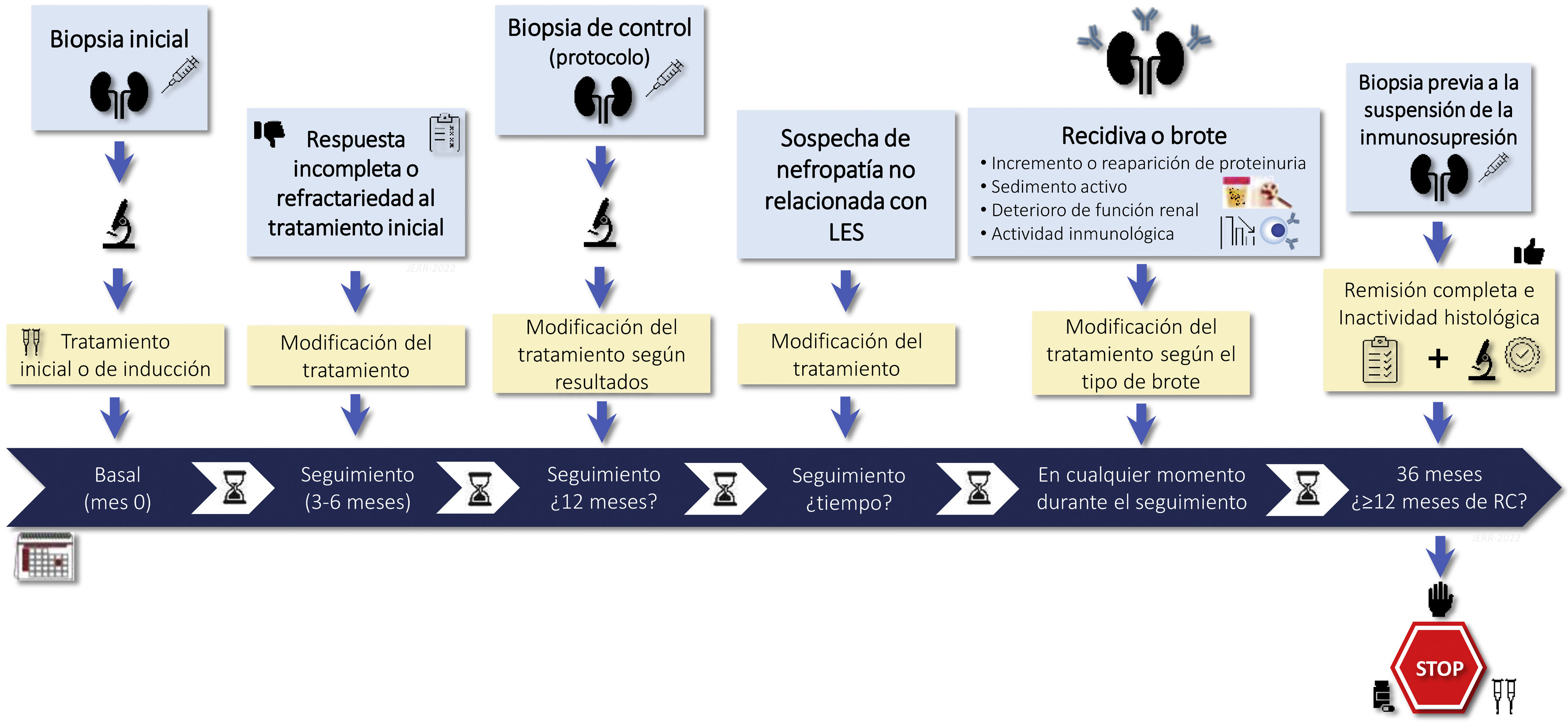
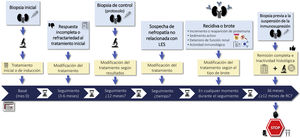
1.2.2 There is no general agreement on the indications for rebiopsy in LN. It may be considered in cases lack of response to treatment, if there are doubts about the predominance of active or chronic lesions in patients with persistent proteinuria or renal failure, in relapses of LN that raising doubts about diagnostic or therapy, or when it is suspected nephropathy unrelated to LN. Rebiopsy could also help in decision making regarding withdrawing immunosuppressive treatment (Figure S1).
RationaleRenal biopsy is necessary to establish the diagnosis of LN and is recommended in patients with a diagnosis of SLE who present with persistent urinary abnormalities not attributable to other causes or detection of unexplained deterioration of renal function (Figure S1).7–10
The greatest strength of the current 2003 N L ISN/RPS classification is its wide acceptance by all scientific societies and the therapeutic implications according to this classification, however, it is not without weaknesses.11 For this and other reasons, the recent 2018 classification has eliminated the LN class IV subdivisions. Semiquantitative assessment of active and chronic lesions is now mandatory because of its prognostic implications2 (Table S2). Finally, the description of vascular and interstitial lesions should be included because of their role in the renal prognosis.12–14
The function of renal rebiopsies is a controversial issue and should be individualized according to the characteristics of each case, its response to treatment and its evolution. Figure S1 summarizes the main causes that may indicate a rebiopsy. There is even less agreement regarding the protocol biopsies. Observational studies suggest that the use of this type of biopsy could help decisions on the maintenance of immunosuppressive treatment.15–17 Protocol biopsies have highlighted the striking discrepancy between clinical and histologic response. Cases of biopsies performed at 6 and 8 months post-treatment with complete clinical remission have shown histological activity between 20 and 50% of cases. Whereas 40–60% of patients with persistent proteinuria (>0.5 g/24 h) showed histological remission.18 Recently, two prospective studies have attempted to explain this clinical-histologic discrepancy. The first study selected 36 patients with complete clinical remission for at least one year who had received at least 36 months of treatment. A protocol renal biopsy was performed before withdrawal of immunosuppression and the patients were followed for 24 months. Ten of the 11 patients who had a recurrence of LN had histologic activity on protocol biopsy and all patients with an activity index greater than 2 had a recurrence.3 In the second study, serial renal biopsies were performed in a cohort of 75 patients with LN and on immunosuppressive therapy for at least 42 months. A new renal biopsy was performed and, depending on the degree of activity, immunosuppression was suspended or maintained. With this protocol, no patient developed advanced CKD and the rate of recurrence of LN was lower than those described in previous studies.19 It has therefore been suggested that a protocol biopsy before withdrawal of immunosuppression and in selected patients could help in making this therapeutic decision. However, larger studies are required to confirm this possibility. On the other hand, the application of genomics, proteomics and metabolomics techniques together with the identification of new biomarkers in renal tissue may improve the individualization of treatment in patients with LN.
Assessment of clinical manifestationsRecommendations1.3.1 The identification, assessment and follow-up of extrarenal clinical manifestations is essential in every patient with NL. The use of the validated SLEDAI (Systemic Lupus Erythematosus Disease Activity Index) scoring system is recommended for the follow-up of these manifestations (Table S3).
1.3.2 In a significant proportion of patients, LN is asymptomatic and is detected by laboratory abnormalities. Particular attention should be paid to the presence of edema and/or de novo development of arterial hypertension in patients with SLE.
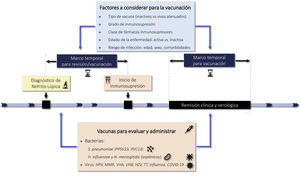
1.3.3 Patients with LN should have the usual clinical data recorded in the clinical history of chronic patients, always specifying the variables involved in the development of cardiovascular complications, history of neoplasia, and data from their vaccination card.
RationaleA significant proportion of patients are asymptomatic at the time of diagnosis. LN is most often discovered after careful examination of urine and laboratory data in patients with SLE. According to the clinical diagnostic criteria for SLE, the diagnosis can be confirmed in any patient presenting with biopsy-confirmed immunocomplex glomerulonephritis without any other secondary cause except the presence of ANA or anti-dsDNA.4,20–22
Assessment of analytical abnormalities (proteinuria, urinary sediment, glomerular filtration rate)Recommendations1.4.1 Urinalysis (to determine the albumin/creatinine and/or protein/creatinine ratio in an isolated urine sample and to assess the urinary sediment) and a determination of serum creatinine and estimated glomerular filtration rate (eGFR) should be performed periodically in all patients with SLE. These determinations should be more frequent (at least 1−2 times a year) in those patients with a higher propensity to develop LN (persistent extrarenal clinical manifestations, serologic markers of activity, early onset of SLE, non-Caucasian race population).
1.4.2 In patients diagnosed with LN, the amount of proteinuria (expressed as proteinuria/creatinine ratio in isolated urine sample or as proteinuria in 24-h urine collection), evaluation of urinary sediment, and determination of serum creatinine and eGFR are the most important parameters for assessing treatment efficacy and disease progression. The clinical manifestations most commonly associated with each histological class are summarized in Table S4.
1.4.3 There are factors independent of SLE activity that can influence the amount of proteinuria, renal function (serum creatinine, eGFR) and urinary sediment and should be carefully evaluated at each screening. The amount of proteinuria is not always directly related to lupus activity (especially in relapsing patients and those with significant chronic lesions) and should be reduced as much as possible with renoprotective antiproteinuric treatments, in conjunction with the indicated immunosuppressive treatment (Table 2).
Main factors that may influence the amount of proteinuria, urinary sediment and renal function regardless of lupus activity.
| Renal parameter | Modifying factor or condition |
|---|---|
| Amount of proteinuria |
|
| Urinary sediment |
|
| Renal function |
|
ARA-II, Angiotensin II receptor antagonists; ACEIs, angiotensin converting enzyme inhibitors; iSGLT2, Inhibitors of the tubular sodium-glucose cotransporter type 2; RAAS, Renin-angiotensin-aldosterone system.
LN represents one of the most severe and frequent complications of SLE.20,23,24 Its early diagnosis is a key element in the management of the disease. Since LN may have an asymptomatic onset in many patients,4 periodic blood and urine tests are recommended to assess renal function (serum creatinine and eGFR), abnormal presence of proteinuria/albuminuria and urinary sediment. Although urine test strips have traditionally been recommended, the exact determination of renal function and the quantification of the amount of the albumin/creatinine and/or protein/creatinine ratio in an isolated urine sample are becoming increasingly important in routine clinical practice, given the impact that the stage of renal function and the amount of albuminuria have on the cardiovascular risk of any chronically ill patient. A higher prevalence and more accelerated development of atherosclerotic complications has been reported in patients with SLE25,26 therefore, these periodic determinations take on added value for the prevention and treatment of cardiovascular risk even in inactive lupus patients. The periodicity of these determinations should be adjusted to the clinical situation of the patients. Given that the incidence of LN is higher in patients with extra-renal lupus clinical manifestations, in those with a very early onset of SLE and in non-Caucasian patients, as well as in patients with serological markers of activity (hypocomplementemia, anti-dsDNA positivity) the periodicity of these determinations should be greater (at least every 6 or 12 months) in these patients.4,20,22–24,26,27
The amount of proteinuria is the most important marker to assess the prognosis and efficacy of treatment in a patient with LN.28–31 Therefore, it should always be expressed with precision: amount of proteinuria in 24-h urine or protein/creatinine ratio in an isolated urine sample, generally collected in the first urination of the morning. Imprecise measurements (urine test strips, quantifications calculated by urine volume, etc.) should be avoided. Regarding urinary sediment, automated techniques have replaced traditional manual techniques in most centers. There are few studies comparing both techniques32,33: the available evidence suggests that automated techniques are valid for routine patient monitoring (red blood cell and leukocyte quantification per field), while manual sediments performed by expert personnel are superior for the detection and characterization of casts, dysmorphic morphology of red blood cells and other urinary abnormalities. For calculation of eGFR, the CKD-EPI formula is nowadays the most widely used. Table 3 summarizes the main analytical determinations recommended for the monitoring of patients with LN.
Clinical and analytical controls in patients with lupus nephritis.
At each visit, it should be assessed:
| |
| Initial evaluation | Periodic controls (frequency depending on the patient's situation and treatments prescribed) |
|
|
eGFR, glomerular filtration rate estimated by the CKD-EPI equation; uACR, urine albumin/creatinine ratio; uPCR, urine protein/creatinine ratio.
Although in general there is a correlation between the severity and type of LN and the analytical manifestations (Table S4), striking discordances are not uncommon (e.g., class IV LN with low proteinuria and hematuria and normal renal function).33 Furthermore not all changes in the analytical parameters of a patient with LN are due to LN activity. Careful evaluation of these changes is essential before attributing them simply to changes in SLE activity. Particularly, changes in body weight have an important influence on the amount of proteinuria. Table 2 summarizes the main conditions that may non-specifically influence the amount of proteinuria, urinary sediment and renal function. A decrease in renal function not accompanied by increases in proteinuria or changes in urinary sediment makes it necessary to think of causes independent of lupus activity. Changes in urinary sediment (hematuria, leukocyturia) without increases/reappearance of proteinuria and without changes in renal function requires to carefully rule out causes independent of LN.
Evaluation of serological markersRecommendations1.5.1 If LN is suspected, it is recommended to determine the serum anti-dsDNA antibody values (together with anti-C1q as available at each center), as well as complement C3 and C4 component values. These determinations should be performed at baseline and at follow-up.
1.5.2 When interpreting elevated anti-dsDNA and anti-C1q values and a decrease in complement, it should be kept in mind that they do not always correlate with histological activity and should not be used by them self alone for decision making. However, when determined together, they will rarely present normal values in episodes of proliferative LN activity (classes III and IV).
1.5.3 SLE patients with suspected renal involvement should have determination of lupus anticoagulant and antiphospholipid antibody – anticardiolipin and anti-β2 glycoprotein 1 (AAF) antibodies.
1.5.4 In cases of proliferative LN (type III or IV) with predominantly segmental, necrotizing lesions and significant extracapillary reaction, it is recommended to determine serum anti-neutrophil cytoplasmic antibodies (ANCA).
RationaleAnti-dsDNA antibodies are included in the diagnostic criteria for SLE, and are regularly used to monitor disease activity; n patients with LN these antibodies correlate with the type and activity of the disease, although with low sensitivity. They appear more frequently in proliferative classes (III and IV) than in mild classes or in membranous (class V).34 More than the amount of antibodies in absolute values, what is more relevant are the variations over time, mainly when evaluating the response to treatment and predicting a recurrence of nephritis.
Anti-C1q antibodies have been postulated as an alternative or a complement to anti-dsDNA in the diagnosis and follow-up of LN.35 They have a higher predictive value for proliferative forms, and, as with anti-dsDNA antibodies, the values are reduced after treatment, although none are predictive of CR.34
The specificity and the predictive capacity of LN activity is better using the combination of anti-dsDNA and anti-C1q antibodies than separately. The presence of both is associated with a high risk of recurrence while the absence of both corresponds to a low risk.34,36 The identification of a decrease in serum complement components C3 and C4 has been classically used as a diagnostic and monitoring tool of immune activity in SLE. However its correlation with LN activity is inexact, with a suboptimal sensitivity and specificity (about 75% and 71% respectively for C3 and 48% and 71% for C4).37 It should not be used as the only marker of activity but always within the clinical context, and preferably associated with the values of anti-dsDNA and/or anti-C1q. None of these previously mentioned serological markers has a sufficient sensitivity to identify renal flares, but they express an acceptable specificity, so that in the presence of normal values of these parameters (anti-dsDNA, anti-C1q, C3 and C4), LN activity is improbable.38
The presence of renal lesions related to a secondary antiphospholipid syndrome (APS) may imply a worse prognosis,39 so its study is recommended in every patient with LN, through the determination of lupus anticoagulant and AAF (anticardiolipin and anti-beta2 glycoprotein 1 antibodies) in serum.
The presence of ANCA in patients with LN correlates with a histological expression of a predominance of segmental proliferative forms and more glomerular necrosis, in addition to more lupus activity and worse baseline renal function.40 Determination of ANCA is recommended in the presence of this clinical and histologic profile.
The ideal biomarker should be one that helps to identify those patients with SLE at risk for developing LN, it should be able to aid in the diagnosis, to determine those patients at risk of progression, to discern between states of activity or irreversible chronic renal damage, and to identify those patients who require more intense and prolonged treatment. Despite the efforts and active research in this field, a marker that meets these requirements is still far from being available. At present, of the numerous candidates tested, none is sufficiently sensitive, specific or easy to measure to be incorporated into daily clinical practice. The answer will probably have to be sought in the future using the combination of various biomarkers, whether serum, urinary, histological and/or genetic.41
Treatment goalsDefinition of complete response, partial response and non-responseRecommendationsTreatment efficacy should be assessed by the achievement of complete remission (CR) or partial remission (PR) of LN, as defined in Table 4.
Renal outcome definitions: complete remission, partial remission, non-response, and relapse.
| Outcome _ _ | Definition |
|---|---|
|
|
|
|
|
|
|
|
eGFR, estimated glomerular filtration rate; CR, complete remission; PR, Partial remission; uPCR, urine protein/creatinine ratio.
