Defined as the unpleasant sensation that causes the desire to scratch, pruritus is the most common skin symptom associated with uremia and appears in almost half of patients with advanced chronic kidney disease (CKD). Beyond its direct impact on quality of life, CKD-associated pruritus (CKD-aP) is an independent predictor of mortality that also has a synergistic effect with other quality of life-related symptoms, such as insomnia, depression, and anxiety. Although different mechanisms have been proposed to explain the origin of Pa-ERC, its etiopathogenesis is still not fully understood. Since new therapeutic targets have been identified and several clinical trials have recently shown promising results, our current understanding of the interrelationships has expanded significantly and the pathophysiological mechanisms underlying CKD-aP are now considered to be multifactorial. The potential triggers of pruritus in patients with CKD are discussed in this review, including hypotheses about skin xerosis, accumulation of uremic toxins, dysregulation of the immune system and systemic inflammation, uremic neuropathy, and imbalances in the endogenous opioid system. Other non-uremic causes of pruritus are also discussed, with the aim of guiding the physicians to apply an adequate aetiopathogenic approach to CKD-aP in their day-to-day clinical practice.
Definido como la sensación desagradable que provoca el deseo de rascarse, el prurito es el síntoma cutáneo más frecuente asociado a la uremia, pudiendo aparecer en casi la mitad de los pacientes con enfermedad renal crónica (ERC) avanzada. Más allá de su repercusión directa sobre la calidad de vida, el prurito asociado a la ERC (Pa-ERC) es un predictor independiente de mortalidad que además ejerce un efecto sinérgico con otros síntomas también relacionados con la calidad de vida, como la depresión y el insomnio. Aunque se han propuesto diferentes mecanismos para explicar su origen, la etiopatogenia del Pa-ERC sigue sin conocerse por completo. Dado que se han identificado nuevas dianas terapéuticas y recientemente varios ensayos clínicos han mostrado resultados prometedores, nuestra comprensión actual de las interrelaciones se ha ampliado significativamente, considerando multifactoriales los mecanismos fisiopatológicos subyacentes al Pa-ERC. En la presente revisión se discuten los potenciales factores desencadenantes de prurito en el paciente con ERC, incluyendo las hipótesis sobre la xerosis cutánea, el acúmulo de toxinas urémicas, la desregulación del sistema inmune y la inflamación sistémica, la neuropatía urémica y los desequilibrios en el sistema opioide endógeno, así como otras causas no urémicas de prurito, con el objetivo de orientar al clínico para realizar un adecuado abordaje etiopatogénico del Pa-ERC en su día a día.
Pruritus is defined as the unpleasant sensation of itching of the skin, either in a specific area or all over the body, producing the urge to scratch.1,2 Uraemic pruritus is the most common skin symptom in patients with chronic kidney disease (CKD), particularly in advanced stages of the disease. Although it is known as uraemic pruritus, the fact that there is no known direct cause-effect relationship with uraemia (since it does not usually occur in patients who present with episodes of acute kidney injury) means it is more accurately referred to as CKD-associated pruritus (CKD-aP), a term that is increasingly employed when discussing this condition.3
The clinical presentation of CKD-aP is variable