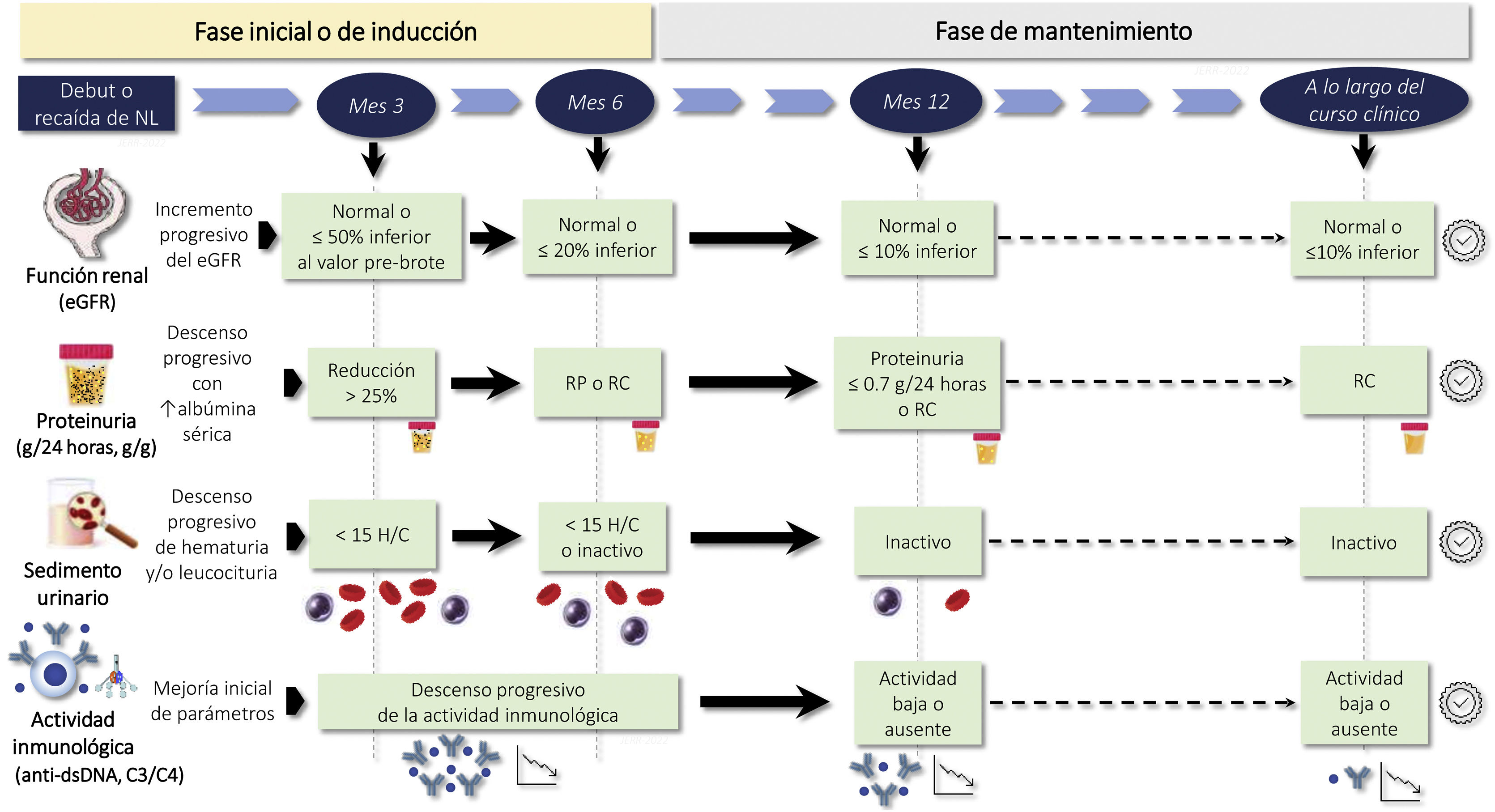
The period of time elapsed until CR or PR is an important prognostic marker, and targets should be set for the achievement of CR or PR.
The time to CR or PR is an important prognostic marker and targets should be set according to the characteristics of each patient (Fig. 2). The desirable goal in all cases is to achieve CR as quickly as possible, since long-term renal survival is significantly longer compared to those who achieve PR alone.
RationaleThere is no universally accepted criterion for defining CR or PR of LN, although all proposed definitions agree in pointing out as fundamental objectives the reduction of proteinuria and the recovery of renal function in those cases presenting with acute renal failure.30,42–44 The value of an improvement or normalization of urinary sediment has been questioned in recent studies and in fact is not included as criteria for CR or PR in some guidelines.44 The opinion of the group is that it is preferable to maintain this criterion, given that hematuria can be a sensitive marker of clinical activity and be useful especially in the evaluation of patients with previous relapses and residual proteinuria associated with histological lesions of chronicity.
A reduction in proteinuria >50% at 6 months is associated with better renal survival5 and a proteinuria level <0.7 g/24 h at 12 months is the best predictor of the likelihood of long-term terminal CKD.28,29 Therefore, in addition to obtaining CR or PR, the time elapsed until they are achieved is important. The evolution of each clinical and analytical parameter should be monitored carefully, verifying their progressive improvement within the time frame shown in Fig. 2. However, the time margins necessary to obtain CR or PR should be adjusted to the previous situation of the patient and to the severity and clinical characteristics of each LN flare.30,45,46 In patients with particularly severe forms these time limits can be less rigid, provided that a progressive clinical and analytical improvement is evident, without adopting treatment changes that are too early and unjustified.
Definition of relapseRecommendations2.2.1 A significant proportion of patients with LN have a relapse after achieving CR or PR, so ongoing periodic revisions are recommended in all patients with LN. The criteria for relapse are shown in Table 4.
RationaleBetween 10% and 50% of patients with LN present relapses and these are more frequent among patients who achieve a PR without reaching CR.47–49 Although the risk of relapse is higher during the first years after an episode of LN (especially when immunosuppression is reduced or suspended), relapses may occur at any time during evolution, even after decades of inactivity. For this reason, ongoing periodic monitoring is recommended in any patient who has undergone LN.
Disagreements in the criteria for defining a relapse are even greater than in the definitions of CR or PR.42–44,50 In general, they are based on the recurrence or increase of hematuria and the amount of proteinuria (Table 4). In the most severe cases, renal function deterioration is observed in addition to hematuria and proteinuria, although in relapses of LN (as in the first episodes) it is exceptional to observe renal function deterioration not accompanied by increased proteinuria and/or recurrence or increase of abnormalities in the urinary sediment (hematuria, leukocyturia, hematic casts). Causes independent of lupus activity are the most likely possibility in patients with deterioration of renal function without changes in proteinuria or urinary sediment (Table 2).
The diagnosis of relapse is more difficult in patients on PR and in those with repeated episodes of LN that have caused extensive lesions of chronicity and residual proteinuria. In these cases, and whenever the clinical and analytical evidence of relapse is doubtful, an additional renal biopsy is important and often decisive to determine the existence and degree of histological activity.
Therapeutic measuresLifestyle and dietary adviceRecommendations3.1.1 It is recommended to use active strategies to quit smoking, since it increases disease activity worsening the quality of life of patients. Likewise, it is recommended regular aerobic physical exercise (bike, walking or swimming) in people with low or moderate lupus activity.
Avoid overweight/obesity and sedentary lifestyle, as well as a diet low in saturated fats and rich in omega-3 fatty acids.
3.1.3 It is suggested to educate and advise patients on photo-protection measures throughout the year and the importance of their use for better control of the disease and to avoid the appearance of symptoms.
RationaleTobacco use has been associated with increased activity and severity of cutaneous lupus lesions.51 The role of tobacco in cutaneous lupus could be related to its influence on the action of antimalarials. In fact, there are some studies showing that smoking interferes with the therapeutic effect of antimalarials on cutaneous lupus.52
Two cohort studies53,54 and a cross-sectional study55 in a Caucasian population with a high percentage of women analyzed the role of tobacco consumption on lupus activity. One of the studies showed an increase in lupus activity measured by the SLEDAI index of 2.17 points (1.0–3.3) in patients who smoke, and in another a 6-point increase compared to non-smoking patients. In addition, tobacco use is associated with an increased risk of discoid rash and skin damage.53
Patients with SLE may initially have difficulty exercising due to asthenia, arthralgias and muscle discomfort, which usually improve over time. A number of studies have shown that aerobic physical exercise have positive effects on symptomatology, functional physical capacity, asthenia and disease activity.56,57
Although there are some studies performed using different diets in SLE patients, most of them are based on the effect of saturated fatty acids on SLE activity and on the positive effect of omega-3 fatty acid supplementation.58 The consumption of the fatty acids eicosapentaenoic acid and docosahexaenoic acid has a positive effect on disease activity in the short term, decreasing both global indices and individual symptoms.59 Low-dose supplementation of these fatty acids improves endothelial function and reduces the level of 8-isoprostanes.60
Photosensitivity is one of the main symptoms of cutaneous and systemic SLE. The role of ultraviolet radiation in the cutaneous manifestations of SLE is well recognized. This is based on the observation that the lesions are present in photo-exposed areas and that they are usually exacerbated in summer or during the weeks after sun exposure.
Recently, the European Society of Cutaneous Lupus Erythematosus evaluated in 1000 patients with cutaneous lupus a questionnaire on different preventive measures. The overall efficacy of photoprotectors in preventing skin lesions is 94.7%.61 Therefore, the use of broad-spectrum photoprotectors with a high sun protection index is recommended, which should be applied to all exposed areas 15−30 min before sun exposure and reapplied every two hours.
Blood pressure control and treatment of dyslipidemiaRecommendations3.2.1 It is recommended to maintain a systolic blood pressure (BP) below 120 mmHg, particularly in patients with proteinuria greater than 0.5 g/24 h. To achieve this goal, general measures and antihypertensive drugs should be prescribed sequentially, starting with ACE inhibitors or ARBs and adding other drugs until the goal is achieved.
3.2.2 Hyperlipidemia, whether or not associated with nephrotic syndrome, should be treated with lifestyle modification and statins with the same guideline indicated in the general population and according to the cardiovascular profile of each patient.
RationaleIntensive BP control in patients with CKD has been shown to increase protection against cardiovascular events and renal progression. This effect is especially relevant in patients with proteinuria >1 g/24 h.62
Lifestyle modification: salt restriction, weight normalization, regular exercise, etc., should be the first step in comprehensive treatment, both for BP and hyperlipidemia control. Salt restriction is a key element; failing to do so is sometimes responsible for not achieving the goal of lowering proteinuria and BP.
In relation to BP control, if the patient presents proteinuria >1 g/24 h, the patient should already be treated with RAAS (renin angiotensin aldosterone system) blockade, ACE inhibitors or ARBs, at the maximum tolerated dose.62
There is no evidence on what should be the next line of treatment in case of poor blood pressure control or intolerance to treatment with ACE inhibitors or ARBs. In case the reason for intolerance is not hyperkalemia or hypotension, a mineralcorticoid receptor antagonist or a direct renin inhibitor can be used.63,64
In the event that treatment with RAAS blockade fails to achieve optimal BP control, non-dihydropyridine calcium antagonists, such as diltiazem or verapamil, may provide additional modest reduction in proteinuria. Likewise, beta-blockers, alpha-1 blockers or non-mineralcorticoid diuretics may also help to control BP, but with minimal effect on proteinuria.
Hyperlipidemia in patients with lupus nephropathy may be related to a nephrotic syndrome, but may also reflect the impact of diet, genetic predisposition of the patient, or be a side effect of the treatment received: corticosteroids, mTOR inhibitors, CNI. There is no specific evidences on the treatment of hyperlipidemia in the patient with LN. In general, it is considered that it should be the same and with the same objectives, according to risk stratification, as in the general population. However, considering the increased risk of atherosclerotic disease in patients with decreased eGFR or proteinuria, as well as in patients with a persistent inflammatory state (as occurs in SLE), patients with LN should be considered as patients at high cardiovascular risk in therapeutic decision-making.65
Statins are generally well tolerated and effective; in case of intolerance, other second-line drugs such as fibrates, nicotinic acid or ezetimibe could be used.
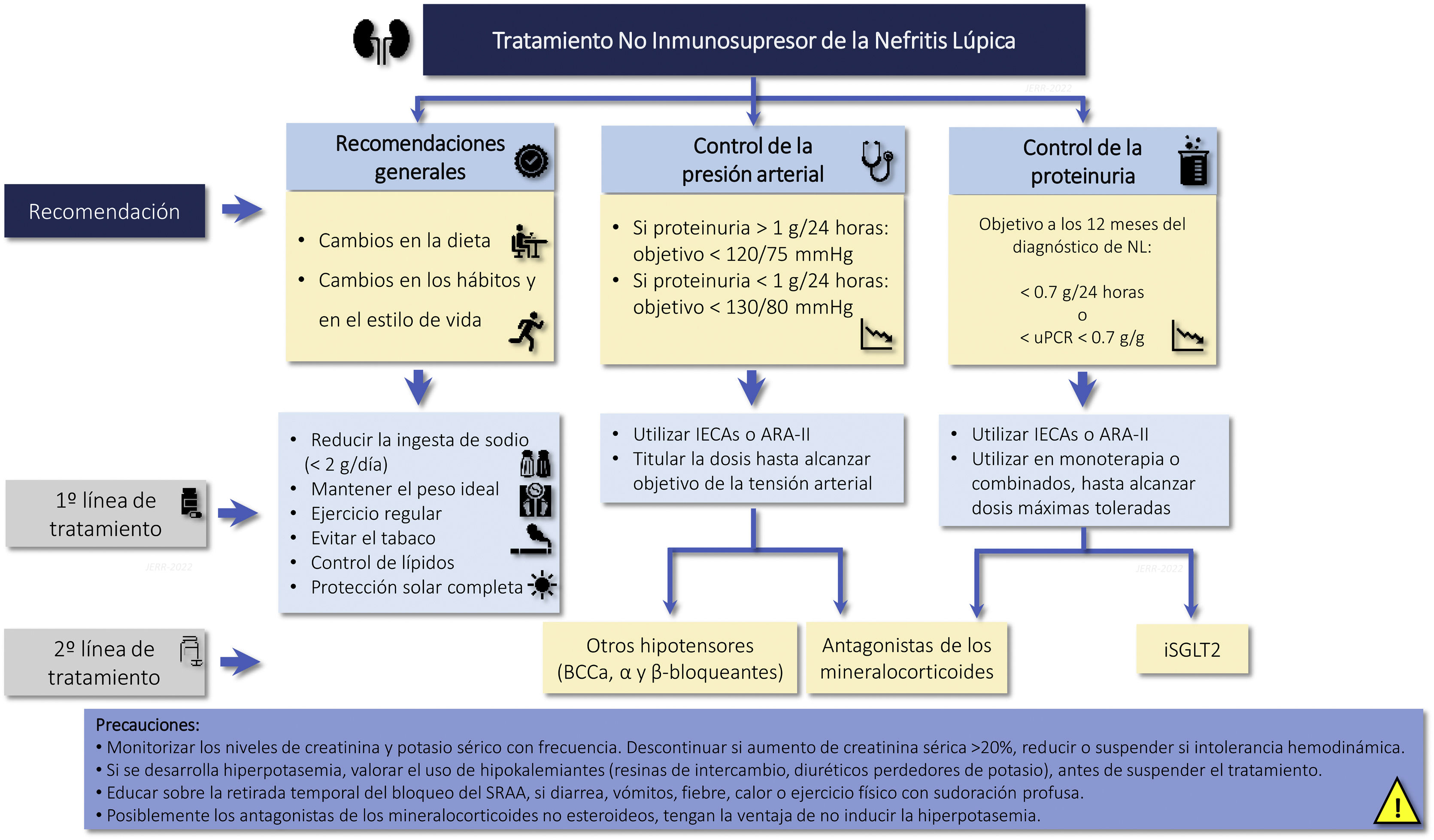
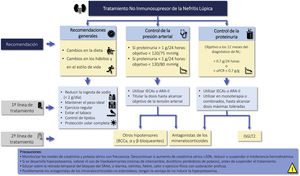
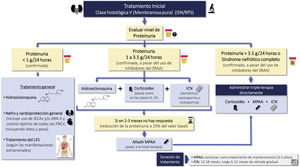
Cardio and renoprotective drugs without immunosuppressive effectRecommendations3.3.1 The amount of proteinuria is a decisive factor in the prognosis of patients with LN. The goal is to achieve proteinuria <0.7 g/24 h (or proteinuria/creatinine ratio <0.7 g/g) within the first year of treatment and a CR (proteinuria <0.5 g/24 h or uPCR <0.5 g/g) throughout the clinical course.
3.3.2 In patients with LN and proteinuria, it is recommended treatment with renin angiotensin-aldosterone system blockade at the maximum tolerated doses, ensuring a sodium-poor diet to optimize its effect.
3.3.3 If the target of proteinuria are not achieved with optimized use of RAAS blockers, it is suggested to consider the introduction of iSGLT2 (Fig. 3).
Renoprotective drugs in lupus nephritis.
ARA-II: Angiotensin II receptor antagonists; CCBs: Calcium channel blockers; ACEIs: angiotensin converting enzyme inhibitors; iSGLT2: Sodium-glucose tubular cotransporter 2 inhibitors; RAAS: Renin-angiotensin-aldosterone system; uPCR: urine protein/creatinine ratio.
Several observational studies have suggested that the renal prognosis of LN improves when proteinuria falls below 0.7 g/24 h.66,67 Until now, renin-angiotension system blocking drugs, ACE inhibitors or ARBs, were the only non-immunosuppressive drugs that had been shown to reduce proteinuria of a glomerular pathology in general. The efficacy of ACEIs or ARBs, as a group, is similar when used at equipotential doses, as are their side effects. Only cough is an exclusive side effect of ACE inhibitors. The objective should be achieved with the maximum tolerated dose, using monotherapy or combined treatment.62 The effect of RAAS blockade on proteinuria is increased by ensuring adequate dietary salt restriction (Na <90 mEq/day, or <2 g/day). Other drugs that block the RAAS, such as direct renin inhibitors or direct mineralcorticoid receptor antagonists, may be considered in patients who do not tolerate ACEIs or ARBs-II.
The hemodynamic effect of these drugs may increase serum creatinine by 10−20%, but this does not mean that they should be discontinued unless renal function deterioration is more intense. In situations of hemodynamic instability, as occurs in massive nephrotic syndrome, the hemodynamic effect can lead to functional renal failure. In these circumstances, the use of these drugs should be delayed or adapted to the hemodynamic situation.
Recently, a clinical trial has shown that treatment with iSGTL2 has an additional effect on proteinuria and cardiovascular protection in patients treated with maximal doses of RAAS blockade in non-diabetic glomerular pathology.65 Although there are no specific clinical trials in NL, their use could be considered when proteinuria has not decreased to the established target.
Similarly, finerenone, a non-steroidal mineralcorticoid receptor antagonist, reduced the risk of renal progression and cardiovascular event in patients with diabetic nephropathy who were already on treatment with RAAS blockade, without a clinically relevant increase in serum potassium.68 Endothelin A receptor antagonists are another pharmacological family that have been shown to reduce renal progression in patients with diabetic nephropathy.69 The concomitant use of these drugs with iSLGT2 may provide complementary nephroprotective mechanisms while decreasing the risk of fluid retention associated with endothelin antagonists due to the diuretic effect of iSGLT2.70
Recommendations for the use of renoprotective drugs in LN are summarized in Fig. 3.
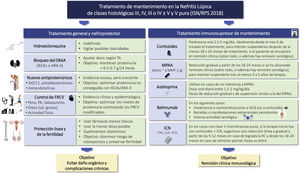
HydroxychloroquineRecommendationsHydroxychloroquine (HCQ) is recommended for all patients with NL who have no contraindications to its use.
3.4.2 The recommended dose of HCQ is 4−5 mg/kg/day up to a maximum of 400 mg/day. In patients with eGFR <30 mL/m2/1.73 m2 (including transplant recipients) or in dialysis, HCQ should be maintained, reducing the dose by up to 50%, without exceeding 200 mg/day (Table 5).
Hydroxychloroquine dose adjustment.
| Hydroxychloroquine dosage | |
|---|---|
| Initial | 4−5 mg/kg/day or 400 mg/24-h |
| Maintenance | 200−400 mg/day |
| Adjustment in renal failure |
|
CKD, Chronic kidney disease; eGFR, estimated glomerular filtration rate.
3.4.3 An ophthalmologic examination should be performed before initiating treatment with HCQ. In patients without risk factors for retinopathy, re-evaluate at 5 years and every year thereafter to rule out macular toxicity. Patients with risk factors for retinopathy should be reviewed annually since the beginning.
3.4.4 Cardiological follow-up of is recommended in patients treated with antimalarials; in case of adverse events, the drug should be withdrawn. In the event of neuromuscular abnormalities, withdrawal of the drug is also recommended. The adverse effects of antimalarials are summarized in Table S5.
RationaleAntimalarials interfere with the function of immunocompetent cells by blocking, among others the TLR 7 and 9 signaling of dendritic cells, inhibiting interferon-α production and decreasing the production of proinflammatory cytokines. All this plays a crucial role in the pathogenesis of SLE.71,72 The most widely used antimalarial due to its greater efficacy and lower toxicity is HCQ. Its administration reduces lupus flares and increases the survival of patients with SLE. It also protects against irreversible organ damage, decreases bone loss, and reduces the risk of thrombosis in patients with APS.72–75
In patients with LN, HCQ delays the onset of renal damage, is inversely associated with the development of stage ≥3 CKD, prevents renal flares, prolongs remission during concomitant treatment with MMF, and improves renal prognosis.76 In the LUMINA study, patients who developed LN during follow-up were compared with those who did not; patients taking HCQ had less frequent class IV LN, less disease activity, and received fewer doses of corticosteroids.77 In another study, 90 patients with LN were analyzed; the use of antimalarials was significantly associated with a lower occurrence of renal damage, defined as reduction of eGFR <50% from baseline and proteinuria >3.5 g/24 h.78
Treatment with HCQ is also associated with recovery of renal function in patients with impaired renal function and a lower probability of CKD.79,80 A longer duration of treatment with HCQ has been shown to be a protective factor against the appearance of lupus flare-ups after withdrawal of corticosteroids.80
In patients with CKD, treatment with HCQ should be maintained, although this practice is not widespread. A recent analysis of the American registry indicates that less than 30% of patients with LN in dialysis were receiving antimalarials.81 In patients with eGFR <30 mL/min/1.73 m2 (including transplanted patients) dose adjustment should be performed, since HCQ is metabolized by the renal route. Thus, the toxicity associated with these drugs increases in renal failure, since it is associated with the dose being accumulated.82 Although there is no consensus on the appropriate dose in patients with eGFR <30 mL/min/m2, a 50% dose reduction with a maximum dose of 200 mg/day is a reasonable estimate. Table 5 lists recommendations about HCQ dosing in patients with SLE.
The HCQ is well tolerated and rarely has to be withdrawn because of adverse reactions. Occasionally it can cause pigmentary changes in the macula, which may result in vision loss. To reduce the risk of maculopathy, an ophthalmological examination is recommended at the beginning of treatment. In the absence of risk factors for maculopathy, it is recommended to repeat the examination after five years and then annually. In the presence of at least one risk factor (dose >5 mg/kg/day, duration of treatment more than 5 years and presence of eGFR <60 mL/min/1.73 m2, the ophthalmological review should be performed annually from the beginning.83,84
The HCQ may produce cardiac abnormalities, mainly left ventricular hypertrophy with conduction disturbances and cardiomyopathies. Elevated of LDH and troponin levels are usually the initial signs that alert to the presence of these alterations, which are confirmed by ECG, echocardiography or magnetic resonance imaging.85 When cardiological alterations appear, withdrawal of the drug is indicated.83 Another adverse effect that requires withdrawal of antimalarial treatment is myopathy or peripheral neuropathy.
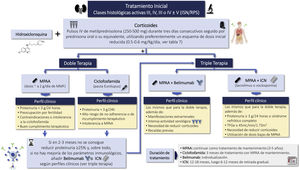
CorticosteroidsRecommendations3.5.1 Currently, corticosteroids are used in all LN treatment regimens. These drugs have immediate immunosuppressive and anti-inflammatory effects.
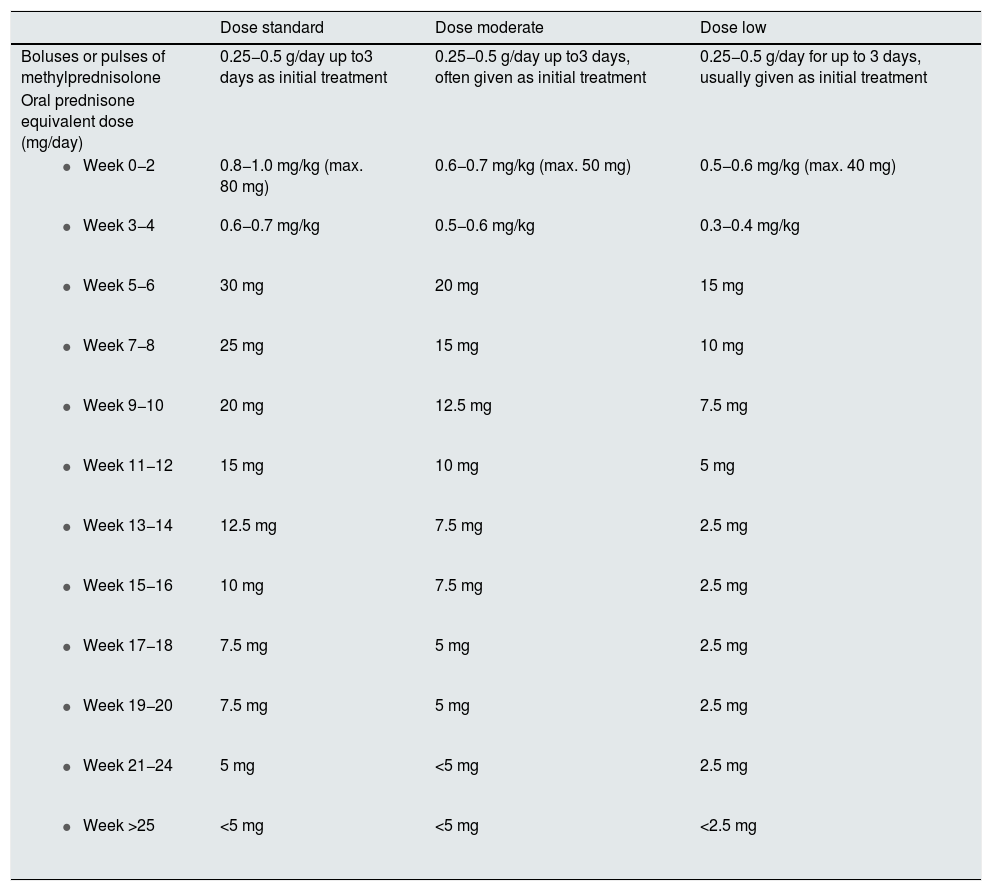
3.5.2 The dose, tapering regimen, and duration of corticosteroid regimens vary considerably and are largely based on opinion. Recent prospective studies have administered reduced doses of oral corticosteroids preceded by intravenous pulses of methylprednisolone in induction therapy.
3.5.3 In classes III, IV and III-IV/V we suggest a schedule with methylprednisolone pulses i.v. (250−500 mg/day) for three consecutive days followed by a reduced dose schedule.
3.5.4 Corticosteroids should be reduced to the lowest possible dose during maintenance, except when it is necessary to control extrarenal manifestations; discontinuation may be considered after patients have maintained clinical remission for at least 18–24 months.
RationaleOral corticosteroids are the mainstay of SLE treatment due to their high anti-inflammatory efficacy. They are used in combination with immunosuppressive drugs for the treatment of organ involvement in SLE, and to prevent the appearance of flares.86 Regarding their mechanism of action, 2 pathways have been described: genomic and non-genomic.87
The genomic pathway is activated by binding the glucocorticoid to an intracellular receptor, with subsequent internalization in the nucleus, and preventing the expression of genes involved in the inflammatory response, a process called "transrepression". At the same time, the transcription of molecules linked to gluconeogenesis, lipid and bone metabolism is stimulated, a process called "transactivation", responsible for most of the adverse effects.88 The genomic route requires hours to begin the effect; the non-genomic route is faster and more potent. Thus, with doses higher than 30 mg/day of prednisone or equivalent, the intracellular receptor is 100% occupied, so that higher doses will have no further effect.
The non-genomic pathway is activated since doses of 100 mg of prednisone or equivalent and has a faster and more intense anti-inflammatory effect, maximal from doses of 250 mg.89 There are no clinical trials comparing the efficacy of different doses of prednisone, so the 1 mg/kg/day regimen is not based on clinical evidence, although it is the most commonly used. The same happens with high doses of prednisone. Thus, in patients presenting with a clinical syndrome of rapidly progressive glomerulonephritis and/or with severe extrarenal manifestations, up to 3 daily doses of 500 mg each (range 250−500 mg/day) of 6-methyl-prednisolone are routinely administered.
In most clinical trials, corticosteroids are used in combination and with few restrictions, without taking into consideration their possible effects on the degree of response or toxicity. Due to the absence of studies in this regard, it is not possible to state that lower doses have similar efficacy. However, indirect data suggest that doses lower than 1 mg/kg/day are effective in the control of severe manifestations of lupus.90–92 Thus, some groups have shown that doses of prednisone lower than 30 mg/day with rapid tapering to 5 mg/day, combined with cyclophosphamide (CYC), HCQ and ACE inhibitors are at least as effective as higher dose regimens, with the added advantage of lower toxicity. To minimize side effects due to high cumulative exposure to corticosteroids, it is being used as an initial IV pulses followed by lower initial dose and/or more rapid tapering of oral corticosteroids.93 Results from a retrospective analysis of the Aspreva Lupus Management Study (ALMS) and the phase 2 AURA-LV trial suggest that lower doses of corticosteroids and mycophenolate than those used in ALMS may offer better long-term safety.94 Overall, there is a trend toward reducing corticosteroid exposure.95
Thus, doses of 2.5−5 mg/day have been proposed for the treatment and control of non-severe manifestations of SLE, although it is premature to recommend these regimens since they have not been evaluated in prospective clinical trials. Regarding the side effects of corticosteroids, prolonged exposure to corticosteroids has been found to be associated with significant irreversible morbidity and organ damage.96 A cumulative oral prednisone dose of 36.5 g (equivalent to 10 mg/day for 10 years) increases by 2-fold the risk of osteoporotic fractures, cataracts, and coronary heart disease.97 In situations where doses >60 mg/day are maintained, the risk of avascular necrosis and stroke increases 1.2-fold for every 2 months of treatment. In contrast, methylprednisolone pulses have not been associated with any of these complications, although a possible neurological involvement has been described. A relationship between oral prednisone dose and irreversible damage has been described, which increases significantly with dosed above 6 mg/day.98
Regarding long-term side effects, it was observed that they were increased after 5 years of treatment and were maintained for up to 15 years.99 In the case of pregnant patients, the use of prednisone has been associated with hypertension, preeclampsia, diabetes and premature delivery.100 A strategy to reduce side effects, it would be a daily dose of prednisone (or equivalent) <7.5 mg, after induction. In a recent open-label controlled trial (Evaluation of discontinuation of maintenance corticosteroid therapy in systemic lupus at rest trial [CORTICOLUP]) in 124 patients with stable and quiescent SLE (history of NL in 34% and 41%, respectively), it was compared continuation of prednisone 5 mg/day vs. discontinuation, and a significantly higher exacerbation rate was observed in those who discontinued prednisone.101 Most of the irreversible complications that have a negative effect on survival are related to oral corticosteroids, and doses above 5 mg/day increase the risk of organ damage. The important esthetic impact caused by prednisone should also be highlighted.
Therefore, the prescription of corticosteroids should be made taking into account the risk-benefit balance, and using schedules that allow saving corticosteroids in an early manner. There are situations where it should be consider whether to use of minimal doses of corticosteroids or even avoid its administration: diabetes, obesity (BMI > 30 kg/m2), latent infections (e.g., viral hepatitis, TB), active peptic ulcer, uncontrolled psychiatric disease and in other risk situations. It is proposed that, the need for high doses of prednisone in induction should be questioned and ensure the rapid tapering to maintenance doses no higher than 5 mg/day.43 In situations of greater severity or need for a rapid response, we should chose methylprednisolone pulses.
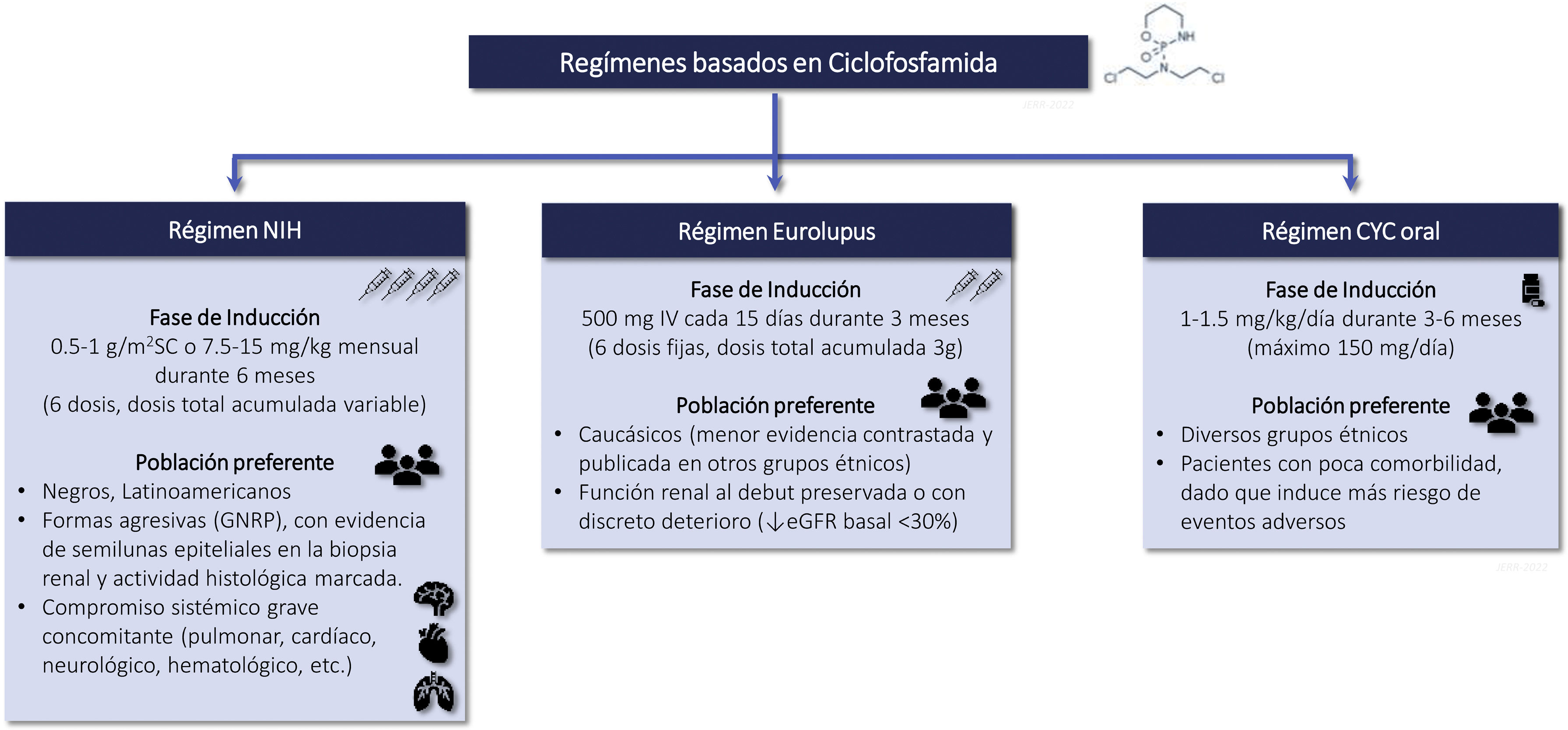
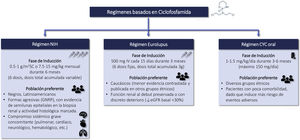
CyclophosphamideRecommendations3.6.1 Intravenous CTX is one of the drugs of choice in the treatment of class III-IV LN (with or without associated class V) in the induction phase. Its use is recommended preferably at low doses, for short cycles and associated with corticosteroids (Eurolupus guideline). CTX is especially indicated in cases of non-adherence to oral treatment. Higher doses of CTX can be used in cases of non-response or resistance to other treatments administered, with evidence of immunological and/or histological activity, (NIH guideline). The different CTX treatment regimens are summarized in Figure S2.
3.6.2 According to the NIH guideline, the dose and duration of oral or intravenous CTX treatment should always be adjusted to the patient's renal function, age and comorbidities in order to minimize its undesirable toxic effects (Table 6 and Table S6).
Cyclophosphamide dose adjustment based on patient characteristics.
| ||||
|---|---|---|---|---|
| IV Cyclophosphamide (bolus dose) | oral cyclophosphamide (daily dose) | |||
| eGFR (mL/min/1.73 m2) | eGFR (mL/min/1.73 m2) | |||
| Age | ≥30 | <30 | ≥30 | <30 |
| <60 years | 15 mg/kg | 12.5 mg/kg | 2.0 mg/kg/day | 1.5 mg/kg/day |
| 60−70 years | 12.5 mg/kg | 10 mg/kg | 1.5 mg/kg/day | 1.0 mg/kg/day |
| >70 years | 10 mg/kg | 7.5 mg/kg | 1.0 mg/kg/day | 0.5 mg/kg/day |
| ||||
|---|---|---|---|---|
|
Dose correction is only done for the NIH regimens (oral and intravenous).
Eurolupus regimen, the patient receives six fixed doses of cyclophosphamide (500 mg IV/dose) every 15 days for 3 months.
eGFR, estimated glomerular filtration rate; SC, body surface.
Mechanism of action and biological effects.
CTX is an alkylating agent derived from mechlorethamine, metabolized by the liver (cytochrome P450) and peripheral tissues into phosphoramide mustard (active molecule) and acrolein (urinary metabolite that may produce hemorrhagic cystitis). Oral administration has high bioavailability (>75%) and clinical efficacy similar to the intravenous route. Eighty percent is metabolized by the liver and 20% undergoes renal excretion. It produces immunosuppression, decreasing T-cell population (CD4+ >CD8+) and B-cell function by 30–40% and the synthesis of pathogenic autoantibodies.
Evidence of the benefit of CTX as an immunosuppressive agent in active and proliferative LN comes from pioneering studies by the NIH in the 70 s–90 s, when it was demonstrated the superiority of this intravenous drug associated with corticosteroids over corticosteroids alone on medium and long-term renal survival. Unfortunately, the doses used and the duration of treatment (up to 2 years) favored the development of important side effects, mainly drug intolerance, premature infertility and serious infections.91,102–105 More recently, the Euro-Lupus Nephritis Trial (ELNT or Eurolupus) demonstrated in a Caucasian population, with active LN (classes III, IV and V), preserved basal renal function (serum creatinine 1.15 ± 0.66 mg/dL) and nonnephrotic proteinuria (3.0 ± 2. 4 g/24 h), that lower doses of CTX (3 g) and a shorter duration of treatment (3 months) followed by azathioprine were equally effective as high doses of CTX for 6 months on clinical remission and progression to end-stage CKD in the short (41 months) and long term (115 months).90,106 The overall safety profile was similar in both groups, with more infections in the high-dose CTX group and more cancer episodes and deaths in the low-dose group, although without significant differences. The predominance of Caucasian population and with preserved basal renal function does not allow extrapolating these results to other ethnic groups (blacks, Latin Americans, Asians) or to more aggressive forms, where clinicians may be inclined to use higher doses CTX and for a longer period of time.
Oral CTX is an effective regimen for achieving short- and long-term CR, even as compared with mycophenolate, but it increases the risk of adverse effects since it involves 3 times more cumulative CTX doses than intravenous regimens.49 It is the least used regimen, but with adequately corrected doses, it is a valid alternative.
Evidence published since the 1970s has positioned CTX as a first-line drug in the initial or induction therapy of proliferative LN (classes III and IV ± V), including the most aggressive (rapidly progressive) forms or in cases that are resistant to other immunosuppressive treatments. The most recent recommendations advocate lower doses and shorter treatments periods, stressing that they should always be adjusted to individual patient characteristics.107,108
Adverse effects of CTX
Adverse effects are directly related to the dose administered, the total cumulative dose, the duration of treatment and the patient's comorbidities. The most frequent and early adverse effects are cytopenias, gastrointestinal symptoms, infertility and infections, while in the long term the risk of neoplasms is increased. Table S6 shows the adverse effects associated with the use of CTX, the preventive measures and the most important risk factors for their occurrence.42,109,110
Mycophenolate mofetil and mycophenolic acid analoguesRecommendations3.7.1 Mycophenolate mofetil mycophenolate (MMF)/mycophenolic acid (MPA), with possible ethnic differences, is together wth CYC and corticosteroids, one of the drugs of choice in the treatment of Class III-IV LN in the induction phase.
MPAA is the treatment of choice for maintenance of LN. It is contraindicated during pregnancy.
The recommended dose for induction is 2 g/day (MPA 1.44–2.16 g/day) and 1−2 g/day during the maintenance phase.
RationaleMMF is a potent, selective and reversible inhibitor of the enzyme IMPDH (ionosinmonophosphate dehydrogenase), a key enzyme in the de novo synthesis pathway of purines required for clonal expansion of B and T cells and thus for T cell-mediated immunity and antibody synthesis. The mechanism of action of MPA primarily affects lymphocytes, as other cells are able to use alternative rescue pathways to synthesize nucleotides. The use of MMF is associated with adverse effects mainly hematological and gastrointestinal.111
The MPA is an enteric-coated prodrug designed to improve gastric tolerance to the drug since mycophenolic acid release occurs in the stomach in the case of MMF and in the small intestine for MPA. Patients on MMF treatment can be safely converted to MPA, in the conversion it must be taken into consideration that as a consequence of differences in their molecular weight, the 720 mg dose of MPA releases a similar amount of mycophenolic acid as 1000 mg of MMF.112
The MMF/MPA is contraindicated during pregnancy because it increases the risk of congenital malformations and miscarriage. Although it is not known whether it is eliminated through breast milk, its use is not recommended during breastfeeding. It is also recommended that both men and woman taking this drug adopt effective contraceptive measures during treatment and for up to 90 days after discontinuation.111
Several studies have shown that treatment with MPAA combined with corticosteroids has similar efficacy of renal response to CTX oral or pulses when administered during induction113–115; the use of bolus CTX should be reserved for patients with poor adherence to oral treatment. There is a certain tendency in routine practice to advise the use of CTX in patients with more clinically aggressive forms of presentation, with rapidly progressive deterioration of renal function, or with histology showing the presence of crescents and/or necrosis. However, in clinical trials these patients have not been included and therefore there is no evidence of superiority of any treatment for this type of patient. In post hoc analyses of the ALMS study, long-term use of CTX did not imply fewer relapses and better long-term prognosis.
In contrast, MMF/MPA has shown superiority in patients with Latin American ancestry; in patients of African ancestry it showed numerical superiority although without statistical significance and, on the other hand, it showed inferiority (numerical, not statistically significant) in the Asian population.49 Its use would be especially indicated in patients with a priori infertility problems, with moderate cumulative doses of CTX.
Although clinical experience has shown that MMF is well tolerated with few side effects, the incidence of side effects reported in the ALMS clinical trial was similar between the two, although with a different profile. The number of deaths in the group that included MMF was nine and five in the group that included CTX, most of them related to infectious complications. In studies performed in populations without LN that received MMF treatment, it was observed that the dose of 3 g/day (the one used in the ALMS study) was associated with more adverse effects without improving its efficacy.
During maintenance, MMF/MPA has been shown to be superior to azathioprine in consolidating the response to induction therapy and preventing relapses in subsequent years.116 It also showed fewer long-term side effects: leukopenia and elevated transaminases. Azathioprine may be an alternative when MMF/MPA is not available, when the patient is intolerant, or during pregnancy.
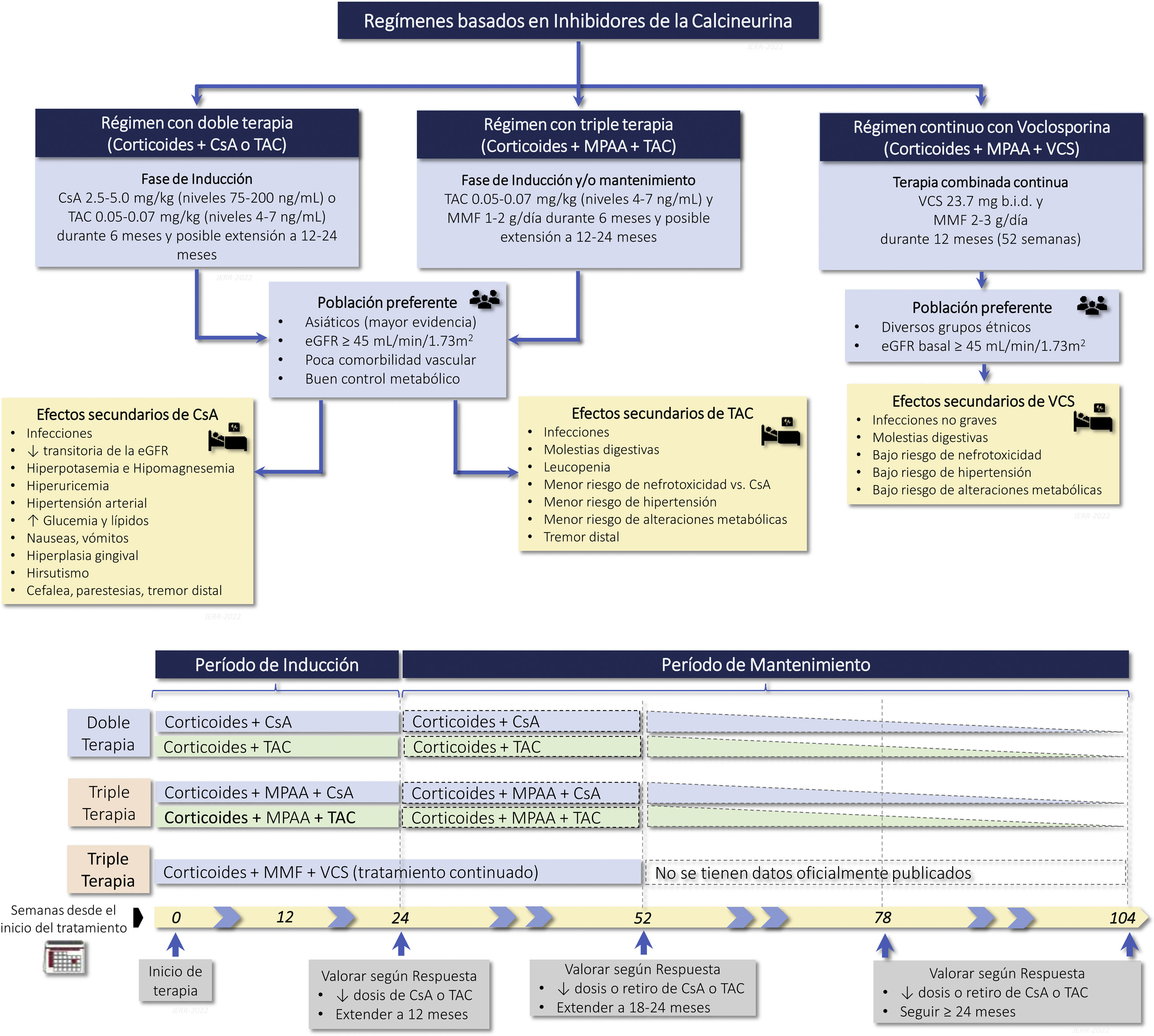
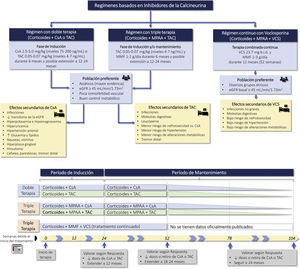
Calcineurin inhibitors (CNI) (cyclosporine, tacrolimus, voclosporin)Recommendations3.8.1 CNI (cyclosporine or tacrolimus) have demonstrated similar efficacy as compared with mycophenolate or CTX therapy in induction therapy. Therefore they are used in association with corticosteroids as initial therapy in class III and IV LN (with or without associated class V), in patients who develop intolerance, adverse effects or limited response to mycophenolate and in whom the use of CTX is not a therapeutic option.
3.8.2 Combination therapy, triple therapy or "multitarget therapy" (corticosteroids, mycophenolate and CNI) has demonstrated significant superiority to corticosteroids and CTX in NIH regimens or to corticosteroids and mycophenolate as induction therapy in patients with class III and IV LN (with or without class V). Figure S3 shows various treatment regimens with CNI, as well as the most frequent adverse effects.
3.8.3 Triple therapy with voclosporin, corticosteroids, and mycophenolate has demonstrated significant superiority over corticosteroids and mycophenolate in inducing remission in patients with class III and IV LN (with or without associated class V) with an estimated baseline glomerular filtration rate equal to or greater than 45 mL/min/1.73 m2, allowing a rapid reduction in the dose of corticosteroids.
RationaleMechanism of action and biological effects.
Calcineurin inhibitors are immunosuppressive drugs que incluyen cyclosporine A (CsA), tacrolimus (TAC) and voclosporin (VCS).
CsA is a lipophilic antibiotic peptide that forms a complex with cyclophilin, inhibiting calcineurin and inactivating T-lymphocyte nuclear transcription factor, which is necessary for the synthesis of proinflammatory cytokines such as IL-2, IL-3, IL-6, TGF-ß and IFN-γ. At the podocyte level it blocks calcineurin-dependent dephosphorylation of synaptopodin, preventing its degradation mediated by cathepsin L. This preserves the actin cytoskeleton and decreases the development of proteinuria.117–119
TAC is a macrolide antibiotic produced by Streptomyces tsukubaensis, structurally different from CsA but with a similar mechanism of action, which is the binding to the FK-binding protein (FKBP). It is 10–100 times more potent than CsA.117–119 It produces the same biological effects and has the same clinical indications as CsA.
VCS is a modified CsA analog recently approved for the treatment of LN. Its mechanism of action is identical to CsA, with greater immunosuppressive potency and better tolerability. It does not modify mycophenolate levels, does not require monitoring or dose adjustment, and produces less nephrotoxicity.120 The pharmacological properties and biological effects of each are shown in Table S7.
CsA significantly reduces proteinuria and lupus activity.104,121–123 The widespread use is limited by its short duration (6–12 months), relapses after discontinuation and nephrotoxicity. It has comparable efficacy to azathioprine as maintenance therapy.124
Tacrolimus has been shown to decrease proteinuria earlier than CTX in the Chinese population with an acceptable safety profile125–127 and it is as effective as mycophenolate in induction therapy.128–130 At 10-year follow-up tacrolimus-azathioprine therapy is comparable to mycophenolate-azathioprine in CR at 6 months, renal and extrarenal flare and renal survival.131 More recently, the so-called "multitargeted therapy" (triple therapy with corticosteroids, mycophenolate and tacrolimus) was also shown in Asian population to be a valid option as induction therapy, using fewer doses of immunosuppressants and with a better safety profile than CTX, although some studies showed a higher incidence of infections.132,133 A recent meta-analysis confirmed the benefit of multitarget therapy in achieving CR and/or PR, improvement in immunological parameters, decrease in lupus activity and fewer adverse events than CTX, except in hypertension.134 As maintenance therapy, it has demonstrated similar rates of complete response and renal relapse in Chinese patients as azathioprine, but with fewer non-serious adverse effects.93
The VCS is a CsA analog with greater immunosuppressive potency that does not alter mycophenolate levels.120 The phase 2 AURA-LV multicenter study showed that oral VCS at a dose of 23.7 mg every 12 h for 48 weeks added to standard therapy was superior to placebo in achieving CR at 24 and 48 weeks (32.6% vs. 19.3% and 49.4% vs. 23.9%, respectively). No nephrotoxicity was observed with VCS, but there were more serious adverse events (28.9% vs. 15.9%), including non-drug-related deaths in the first 2 months of treatment. The phase 3 AURORA 1 study in 357 nephrotic patients with active lupus nephritis and baseline eGFR ≥45 mL/min/1.73 m2 confirmed the superiority of oral VCS over placebo after 1 year of treatment in inducing CR and PR (41% vs. 23% and 70% vs. 52%, respectively).135 This benefit was consistent across diverse ethnic groups, independent of age and histologic class. It also induced an earlier reduction in proteinuria (<0.5 mg/mg). Importantly, there were no differences in adverse events or deaths. VCS did not induce metabolic abnormalities or nephrotoxicity. It remains to be evaluated the risk of relapse, its long-term effectiveness and safety and the possibility of subclinical nephrotoxicity assessed by renal biopsy and its efficacy in more severe cases.
Adverse effects are numerous and common to all CNIs, although there may be some differences among them. In general, they induce nephrotoxicity, hyperkalemia, hypomagnesemia, hyperuricemia, hypertension, hyperglycemia, hyperlipidemia, gastrointestinal discomfort, hepatic dysfunction, hirsutism, gingival hyperplasia, distal tremor, headache, altered mental status, seizures, increased infections and possible development of neoplasms.117 The incidence of nephrotoxicity, hypertension and metabolic alterations is lower with VCS.120,135
AzathioprineRecommendationsAzathioprine is not a drug of first choice in LN. It is a good choice as a substitute for MMF/MPA in intolerant patients or in pregnant women.
3.9.2 The starting dose of azathioprine in lupus nephritis is 1.5−2 mg/kg/day. In patients with renal insufficiency, the dose should be adjusted according to clinical criteria based on hematologic response.
3.9.3 The main adverse effect of azathioprine is bone marrow depression, which usually presents with leukopenia, but anemia and/or thrombocytopenia may also occur.
The joint use of azathioprine and allopurinol should be avoided because it is associated with a high risk of myeloablation.
RationaleAzathioprine is an immunosuppressive agent, an analog of 6-mercaptopurine, which acts as an antimetabolite, interfering with purine synthesis, arresting the cell cycle and reducing IL-2 secretion. The effect of 6-mercaptopurine, which is obtained from azathioprine through different metabolic pathways, most notably xanthine oxidase, causes a reduction in intracellular purine synthesis, resulting in a decrease in circulating T and B lymphocytes. It is also responsible for apoptosis of circulating T lymphocytes.136
The initial dose of azathioprine in LN is usually 1.5−2 mg/kg/day, and blood count monitoring is recommended 2 weeks after initiation of treatment. Maximum doses are 3–3.5 mg/kg/day. During dose escalation, a CBC is recommended every 4–6 weeks and, after reaching a stable dose, at least every three months during the first year. If the patient presents leukopenia or thrombocytopenia, the dose should be reduced by 50 mg or withdrawn if necessary. There are no specific data regarding dose reduction in renal insufficiency, but dose adjustment is recommended according to clinical criteria and response.43,137
The main adverse effect of azathioprine is bone marrow depression, which is reversible and dose-dependent. It is usually expressed as leukopenia, although it can also produce anemia or thrombocytopenia. Close monitoring of the blood count is recommended at the start of treatment or after dose increase.136
Occasionally, the drug may cause nausea, vomiting and gastrointestinal discomfort, which generally disappear if the drug is taken after meals. In a small percentage of patients, there have been described alterations in liver biochemistry, with a pattern of cholestasis, for which reason analytical monitoring of liver enzymes and, rarely, pancreatitis are also recommended.136
Patients with thiopurine S-methyltransferase deficiency (genetic polymorphisms) and those with a slow metabolizing phenotype for NUDT15 present a greater risk of hematologic toxicity due to azathioprine,138,139 but it has not been studied whether there is benefit in performing a genetic study of these patients prior to initiating this treatment.
The major drug interaction to take into account when starting azathioprine is allopurinol, as their co-administration significantly increases the risk of adverse effects of azathioprine. Co-administration should be avoided and, if necessary, the dose of azathioprine should be significantly reduced (50–75%) and closely monitored (blood count at two weeks and monthly for at least the first three months). There is not enough published experience on the joint use of azathioprine and febuxostat.140
Currently, azathioprine is not a drug of first choice in LN, since MMF/MPA has shown superiority in both induction and maintenance therapy in preventing relapses.116,141 Therefore, the use of azathioprine is a good alternative when MPAA is not available (economic cost), in patients intolerant to MPAA or in pregnancy.
In women with LN who wish to become pregnant or are pregnant, azathioprine is a good option because, although it crosses the placenta, the fetal liver lacks the enzyme that converts azathioprine to 6-mercaptopurine, its active metabolite, and therefore, it does not produce effects at the fetal level. Azathioprine exposure has been associated with increased risk of low birth weight and preterm delivery.142
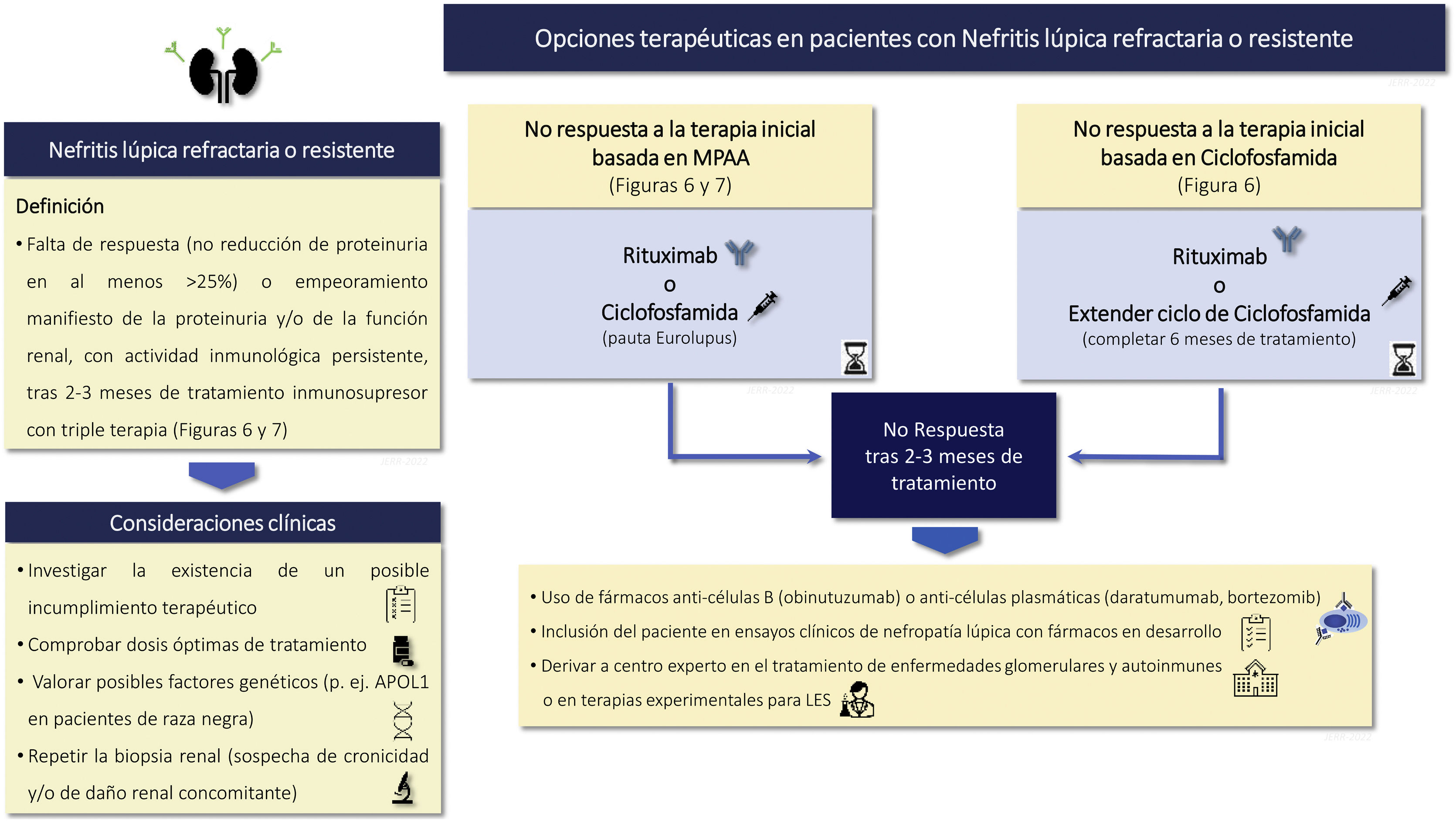
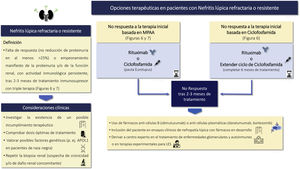
Rituximab and other anti-CD20Recommendations3.10.1 Although not proven in controlled clinical trials, rituximab may be an effective and safe alternative for the treatment of LN refractory to first-line therapy.
3.10.2 In the coming years, the profile of the patient with LN who is a candidate for treatment with new anti-CD20 agents such as obinutuzumab should be established.
RationaleMechanism of action and pharmacology.
Rituximab is a glycosylated immunoglobulin (Ig) containing the constant regions of human IgG1 and the variable region sequences of murine light and heavy chains that binds specifically to the CD20 antigen, a non-glycosylated transmembrane phosphoprotein expressed on mature pre-B and B lymphocytes.
The Fab domain of rituximab binds to the CD20 antigen on the surface of B lymphocytes, whereas the Fc domain can recruit effectors of the immune response to mediate lysis of B lymphocytes. Possible mechanisms of effector-mediated cell lysis include complement-dependent cytotoxicity as a result of C1q binding and antibody-dependent cellular cytotoxicity mediated by one or more Fcγ receptors on the surface of granulocytes, macrophages, and NK cells. Binding of rituximab to the CD20 antigen of B lymphocytes has also been shown to induce cell death by apoptosis.
Biological effects
In patients with SLE, an immediate decrease in the number of B lymphocytes in peripheral blood is observed after two 1000 mg infusions of rituximab separated by an interval of 14 days. The number of B lymphocytes in peripheral blood begins to increase from week 24 and evidence of repopulation is observed, in most patients, at week 40, regardless of whether rituximab is administered in monotherapy or in combination.143–145
B lymphocytes play a critical role in the pathogenesis of SLE, which makes rituximab, a B lymphocyte depletion therapy, an attractive therapeutic option in SLE and LN. Despite failure in one clinical trial, rituximab has shown encouraging results in the treatment of treatment-refractory LN in real-life clinical scenarios. In meta-analyses, rituximab has been shown to be effective, especially in LN that is refractory to standard therapy. Most studies have been retrospective, but a single-center prospective observational cohort study demonstrated the efficacy of a corticosteroid-sparing regimen of rituximab and MMF for NL.143–145 Moreover, rituximab can be used in combination with other immunomodulators such as CTX, calcineurin inhibitors or belimumab and is a mainstay of sequential therapy in refractory LN. Results from the French autoimmunity and rituximab registry revealed a 74% response rate when rituximab was added to another immunosuppressive agent.146,147
Despite the reported evidence of rituximab efficacy in refractory disease, a recent case series showed that five out of seven patients had no improvement after rituximab when accompanied by incomplete B-cell depletion.148 Thus the role of monitoring lymphocyte subpopulations in establishing the dose and frequency of rituximab administration remains to be clarified.
Adverse effects
Most of the adverse effects are mild to moderate in intensity, but some of them may require treatment. The most frequent are:
- (A)
Reactions during the infusion or after 2 h of the first dose the patient may have, fever, chills and tremors. Other less frequent adverse effects are: flushing and itching of the skin, nausea and vomiting, tiredness, headache, difficulty breathing, edema of the tongue or throat, itching and nasal congestion, vomiting, or palpitations.
In some patients, a decrease in the number of red blood cells, white blood cells or both may also occur.
In patients with heart disease or angina pectoris, they may worsen.
After the second infusion, these reactions are less likely to occur.
- (B)
Infections: infections may be more easily developed after treatment with rituximab. Cases of reactivation of hepatitis B and other viral diseases have been reported,
- (C)
Progressive Multifocal Leukoencephalopathy.143–147
Obinutuzumab, instead, produces direct B-cell death and cell-mediated cytotoxicity with significantly higher antibody dependence compared to rituximab and also exhibits lower complement dependence to produce for B-cell depletion. The role of obinutuzumab in LN has recently been evaluated in the NOBILITY study. An improved renal responses up to week 104 were observed in patients with LN who received obinutuzumab plus standard therapies compared to standard therapies alone. Obinutuzumab was well tolerated and had no more side effects than the control group. These results are pending confirmation in the ongoing phase 3 REGENCY study.149
BelimumabRecommendations3.11.1 Belimumab, added to standard LN therapy, has demonstrated significant superiority over placebo in obtaining improvement in LN, with an acceptable safety profile.
Belimumab has demonstrated efficacy in reducing extrarenal clinical manifestations of SLE and preventing relapses.
RationaleBelimumab is a human IgG1λ monoclonal antibody that specifically binds to the soluble form of human B-lymphocyte stimulatory protein (BLyS, also known as BAFF and TNFSF13B). BLyS levels are elevated in some SLE patients and may play a role in the pathogenesis of the disease.150 Belimumab blocks the binding of soluble BLyS (B-cell survival factor) inhibits B-cell survival and reduces B-cell differentiation to immunoglobulin-producing plasma cells.151,152 It was approved by the FDA for patients with SLE following the results of two phase 3 studies (BLISS-52 and BLISS-76).153 In both studies, belimumab demonstrated a ≥4-fold reduction in SLE activity indices (SLEDAI) and improvement in SLE response index (SRI) at week 52. These studies excluded patients with severe renal involvement demonstrated by renal biopsy. However, a post-hoc study analyzed the renal response of 267 patients over 52 weeks and demonstrated significantly greater reductions in proteinuria in belimumab-treated versus placebo-treated patients, which was the main rationale for the phase III Belimumab International Study in LN (BLISS-LN).153,154
The BLISS-LN assigned 448 patients with class III-V NL biopsy in a 1:1 ratio to intravenous belimumab 10 mg/kg or placebo, added to standard therapy (oral corticosteroids and MMF or IV CTX followed by azathioprine for a total of 104 weeks).155 Randomization was according to induction regimen and racial group. Belimumab was initiated within the first 8 weeks after renal biopsy and was continued on a regular basis (monthly) for 2 years of follow-up. The main objective of the study was to analyze renal response (defined as a urine proteinuria/creatinine ratio ≤0.7 g/g, a reduction in eGFR of no more than 20% from baseline or an eGFR ≥60 mL/min/1.73 m2 and not requiring rescue therapy) at week 104 of the study. At the end of the study, 43% of patients treated with belimumab achieved renal response compared to 32% in the placebo group (OR 1.6, p = 0.03), this difference was also significant at week 52 (47% vs. 35%). Regarding, the criterion of complete renal remission (defined as a proteinuria/creatinine ratio ≤0.5 g/g, an eGFR reduction of no more than 10% from baseline or an eGFR ≥90 mL/min/1.73 m2), patients treated with belimumab achieved 30% compared to 20% of the placebo group (OR:107, p = 0.02). The group of patients receiving belimumab had a lower incidence of renal events and/or death during the study. While these results can be considered favorable, it should be noted that observations from subgroup analyses highlighted a benefit of belimumab for patients who received it together with MMF, but not for patients who received belimumab together with CTX/AZ (patients with a more severe renal disease profile) and in black patients.156,157 Belimumab did not demonstrate benefit in patients with higher amounts of proteinuria (>3 g/g).153,154 However, in a recent post-hoc analysis of BLISS-LN, belimumab had a dual beneficial effect, decreasing the number of recurrences and slowing the decline of eGFR thus preserving renal function.158 Finally, it should be highlighted the role of belimumab in the reduction or withdrawal of corticosteroids in patients with LN11 and its possible combination with rituximab in refractory LN.159,160 Although further studies are needed to define the profile of the candidate for treatment with this drug,161 belimumab is the first biologic agent approved for the treatment of NL, constituting an effective and safe alternative for the treatment of LN.
Other drugsRecommendations3.12.1 It is recommended to participate and include patients with NL in controlled clinical trials with emerging treatments in order to explore and consolidate new therapies in this pathology. Table S8 summarizes the drugs under study for the treatment of NL at the time of writing.
RationaleThe improved pathophysiological understanding of SLE and LN has led to a significant increase in interest during recent years in exploring new therapeutic targets through clinical trials with new drugs.162
Interferon is one of these targets. It is known that mainly type 1 interferon is actively involved in the pathophysiology of SLE and LN. For this reason it has been considered of interest to block its effect with the use of anifrolumab, an anti-interferon type 1 receptor monoclonal antibody. Phase III studies (TULIP-2) in SLE have shown a greater efficacy than placebo, together with the standard base treatment, in reducing activity in moderate-severe lupus. Its use in this patient profile has been approved in the USA. A phase II study in LN, together with corticosteroids and mycophenolate (TULIP-LN study NCT02547922) is being currently completed. Preliminary results, pending definitive publication, indicate that the primary endpoint of proteinuria reduction at week 52 has not been achieved, with the CR rate at week 52 (secondary endpoint) being numerically higher with the high dose of anifrolumab, although without statistical significance.163
Other therapeutic targets currently being explored in various clinical trials are:
- -
Complement blockade, based on a strong evidence of pathogenic involvement. There are ongoing Clinical trials in proliferative LN with ravulizumab (an anti-C5 monoclonal antibody; NCT04564339), narsoplimab (an anti-MASP-2 monoclonal antibody; NCT02682407), APL-2 (a C3 inhibitor; NCT03453619), and vemircopan (ALXN2050, an oral factor D inhibitor; NCT05097989). Eculizumab, the most experienced anti-C5 monoclonal antibody, has not been explored in a controlled manner in LN and its use is limited to cases with APS and/or signs of refractory TMA.164
- -
Co-stimulation blockade. There are clinical trials in proliferative LN with Iscalumab (CFZ533, an antiCD40 monoclonal antibody; NCT03610516), and BI 655064 (an antiCD40 monoclonal antibody; NCT03385564 and NCT02770170).
- -
Various cytokines. There are clinical trials in proliferative LN with secukinumab (anti-IL-17A monoclonal antibody; NCT04181762), and guselkumab (anti-IL-23 monoclonal antibody; NCT04376827).
4.1.1 In cases with class I LN we recommend treatment with HCQ, general nephroprotective measures and treatment of SLE according to the extrarenal manifestations that may present (Fig. 4).
Therapeutic attitude and general measures in histological classes I and II.
ARA-II: Angiotensin II receptor antagonists; CVRF: Cardiovascular risk factors; ACEIs: angiotensin converting enzyme inhibitors; ISN/RPS: International Society of Nephrology/Renal Pathology Society; SLE: systemic lupus erythematosus; MPAA: Mycophenolic Acid Analogues; RAAS: Renin-angiotensin-aldosterone system.
4.1.2 In patients with class II LN presenting proteinuria <1 g/24 h and normal urinary sediment we recommend the same treatment as in class I LN (Fig. 4).
4.1.3 In patients with class II LN presenting proteinuria >1 g/24 h and/or sediment with hematuria of glomerular origin despite optimized RAAS blockade, we recommend re-evaluating the renal biopsy or performing a new biopsy to rule out another class of LN. If class II LN is confirmed, we suggest adding corticosteroids and MPAA to the general treatment for 6–12 months with subsequent gradual withdrawal according to evolution (Fig. 4).
RationaleType I and II LN are usually considered mild forms of renal involvement with a good prognosis.165 There are no controlled studies that support a specific therapeutic regimen in this group of patients; although it is recommended that all patients be treated with an immunomodulatory regimen with HCQ. There is consensus that cases with mild proteinuria <1 g/24 h can be managed conservatively by instituting or intensifying RAAS blockade with ACEI or ARB-II independently of BP, both for their renoprotective effect and their potential anti-inflammatory effect.165,166 In addition, strict control of BP and dyslipidemia and other cardiovascular risk factors should be insisted upon (sections 3.1–3.4).
It should be noted that the classes of LN are not fixed in one category, they can evolve and progress over time. The presence or progression of proteinuria to >1 g/24 h in a persistent manner despite measures instituted with optimized RAAS blockade, with or without hematuria or renal dysfunction, makes it advisable to reconsider the diagnosis with a directed re-reading of the previous renal biopsy or by performing a new renal biopsy to rule out progression to more severe forms. In the different series of repeated biopsies studied, around 70–78% of cases of non-proliferative LN it is observed a progression to proliferative or membranous forms, which changes completely their therapeutic approach and prognosis.16
The presentation of LN type I and II with established nephrotic syndrome is considered within the setting of PL (see section 4.2).
Patients who after histological reassessment remain in class II with nephrotic proteinuria despite optimized RAAS blockade may benefit from corticosteroid regimens in monotherapy or preferably in combination with other immunosuppressants such as MMF.167,168
Lupus podocytopathyRecommendations4.2.1 In cases diagnosed with LP we recommend treatment similar to that of nephrotic syndrome due to minimal lesions, including corticosteroids as first line of treatment, calcineurin inhibitors as second line in corticoid-resistant patients and rituximab, CTX (Eurolupus guide), MPAA or calcineurin inhibitors as therapeutic alternatives in corticoid-dependent or frequently relapsing patients (Fig. 5).
4.2.2 In cases of corticosteroid-resistant LP in which there is deterioration of renal function not explained by other causes and/or hematuria of glomerular origin, we recommend performing a new renal biopsy to rule out a change in histologic class (Fig. 5).
RationaleThere are currently no randomized clinical trials on LP. Observational studies show that similar to minimal lesion (ML) nephrotic syndrome, they usually respond to short courses of high doses of corticosteroids (76% CR, 90% PR at 4 weeks of treatment),169,170 but with a high rate of relapse.170,171
The forms of LP associated with focal segmental glomerulosclerosis (FSGS) seem to have worse response to corticosteroid monotherapy (CR 22.2%, PR 55.6%) and need longer time to reach remissions (up to 8 weeks of treatment).172 This leads us to consider the combination of corticosteroids with other immunosuppressants.
The collapsing form is the most aggressive histological variant of LP, and may require greater immunosuppression. African-American patients (high-risk genetic variant APOL1) may not respond to immunosuppression.173,174
Relapses occur in more than half of patients with LP170 and usually coincide with extrarenal manifestations and/or serologic activity of SLE. No difference has been observed in the rate of relapses according to the histologic group of LP. Between 30 and 50% of patients with LP present acute renal failure, being more likely to occur in cases of GSFS.
Relapses with corticosteroids (monotherapy) can reach 89.5%, but decrease significantly to 50% if associated with other immunosuppressants.175
As with treatment of ML, it is suggested the administration of prednisone in single doses of 1 mg/kg/day (maximum 80 mg) or single doses on alternate days of 2 mg/kg (maximum 120 mg). Depending on the degree of tolerance, these doses can be maintained for a minimum of 4 weeks if CR is achieved and, up to a maximum of 16 weeks if not. Corticosteroid tapering should be slow, up to 6 months post-remission.176,177
Similar to other glomerular pathologies, in the corticosteroid-resistant forms the combination corticosteroids-CNI should be considered. In corticoid sensitive patients or with frequent relapses, the combination of corticosteroids with other immunosuppressors, such as MPAA, azathioprine, CTX, CNI or rituximab may be considered.119
Classes III/IV ± V. Initial or induction treatmentRecommendations4.3.1 All patients with classes III, IV, or III/IV + V should receive corticosteroids as initial treatment (unless there are contraindications) together with other immunosuppressants. As a preferred steroid treatment schedule we suggest i.v. pulses of methylprednisolone (250−500 mg/day) for three consecutive days followed by a reduced dose regimen (oral prednisone or equivalent 0.5−0.6 mg/kg/day) (Table 7).
Steroid regimens or regimens for patients with lupus nephritis.
| Dose standard | Dose moderate | Dose low | |
|---|---|---|---|
| Boluses or pulses of methylprednisolone | 0.25−0.5 g/day up to3 days as initial treatment | 0.25−0.5 g/day up to3 days, often given as initial treatment | 0.25−0.5 g/day for up to 3 days, usually given as initial treatment |
| Oral prednisone equivalent dose (mg/day) | |||
| 0.8−1.0 mg/kg (max. 80 mg) | 0.6−0.7 mg/kg (max. 50 mg) | 0.5−0.6 mg/kg (max. 40 mg) |
| 0.6−0.7 mg/kg | 0.5−0.6 mg/kg | 0.3−0.4 mg/kg |
| 30 mg | 20 mg | 15 mg |
| 25 mg | 15 mg | 10 mg |
| 20 mg | 12.5 mg | 7.5 mg |
| 15 mg | 10 mg | 5 mg |
| 12.5 mg | 7.5 mg | 2.5 mg |
| 10 mg | 7.5 mg | 2.5 mg |
| 7.5 mg | 5 mg | 2.5 mg |
| 7.5 mg | 5 mg | 2.5 mg |
| 5 mg | <5 mg | 2.5 mg |
| <5 mg | <5 mg | <2.5 mg |
Taken and adapted from:
KDIGO 2021 Clinical Practice Guideline for the management of glomerular diseases. Kidney International (2021) 100, S1–S276.
Rovin BH, Onno Teng YK, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA-1): a double-blind, randomized, multicentre, placebo-controlled, phase 3 trial. Lancet 2021;10289: 2070-80.
4.3.2 In patients with proteinuria <3 g/24 h, good compliance, infertility concerns or contraindications/intolerance to CTX, we suggest initial double immunosuppressive therapy with corticosteroids and MPAA (in doses equivalent to 2 g/day of mycophenolate mofetil) (Fig. 6).
Initial or induction treatment in histological classes III/IV ± V.
eGFR: estimated glomerular filtration rate; CNI: Calcineurin inhibitors; ISN/RPS: International Society of Nephrology/Renal pathology Society; MMF: mycophenolate mofetil; MPAA: Mycophenolic Acid Analogues; TAC: tacrolimus; VCS: Voclosporin.
4.3.3 In cases of proteinuria <3 g/24 h, risk of therapeutic noncompliance or contraindications/intolerance to MPAA we suggest initial double immunosuppressive treatment with corticosteroids and intravenous CTX (Eurolupus guide). After the administration of the sixth and last pulse of CTX, treatment with MPAA will be started or azathioprine in case of intolerance to MPAA (Fig. 6).
4.3.4 In those cases treated with corticosteroids + MPAA or corticosteroids + CTX that have not shown a reduction in proteinuria of at least 25% after 2–3 months of treatment with corticosteroids + MPAA or at the end of CTX boluses, we suggest adding to the treatment belimumab (especially if immunological activity persists) or CNI (especially if significant proteinuria persists) (Fig. 6).
4.3.5 We suggest initial triple immunosuppressive therapy with corticosteroids, MPAA and belimumab in those patients who fit the profile described in recommendation 4.3.2 and who also present extrarenal manifestations of SLE, intense serologic activity, need to reduce corticosteroids more rapidly or who have had previous flares og LN (Fig. 6).
4.3.6 In those patients presenting with proteinuria >3 g/24 h or complete nephrotic syndrome, adjusting for the other features described in recommendation 4.3.2, we suggest initial triple immunosuppressive therapy with corticosteroids + MPAA + CNI (tacrolimus, voclosporin) provided that the eGFR is ≥45 mL/min/1.73 m2.
4.3.7 In cases with acute renal function deterioration we suggest applying the treatment schemes adjusted to the patient profile shown in Fig. 5, but avoiding CNIs in patients with eGFR <45 mL/min/1.73 m2.
RationaleCorticosteroids significantly improve acute inflammation and immune activity in patients who debut or relapse with SLE and/or LN.83,86 There is a tendency to use lower doses of corticosteroids during the initial phase because of the lower risk of adverse effects.42,50,178
MPAA represent the first line of treatment in proliferative or mixed LN (Fig. 5). As induction therapy, they show similar114,179 or superior efficacy to CTX,113 with better safety profile. This benefit occurs in various ethnic groups, including black and Hispanic American populations.115 Thus, they are the first choice in compliant patients and/or when CTX is not a viable alternative (Fig. 6).42,50
CTX is still a first-choice therapeutic option (section 3.6), especially if there is non-compliance or contraindications to MPAA. It has been consistently demonstrated its superiority over corticosteroid monotherapy in inducing remission and preserving renal function.91,105 Intravenous administration is preferred due to its lower risk of adverse effects (Table 6). The oral route, although effective, is less used due to higher doses and accumulated toxicity.107,108 The "Euro-Lupus Nephritis Trial" clinical trial, conducted in European and predominantly Caucasian patients with class III, IV or V LN, showed that low doses of CTX (500 mg-IV/weekly for 3 months), followed by oral azathioprine, have the same short- and long-term clinical and immunologic efficacy as high doses, with less risk of infections.90,106 Although this "Eurolupus guideline" evaluated patients with relatively preserved renal function and had underrepresented ethnic groups with more aggressive clinical presentations, it is an effective, safe and frequently used approach in patients with proliferative LN.42,50,106
Several studies in recent years have shown superiority of so-called triple immunosuppressive therapy over double immunosuppressive treatment with corticosteroids plus another immunosuppressive agent. In the BLISS-LN study it was shown that the combination of corticosteroids, belimumab and MMF or CTX was significantly superior to treatment with corticosteroids and MMF or CYC in inducing renal response (section 3.11).155 This beneficial effect of belimumab was not observed in subgroups with significant proteinuria, black patients, or those who had received CTX. Further analysis of the study showed that belimumab also prevented relapses and slowed down the loss of renal function.158 Moreover, several studies have demonstrated the efficacy of belimumab in controlling extrarenal manifestations, decreasing lupus immune activity and early reduction of corticosteroid doses.154,180–182
CNIs have shown a significant antiproteinuric and cytoprotective effect of podocyte in various glomerular diseases, including LN (section 3.8).183 Studies in China showed that the combination of corticosteroids, MMF and tacrolimus was superior to corticosteroids plus CTX in obtaining complete remissions.132,133 Prospective studies with VCS have shown that this drug added to corticosteroids and MMF induces significantly greater numbers of CR and PR of LN than corticosteroid plus MMF therapy without increasing adverse effects.93,135 In patients with reduced renal function, the acute and chronic nephrotoxicity that can be induced by CNI makes it advisable to choose other drugs or to use them with close monitoring.
Although there are no studies designed in this sense, we suggest adding belimumab (especially if serological activity persists) or a CNI (especially if significant proteinuria persists) in those patients treated with dual therapy who do not show >25% reduction in proteinuria after 2–3 months of treatment.
In the subgroup of patients who debut with acute deterioration of renal function, we do not have sufficient data to support a specific treatment. In 2013, a post-hoc analysis of the ALMS (Aspreva Lupus Management study) clinical trial was published, comparing induction treatment with CTX vs. mycophenolate mofetil in 32 patients with eGFR <30 mL/min/1.73 m2.184 Of these 32 patients, 12 were randomized to receive CTX and 20 to mycophenolate. Four patients (20%) treated with MMF responded and only two (17%) those treated with CTX. Renal function in the MMF-treated group improved more rapidly than in the CTX-treated group. Serious adverse events were more frequent in the CTX-treated group (63.6 vs. 45%). However, this subanalysis is based on a sample size that is too small to draw definitive conclusions in favor of one treatment or the other. This study also shows that patients with low eGFR respond poorly to any treatment (19% compared to 55% in patients with higher eGFR) and generally have greater adverse effects as compared to patients who debut with better renal function (52% vs. 25%).
The acute and chronic nephrotoxicity that CNI can induce makes it advisable to choose other drugs or to use them with close monitoring in patients with reduced renal function.
Class V (Pure Membranous). Initial or induction treatmentRecommendations4.4.1. In patients with proteinuria <1 g/24 h we suggest treatment with HCQ, general nephroprotective measures and treatment of SLE according to the extrarenal manifestations that may present (Fig. 7).
Induction treatment in class V (pure membranous).
ARA-II: Angiotensin II receptor antagonists; CsA: Cyclosporin A; CVRF: Cardiovascular risk factors; CNI: Calcineurin inhibitors; ACEIs: angiotensin converting enzyme inhibitors; ISN/RPS: International Society of Nephrology/Renal pathology Society; SLE: systemic lupus erythematosus; MPAA: Mycophenolic Acid Analogues; RAAS: Renin Angiotensin-Aldosterone System, TAC: Tacrolimus; VCS: Voclosporin.
4.4.2. We suggest initial double immunosuppressive treatment with corticosteroids and CNI (tacrolimus, voclosporin, CsA) in those patients with proteinuria between 1 and 3.5 g/24 h. If after 3−4 months of treatment (especially if accompanied by persistent immunological activity) a reduction in proteinuria of at least 25% has not been obtained, we suggest adding MPAA to the treatment (Fig. 7).
4.4.3. In patients presenting with proteinuria >3.5 g/24 h or complete nephrotic syndrome, we suggest triple immunosuppressive treatment from the start with corticosteroids, CNI and MPAA (Fig. 6).
RationalePure lupus membranous nephropathy is a rare disease, usually associated with nephrotic syndrome. Progression to CKD is slow, but may be accelerated by the frequency of flares. Persistent nephrotic syndrome and/or frequent use of corticosteroids can lead to a number of potentially serious complications.
There is agreement that patients with persistent nephrotic syndrome should receive immunosuppressive therapy, while there is controversy about the use of immunosuppression in patients with proteinuria below nephrotic range, however its use seems advisable when proteinuria persists >1 g/24 h despite optimal treatment with RAAS inhibitors.185
Different immunomodulatory treatments have been shown to be effective, but still remain several uncertainties about the treatment and there with no much data from controlled clinical trials. The use of Corticosteroids alone are not very effective. Mycophenolic acid derivatives may induce renal remission but require more time to achieve the therapeutic goal than CNIs.130 In patients with severe nephrotic syndrome, a more rapid and sustained reduction in proteinuria can be obtained with triple therapy with CNI, MPAA and corticosteroids.135,186
Maintenance therapy for Class III/ IV ± V and Class VRecommendations4.5.1 We suggest that patients with LN reach doses of corticosteroids around 2.5−5 mg/day 6 months after starting treatment, and try to withdraw them between 18 and 24 months of treatment, provided that they are in clinical and serological remission (Fig. 8).
Maintenance treatment in classes III/IV ± V and V.
ARA-II: Angiotensin II receptor antagonists; CsA: Cyclosporine A; HCQ; Hydroxychloroquine; FRCV: Cardiovascular risk factors; CNI: Calcineurin inhibitors; ACEIs: angiotensin converting enzyme inhibitors; iSGLT2: Sodium-glucose tubular cotransporter 2 inhibitors; BP: blood pressure; MMF: mycophenolate mofetil; MPAA: Mycophenolic Acid Analogues; TAC: tacrolimus; VCS: Voclosporin.
4.5.2 In patients with LN who have achieved clinical remission and especially in the presence of serological remission, we recommend a gradual and progressive reduction in the dose of MPAA from 18 to 24 months, with the ultimate goal of withdrawal after 3–5 years of treatment (Fig. 8).
4.5.3 We suggest azathioprine as maintenance treatment in patients who do not tolerate MPAA, with a daily oral dose between 1.5 and 2 mg/kg/day. A tapering and discontinuation pattern similar to that recommended for MPAA should be followed (Fig. 7).
4.5.4 We suggest administering belimumab during the maintenance phase in patients with intolerance or contraindications to HCQ and/or corticosteroids, in patients with frequent relapses, with persistent extrarenal manifestations and/or with intense or persistent serological activity (Fig. 8).
4.5.5 In patients with class V (pure membranous) initially treated with corticosteroids plus CNI (Fig. 7), the reduction of corticosteroids should follow the recommendation 4.5.1 (of this section). Regarding CNIs, we suggest starting a slow and gradual reduction from 9 to 12 months in cases with CR and from 18 to 24 months in cases with PR until discontinuation.
RationaleReduced-dose corticosteroid schedules have similar efficacy as higher doses, but fewer side effects. In patients in clinical and serologic remission it is advisable to try to discontinue treatment after 18–24 months, especially if they are receiving HCQ and another immunosuppressant. Intravenous CTX pulses are not usually used as maintenance therapy because of their high cumulative dose and MPAA or azathioprine are available which have a lower incidence of adverse effects and greater efficacy.187 In an extension of the ALMS study in 227 patients who had achieved initial remission, MMF was superior to azathioprine in preventing relapses (12% vs. 23%, respectively) and maintaining clinical remission after 36 months of maintenance therapy.116 In the MAINTAIN study, conducted in 105 patients, there were no differences in the number of relapses, although there was a higher incidence of leukopenia with azathioprine.106 For all these reasons, MPAA are the drugs most frequently used as maintenance treatment, although azathioprine is an effective alternative in those who do not tolerate MPAA. It is advised a minimum duration of 3 years for both drugs, with a gradual and cautious tapering of the dose before discontinuation. In a post-hoc analysis of the BLISS-LN study, belimumab was found to decrease significantly the risk of relapse,155,158 so its use during the maintenance phase may be beneficial in patients with frequent relapses, intolerance or contraindications to HCQ, or persistent extrarenal manifestations.
The optimal maintenance regimen in class V (pure membranous) is not established, given the paucity of specific clinical trials. The evidence comes mainly from trials involving proliferative or mixed classes using MPAA-based regimens.5 A randomized study in patients with classes IV and V compared maintenance therapy with CsA or azathioprine, and the incidence of relapse was similar.124 In a randomized trial in patients with pure class V, CsA induced more remissions at 12 months than CTX or corticosteroids (83% vs. 60% and 27%, respectively) but the incidence of relapse was significantly higher with CsA after extended follow-up (120 months).188 No significant differences were observed in another study comparing MPAA and tacrolimus as maintenance therapy.128 We suggest a slow and progressive tapering of CNI dose in those patients with class V LN who have achieved clinical remission after initial therapy with corticosteroids plus CNI (Fig. 7).
Special situationsRefractory or resistant lupus nephritisRecommendations5.1.1 We suggest defining refractory or refractory LN as a lack of response or manifest worsening of proteinuria and/or renal function after at least 3 months of treatment with triple immunosuppressive therapy, according to the schemes proposed in Figs. 6 and 7 (Fig. 9).
5.1.2 In cases of refractory LN after treatment with immunosuppressors based on MPAA we suggest adding rituximab or changing MPAA for CTX (Eurolupus guideline) (Fig. 9).
5.1.3 In cases of refractory LN after treatment with immunosuppressors based on CTX, we suggest adding rituximab or extending the i.v. pulses of CTX until the completion of 6 months of treatment (Fig. 9).
5.1.4 In cases of no response after the measures described in the previous recommendations, we suggest treatment with the new anti-CD20 drugs (obinutuzumab) or anti-myeloma drugs (daratumumumab, bortezomib) or the inclusion of these patients in clinical trials with new drugs under evaluation.
RationaleRefractory or resistant LN is an inadequate or insufficient response to the treatment and represents up to 30% of the patients treated.159 There is currently no uniform and consensual definition of refractory LN,145 but there are ongoing initiatives (http://lupusnephritis.org/), which should include failure to achieve goals in certain clinical parameters (reflecting persistent disease activity and progression of renal damage), after drug exposure and a period of observation.143 Before making this diagnosis, we must rule out therapeutic noncompliance, suboptimal treatment doses, probable genetic factors and possible irreversible renal damage or concomitant renal pathology (Fig. 8),189 For the latter, the performance of a second renal biopsy is critical because of its prognostic and therapeutic implications15–17 (Figure S1, section 1.2).
In truly refractory cases, treatment depends on the previously administered regimen. Those based on MPA may benefit from switching to CTX (Eurolupus regimen) or rituximab.190 CTX-based regimens could extend their duration or switch to rituximab (Fig. 8). Although rituximab has not demonstrated superiority as add-on therapy to MPAA-corticosteroids,190 several observational studies with ethnically diverse patients, albeit with different definitions of refractory LN, different doses, and varying treatment times, have shown its effectiveness as rescue therapy.191–196
Patients who do not respond to rituximab or extended CTX treatment may benefit from newer anti-CD20 (obinutuzumab) or anti-plasma cell (bortezomib, daratumumumab) therapies.126,149,197,198 Ideally, they should be included in new clinical trials or preferably referred to centers with expertise in the experimental treatment of glomerular and/or autoimmune diseases.199
Prevention and treatment of relapsesRecommendations5.2.1 We suggest treating relapses of LN according to the patient's clinical profile and relapse characteristics, following the algorithms shown in Figs. 6 and 7.
5.2.2 In patients with relapses it is essential to investigate and ensure adequate therapeutic compliance. In cases with repeated relapses we suggest to consider to obtain a new renal biopsy to quantify activity, chronic irreversible lesions and changes in histological class.
5.2.3 After achieving remission of the relapse, we suggest to extend the duration of treatment with MPAA. In cases with repeated relapses we suggest maintaining MPAA at reduced doses indefinitely.
5.2.4 We suggest adding belimumab to the treatment of relapses and maintaining it for about 2 years. In case of repeated relapses, consider treatment with belimumab indefinitely. Recommendations for the treatment of frequent relapses of LN are summarized in Fig. 10.
RationaleThere are no studies specifically designed to compare treatments in relapsing LN, but it seems reasonable to suggest that the clinical profile and the characteristics of the relapse should guide the immunosuppressive treatment chosen according to the schemes in Figs. 6 and 7. In patients with relapse, it is essential to ensure strict compliance with prescribed therapies. HCQ has been associated with a lower incidence of relapse,76–78 so it is important to maintain this drug indefinitely, unless there are contraindications (section 3.4).
When the relapse has clinical characteristics different from those of the first outbreak, a new renal biopsy can rule out changes of histological class. In patients with repeated relapses it can be very difficult to differentiate residual proteinuria due to scarring of previous lesions from that produced by active lesions. In these cases a new biopsy can provide very useful data3,16,19 (section 1.2).
In cases with relapses, the duration of treatment with MPAA (or azathioprine in case of intolerance to the latter) could be extended beyond the 3–5 years that is recommended for the initial outbreak, and even maintain MPAA at a reduced dose indefinitely in case of multiple relapses. In cases of relapses after cyclophosphamide treatment, the use of MPAA could also be a viable alternative,200 although there are no formal clinical trials in this regard. A subanalysis of the BLISS-LN study158 showed that treatment with belimumab reduced the risk of relapses of LN by 55% and that this protective effect was independent of the initial immunosuppressive treatment, so that prolonged treatment with this drug may be useful in patients with persistent immune activity and relapses of NL (section 3.11).
Lupus nephritis and pregnancyRecommendations5.3.1 Pregnancy in women with LN is associated with an increased risk of maternal and fetal complications, such as preeclampsia, preterm delivery, intrauterine growth retardation, and fetal loss. It should be managed by multidisciplinary teams and high-risk pregnancy units. Preconception counseling and pregnancy planning in patients with stable NL (a minimum of six months in remission) may improve the chances of pregnancy success. Table 8 summarizes the differences between LN and preeclampsia.
Differential diagnosis between a flare of lupus nephritis and preeclampsia in pregnant women.
| Clinical feature | Lupus nephritis | Preeclampsia/HELLP1Syndrome |
|---|---|---|
| Often | Occasionally (HELLP) |
| Occasionally | Yeah |
| Yeah | Yeah |
| Yeah | No |
| Yeah | No |
| Yeah | No |
| Occasionally | Occasionally |
| Occasionally | Occasionally |
| Occasionally | No |
| No | Occasionally |
| Yeah | No |
| Occasionally | No |
| During pregnancy | From week 20 |
5.3.2 Pregnancy planning in a woman with LN should adapt her immunosuppressive medication to maintain controlled renal and systemic activity, avoiding the use of drugs that are teratogenic or have negative effects on gestation. Corticosteroids, azathioprine and CNI are drugs considered safe during pregnancy (Table S9).
5.3.3 To reduce the risk of complications during pregnancy, it is recommended to continue HCQ, and to use low-dose aspirin (100 mg/day) before week 12 of pregnancy.
RationaleAlthough the prognosis has improved markedly in recent years, pregnancy in women with SLE conditions a series of additional problems such as a greater probability of presenting HTN, preeclampsia, prematurity and preterm delivery; for this reason an adequate control of the disease for a minimum of six months before conception is recommended.201,202 Pregnancy in patients with LN should be considered a risk situation and should be attended by multidisciplinary units, which allow adequate coordination and strict maternal-fetal monitoring.202,203
For the diagnosis of LN during pregnancy, it should be used the same criteria as in non-pregnant patients. The presence of extrarenal activity, the anti-dsDNA antibodies turning positive or the increase of their titer and/or the presence of low complement levels would support the diagnosis of suspected LN. When clinical and laboratory parameters are not sufficient for diagnosis or there is not an adequate response to treatment, we should consider performing a renal biopsy if there is no contraindication (it can be safe until the 20th week of gestation).204,205 Increased proteinuria, arterial hypertension or the presence of edema may also be present in patients with preeclampsia. Patients with SLE have a higher incidence of preeclampsia, ranging from 11 to 35% according to different series, compared to a 5% risk in the general population. It is difficult to establish the differential diagnosis between a flare of LN and preeclampsia in pregnant lupus women and it is of great importance because the therapeutic approach is very different (Table 8). The LN flare mayoccur at any time during pregnancy. Although there are clinical or analytical data that guide the differential diagnosis between LN or preeclampsia, currently the combined indices of angiogenic markers (increased sFlt-1 and endoglin, decreased PlGF and increased sFlt-1/PlGF ratio) and ultrasound findings (uterine artery Doppler ultrasound) have shown the best ability to detect early preeclampsia (<34 weeks).206,207 Finally, low-dose aspirin (100 mg/day) is recommended before 12 weeks of pregnancy because it reduces the risk of preeclampsia and intrauterine growth retardation.42
Some of the immunosuppressants used in LN are contraindicated during pregnancy such as CTX and MPAA due to their teratogenic capacity (Table S9). Azathioprine is the immunosuppressant of choice both for maintenance therapy in patients with previous LN as well as for induction of treatment in new cases. Patients who were on MPAA should be discontinued and azathioprine initiated. Corticosteroids do not pose a problem of fetal toxicity, since they are inactivated in more than 90% by 11β-hydroxy-steroid hydrogenase in the placenta; however, we should not forget the possible maternal complications. Treatment with CNI (CsA and TAC) has also been shown to be safe during pregnancy. In exceptional cases in which there is a vital risk to the mother and the fetus is not yet viable, some authors suggest the possibility of using CTX in the second-third trimester, since it has been seen that in studies in patients with breast cancer there are no significant fetal complications. Current evidence indicates that the use of rituximab in the first trimester of pregnancy does not increase the risk of fetal malformations. However, there is a significant risk of B-cell depletion and cytopenias in the newborn with a consequent risk of infection, so it should only be used in very exceptional cases. HCQ is considered safe during pregnancy and may decrease the risk of prematurity and intrauterine growth retardation and reduce the risk of fetal heart block in up to 50% of anti-Ro positive mothers. Its withdrawal has been associated with an increased risk of flares.9,73,208,209
The BP should be controlled with labetalol, nifedipine, methyldopa, or hydralazine.210
It is important to discuss contraception with fertile women with an LN to avoid unplanned pregnancies, which could be the origin of a large number of maternal-fetal complications. Estrogen contraceptives should be avoided in patients with APLAS or a previous history of thrombosis or risk of pulmonary thromboembolism. Fertility preservation with ovarian or sperm cryopreservation should be considered in patients to be treated with CTX.
Lupus nephritis in the pediatric patientRecommendations5.4.1 SLE is more aggressive in children and adolescents. LN is more frequent and plays a fundamental role in the prognosis, morbidity and mortality in this age group.
5.4.2 Diagnosis based on histology findings is key to establish the prognosis and planning treatment. Biopsy is indicated in the presence of sediment alteration, proteinuria and/or renal failure (Table S10).
5.4.3 Treatment is based on a remission induction phase, in which methylprednisolone boluses remain the cornerstone in severe pediatric cases. MPAA and CTx are the main corticosteroid-sparing drugs, with equivalent results. There are not randomized controlled trials on induction nor in maintenance, so the adult guidelines are being used. Early and sometimes, more aggressive, treatment in induction improves prognosis (Table 9).
Immunosuppressive treatment in infant and adolescent lupus nephritis.
| Drug | Dose | Indication | Pattern | Secondary effect |
|---|---|---|---|---|
| Methylprednisolone | 30 mg/kg(maximum 1 g) | Classes III, IV | 3 consecutive days | WaterfallosteopeniaInfectiongrowth retardationObesitycardiovascular risk |
| Prednisone | 1−0.5 mg/kg0.5 mg/kg | Maintenance for classes III, IVClass II | 4 weeks3 months if proteinuria | |
| Mycophenolatemofetil | 1.2−1.8 g/m2/day.(maximum 3 g) | Induction and maintenance for classes III, IV and V | DiaryEvery 12 h | Infection and bone marrow suppression |
| Cyclophosphamide | 500 mg/dose or500−750 mg/m2/dose | Induction and maintenancefor classes III, IV (3 years) | 6 biweekly IV doses6 monthly IV doses | Infectiongonadal toxicitymarrow suppressionCarcinogenic |
| Rituximab | 750 mg/m2/dose375 mg/m2/dose | Resistant and severe cases | 2 IV doses with an interval of 14 days4 IV doses with an interval of 7 days | InfectionAllergic reactionsPersistent hypogammaglobulinemia. |
| Tacrolimus | 0.15 mg/kgMeasure serum levels | Combination resistant cases | Diary | Diabetes, infectionsCarcinogenic |
| Belimumab | 10 mg/kg | Moderate lupus from 5 to 17 years | IV every 4 weeks | Infection |
IV, Intravenous route.
SLE affecting children and adolescents (pSLE) differs mainly from adult SLE in being a more severe disease and more frequently having renal involvement (60–80%). Renal involvement is the strongest predictor of overall morbidity and mortality in patients with pSLE. There are no specific guidelines for the pediatric population regarding renal biopsy, and although adult guidelines are followed, there is controversy regarding the indications for biopsy in pSLE. In general, the consensus is to perform a first renal biopsy when renal involvement is suspected in patients with SLE: active sediment (red blood cells, casts, leukocytes), renal failure and the presence of proteinuria, once orthostatic proteinuria has been excluded, >0. 5 g/24 h (or protein/creatinine ratio in morning sample ≥0.5 mg/mg or ratio ≥0.5 mg/mg calculated in 24-h urine), although some groups recommend performing the kidney biopsy with proteinuria ≥0.3 g/24 h if accompanied by active sediment and/or renal insufficiency, without any alternative explanation. There is more doubt regarding a second biopsy. In any case, it is recommended to perform a previous renal ultrasound and to rule out contraindications for renal biopsy.211–213
There are very few randomized controlled trials in children with SLE. The few uncontrolled adolescent case series are taken as supportive evidence. Guidelines generally advise the same treatment strategies as in adults, with no safety and efficacy studies in this subpopulation. Non-immunosuppressive treatment consists of HCQ (5 mg/kg/day, maximum 400 mg) and ACE inhibitors. Class I nephropathy does not require specific treatment, it will be treated according to the systemic manifestations. Class II, according to systemic manifestations, or in case of proteinuria, with corticosteroids at a dose of 0.5 mg/kg/day. Azathioprine and methotrexate are used in mild-moderate cases, the latter especially if there is neuromuscular involvement. In severe forms of LN class III and IV (with or without class V), it is recommended to start with 3 i.v. pulses of methylprednisolone (dose: 30 mg/kg, maximum 1 g), on 3 consecutive days, followed by oral prednisone at a dose of 0.5−1 mg/kg/day for 4 weeks, which is then gradually reduced. According to the latest evidence, it is possible that initial prednisone doses of 1 mg/kg/day may not be required and that lower doses, together with methylprednisolone pulses, may be sufficient to achieve remission in patients with proliferative LN, thus decreasing the associated toxicity. At the moment there are no evidences to support these recommendations and there are studies that demonstrate a higher risk of readmission in patients in whom methylprednisolone boluses have not been used. The long-term toxicity of corticosteroids makes it necessary to look for other therapeutic options. The drugs that are added to reduce corticosteroid doses are mainly MPAA and CTX with similar results of the two of them, even in the induction of remission. Alternative treatments such as CNI, immunoglobulins, rituximab and belimumab are approved in children and a clinical trial with voclosporin is currently underway. The rest of the therapeutic arsenal is not currently approved.214–222
Antiphospholipid syndrome and thrombotic microangiopathyRecommendations5.5.1 In asymptomatic patients with positive APLAS and a high-risk profile (high and sustained titers), antiplatelet therapy with aspirin 75−100 mg/24 h is recommended. In case of thrombosis of large arterial or venous vessels, anticoagulation should be established.
5.5.2 In patients with APLAS-associated nephropathy (NAPS) we suggest, in severe cases, starting anticoagulation with vitamin K antagonists, added to the usual treatment. In mild cases or when there is a contraindication to anticoagulant treatment, it is suggested to start antiplatelet treatment.
5.5.3 In patients with catastrophic AP syndrome (CAS), it is recommended treatment with high doses of corticosteroids and heparin plus plasmapheresis or gammaglobulin. Non-responders to this treatment can receive anti-B cell therapies (rituximab) or complement inhibitors (eculizumab).
5.5.4 In patients with SLE and TMA, we recommend measuring the activity of ADAMTS-13, APLAS and ruling out other etiologies associated with TMA (drugs, neoplasms, vasculitis, genetic dysregulation of the complement). We recommend treatment with eculizumab (short cycles of 1−2 months) in cases with persistence of TMA despite immunosuppressive treatment or if complement dysregulation is suspected or confirmed. Fig. 11 summarizes the diagnostic and therapeutic algorithm for patients with SLE and TMA.
Study and treatment of patients with SLE and TMA.
*Consider to associate treatment with mycophenolate or cyclophosphamide
AAF: antiphospholipid antibodies; AC: anticoagulation; CS: Corticosteroids (in boluses); IV Ig: intravenous immunoglobulin; SLE: systemic lupus erythematosus; TMA: thrombotic microangiopathy; APSN: antiphospholipid syndrome-associated nephropathy; PE: plasmapheresis; TTP: Thrombotic Thrombocytopenic Purpura; RTX; Rituximab; CAPS: catastrophic antiphospholipid syndrome.
About 30–40% of lupus patients have circulating APLAS, many of them asymptomatic. However, the presence of these antibodies is considered a fundamentally risk factor of vascular damage that determines a characteristic clinical, analytical, and histological presentation in patients with SLE.14,223–228
It is important to distinguish these 4 situations in patients with SLE and APS, although many times these pictures overlap, making their diagnosis difficult:
- 1
APS is defined as the presence of thrombosis (arterial, venous and/or small vessels) and/or obstetric complications (abortions, fetal deaths and/or prematurity due to placental insufficiency), together with sustained positivity for APLAS (lupus anticoagulant, anticardiolipin and anti-beta2-glycoprotein I antibodies). APS may be asymptomatic, affect the great vessels of the kidney (thrombosis of the renal arteries or veins, renal artery stenosis) or present as APS-associated nephropathy (APSAN).
- 2
APSAN represents a specific and rare entity associated with lupus nephropathy where arteriolar or interlobular glomerular thrombotic lesions (TMA) predominate over the involvement of large vessels.
- 3
Catastrophic APS (CAS) is characterized by an often rapid course characterized by thrombosis affecting various organs and with high mortality.
- 4
TMA associated with SLE/APS is a more severe expression of APSAN (at the analytical and histological level) and it is practically identical to that which can occur in thrombotic thrombocytopenic purpura (PTT)/haemolytic uremic syndrome (HUS). A typical, malignant arterial hypertension, or complement-mediated TMA.
In asymptomatic and APA-positive patients, antiplatelet therapy with aspirin 75−100 mg/24 h is only recommended in those with a high-risk antibody profile (high and sustained APA titers or double or triple positivity).229,230
The APSAN may affect more than 30% of patients with SLE and AAF, as well as in individuals with primary APS. It may have a broad histopathological spectrum, although its characteristic finding is the presence of thrombosis. In the acute forms, thrombosis is observed at the level of arterioles and glomerular capillaries as in MAT, and in the chronic form it can be found lesions with thickening and fibrosis of the intima, and sclerosis and capsular atrophy. APSAN lesions may overlap and are independent of LN. These autoantibodies condition worsens the renal survival in patients with LN. Likewise, during subsequent follow-up of patients with APSAN. it has been observed the development of vascular thrombosis, especially at the arterial level.
In addition to the typical small vessel lesions of APSAN, thrombosis of large renal vessels, arteries, and veins may develop in the clinical context of APS. Renal vascular hypertension associated with renal artery stenosis is also characteristic.
There are no established guidelines for the treatment of APSAN. A retrospective study231 showed a beneficial effect of adding warfarin anticoagulation to their usual immunosuppressive therapy. Direct oral anticoagulants are not recommended since they were inferior to warfarin in preventing thromboembolic events.231,232
The CAPS is characterized by an often rapid course characterized by thrombosis that affects various organs and with high mortality.233 Treatment includes anticoagulation and high doses of corticosteroids. Plasmapheresis has shown a beneficial effect in retrospective studies, which is why it is usually added in these severe cases. Rituximab and eculizumab234 have also recently been used in isolated cases.
In many SLE patients with APS, can develop MAT acutely with thrombocytopenia and acute renal failure. Most of these patients have active LN, so the basic induction treatment will be corticosteroids with MPAA or CTX.
It is necessary to distinguish in these patients the 3 most frequent situations of TMA: TTP, APS and complement-mediated TMA, without forgetting the other causes of TMA (HUS associated with Shiga toxin, pregnancy, drugs, infections, neoplasias, associated systemic diseases, etc.).
In these cases, the first step is to request the antibodies/functional activity of ADAMTS13, APA and calculate the PLASMIC score234 (Table S11). If the PLASMIC score in adults is high (>5), the diagnosis of TTP can be assumed and treatment with corticosteroids and plasmapheresis can be started while waiting for the other tests to be available. In children, it is preferable to wait for the results of ADAMTS13 before initiating plasmapheresis due to its lower frequency and high morbidity of the treatments. In cases of SLE/PTT that do not respond to corticosteroids/plasmapheresis, it can be associated rituximab and/or caplacizumab (a von Willebrand factor inhibitor).
Treatment of TMA associated with NAPS or APS has been previously discussed. In patients with SLE, TMA (normal ADAMTS-13) and APA negative, it should be performed an etiological study and considered the use of eculizumab if complement dysregulation is suspected or in cases with persistence of TMA despite immunosuppressive treatment. A retrospective study suggests that short cycles of eculizumab could be beneficial in cases of secondary TMA that do not respond to usual treatment.235 It should also be noted that in a series of TMA in patients with SLE it was found mutations in the complement regulatory genes that in 6 of the 10 cases studied.227
Advanced CKD and dialysisRecommendations5.6.1 When LN progresses to a stage of advanced CKD (ACKD), patients should be evaluated in specialized ACKD clinics. In cases of ACKD without signs of renal activity, it is recommended a slow decrease in immunosuppression until it is discontinued, and continuing only in the case of extrarenal manifestations.
5.6.2 All patients with SLE, even with ACKD and on renal replacement therapy (RRT), should receive treatment with HCQ. In patients with eGFR <30 mL/min/m2 or on dialysis, it is not recommended to increase the dose above 200 mg/day. It is suggested to maintain RAAS blockers in patients with LN and ACKD.
5.6.3 It is recommended to offer any dialysis modality to patients with LN and ACKD; renal transplantation is the option with the highest long-term survival.
RationaleA meta-analysis of 187 articles that included 18,309 patients showed that the 5-year risk of ESRD secondary to LN has decreased in developed countries from 16% in the 1970s–1980s to 11% and subsequently, in the 1990s, has been stable.236 The patients that reach the final stage of the disease (eGFR <15 mL/min/1.73 m2) are the result of the progression of the different types of LN, showing at least 90% sclerotic glomeruli and interstitial fibrosis in the histology.11 Histological signs of immunological activity are usually minimal, and immunofluorescence only reveals some deposits of immunoglobulin or complement. Therefore, in this stage of the disease it does not make sense to maintainin aggressive immunosuppression, since progression to ESRD is inevitable. When the patient starts renal replacement therapy, lupus activity generally decreases, so both renal and extrarenal flare-ups are much less frequent. This is documented in old studies showing that initiation of renal replacement therapy significantly decreased immunological activity,237 and therefore reduction and even suspension of immunosuppressive treatment was recommended in the absence of extrarenal manifestations.
The objective is to minimize the side effects associated with immunosuppressive treatment. Medication adjustment should always be made based on immunological activity, since cases of persistent lupus activity have also been described during the first year of dialysis.238 Thus, all patients will should receive HCQ to avoid flare-ups and extrarenal manifestations.50 A recent study in kidney transplant recipients also supports its use, with routine cardiological monitoring due to the possible development of arrhythmias.239
In cases where the initiation of renal replacement therapy occurs after a disease flare, there is usually significant immunological activity with active renal lesions that persist during the first months of RRT. These patients are therefore likely to improve with immunosuppressive treatment. Induction treatment should be maintained for at least 6 months after starting renal replacement therapy, until the absence of recovery is confirmed. In fact, an improvement in renal function has been described in 10−20% of these cases, which allows abandonment of dialysis, at least temporarily.240
In LN, RAAS inhibitors may be used and in fact they should be used, with caution, to control BP and reduce proteinuria to delay the the need of renal replacement therapy. In these patients, caution must always be taken with the possible appearance of hyperkalemia in the final stages.
Regarding the modalities of renal replacement therapy, three studies compared hemodialysis with peritoneal dialysis, finding no differences on patient survival.241 Two other studies have compared the two modalities of dialysis with renal transplantation; best survival at 1.5 and 10 years was observed with transplantation.242,243 Initially it was thought that after entering dialysis it would be necessary to wait about 6 months before receiving a transplant, but recent studies suggest that early transplantation results in lower morbidity and better prognosis.244,245
Kidney transplantation in lupus nephritisRecommendations5.7.1 Kidney transplantation, from a living donor or cadaver, is the renal replacement therapy of choice in patients with ESRD secondary to LN. It is recommended to include all these patients on the kidney transplant waiting list if there are no contraindications.
5.7.2 It is suggested to postpone renal transplantation in ESRD until lupus activity ceases for at least 3–6 months. Once this has been achieved, there is no contraindication for performing a kidney transplant as soon as possible. Preemptive kidney transplantation may be performed if there is no activity.
Clinically significant recurrence of LN in the renal graft is infrequent and rarely leads to loss of the renal graft. Therefore, patients with LN do not require additional immunosuppressive treatments to the usual rejection prevention treatments.
5.7.4 Anticoagulation with dicoumarins prevents graft thrombosis in patients with LN and APA, but it is recommended to apply treatment based on the individual risk of thrombosis/bleeding in the immediate post-transplant period
5.7.5 If there are no contraindications, HCQ should be maintained in transplant patients at the recommended doses according to renal function (section 3.4).
RationaleBetween 10% and 30% of patients with class III/IV LN progress to CKD fifteen years after diagnosis, although additional studies indicate that in recent years the progression is <10%.236,246 As compared with hemodialysis or peritoneal dialysis, renal transplantation is offers better survival in patients with LN, although it presents an increased risk of certain complications such as infections, graft thrombosis, or recurrence of nephritis after transplantation.244 In a study of 9,659 patients with LN undergoing renal replacement therapy, of whom 59% had received a kidney transplant, it was observed a significantly lower mortality in transplant recipients as compared with those on hemodialysis or peritoneal dialysis, especially due to the decreased in cardiovascular and infectious mortality.
The survival of patients with LN who receive a kidney transplant is similar to the rest of transplanted patients (85–90% at 1 year and 66% at 10 years)247–251; although in some analyzes such as that of the Australian registry, survival of LN patients is lower252 Kidney graft survival is the same247–250,253 or higher251 than in transplant recipients for another reason. These discrepancies are due to differences in the populations included, follow-up times, and heterogeneity in the control groups. Regarding the type of donor, living donor recipients have better survival than cadaver donor.251 Renal transplantation offers better quality of life as compared to dialysis and lower cost in the medium-long term. These data support the inclusion of all patients with CKD secondary to LN in the transplant waiting list except those that present contraindications for renal transplantation.
The recurrence of LN after renal transplantation ranges between 0.5 and 52% in the different studies analyzed, being more frequent if protocol biopsies are performed. Clinically significant recurrence is less frequent (1–13% after 5 and 10 years post-transplant), and it rarely causes renal graft loss (0.5 and 1%).247–249,251,252,255 The main causes of graft loss in patients with SLE are chronic graft nephropathy and death with a functioning graft due to cardiovascular or infectious causes.254
The risk of graft loss due to LN recurrence has led to questions about the ideal timing to perform a kidney transplant. Although it has been described the cessation of lupus activity at the start of dialysis and during kidney transplantation due to uremia and immunosuppressive treatment, this does not occur in all patients, and the persistence of activity at the time of transplantation is associated with lower graft survival. For this reason, it is recommended to wait 3–6 months after the cessation of lupus activity to perform the kidney transplant with the idea that if the lupus becomes quiescent, the risk of recurrence is reduced.50,245,251,254 However, some authors indicate that the degree of lupus activity at the time of renal transplantation is not a risk factor for LN recurrence; by contrast, risk factors of LN recurrence are being African descent, younger age, female gender, and persistence of APA at the time of transplantation.249 Instead, Ntatsaki et al. observed that a prolonged stay on dialysis (>24 months) was associated with lower graft survival.256 Taking into account all these facts, it is suggested that patients with ESRD secondary to LN without activity can receive a transplant without a waiting period, even preemptive. In contrast, in patients with activity at the start of dialysis or who have presented acute deterioration of renal function before ERCT, the waiting period may not only allow the cessation of this activity, but also allows a chance to recover.
The positivity of APA in patients undergoing renal transplantation increases the risk of graft thrombosis and TMA (10.4% vs. 1.7%, and 10.2% vs. 0%, respectively). It was also associated with a higher probability of extrarenal thrombosis and lower graft survival but was not associated with an increase in delayed graft function or acute rejection.257 Anticoagulation with dicoumarins effectively prevents graft thrombosis, but the benefit obtained must be carefully weighed against the risk of bleeding in the immediate post-transplant period.258
Due to all these determining factors, it is suggested that the determination of markers of lupus activity and APA be included in the pre-kidney transplant evaluation to facilitate individualizing treatment according to thrombotic risk.
Conflicts of interest- -
Jorge E. Rojas Rivera declares being paid for talks and scientific advice. GSK, Otsuka and Alexion.
- -
Clara García-Carro declares that she has been paid for talks and scientific advice. Astra Zeneca, Esteve, Novonortis, Boehringer Ingelheim, Astellas, Otsuka, Novartis, Mundifarma, Baxter and Vifor.
- -
Ana I. Ávila declares that she has no conflict of interest.
- -
Mar Espino declares having received paid for talks. Alexion.
- -
Mario Espinosa declares that he has been paid for consultancies and talks. Alexion.
- -
Gema Fernández-Juárez declares being paid for talks. GSK and Otsuka.
- -
Xavier Fulladosa Oliveras states that he has been paid for providinf advice. Otsuka and Novartis.
- -
Marian Goicoechea declares that she has been paid for consultancies and talks. GSK.
- -
Manuel Macía declares that he has no conflict of interest.
- -
Enrique Morales declares that he has been paid for talks. GSK.
- -
Luis F. Quintana Porras states that he has been paid for talks. GSK and Otsuka.
- -
Manuel Praga Terente declares that he has been paid for consultancies and talks. GSK, Osuka, Novartis, Apellis, Alexion, Sanofi, Vifor and Travere.
- -
All authors participated in the intermittent consensus meetings. All developed at least one specific section of the main document, including the search and review of the publications. All reviewed, approved and signed the final document, including the main manuscript with tables and figures and the supplementary material before being submitted.
- -
Manuel Praga carried out the design and general coordination of the study.
- -
Manuel Praga and Jorge E. Rojas Rivera designed and made the main and supplementary figures and the correction, edition and layout of the main and supplementary tables.
- -
Jorge E. Rojas-Rivera and Manuel Macía, in addition to their specific sections in the main document, developed the sections of the supplementary material.
- -
Jorge E. Rojas-Rivera and Clara García-Carro, in addition to their specific sections in the main document, they made the final edition of the final manuscript.
None.
The authors of this document would like to thank the support and collaboration of the Carlos III Health Institute (ISCIII), the RICORS2040 program (RD21/0005/0001) financed by the European Union (European Union - Next Generation EU), Mechanism for Recovery and Resilience (MRR) and especially to the members of the GLOSEN working group for establishing a space of reference in the study of glomerular pathology, from which it has been possible to carry out the consensus and drafting of this manuscript.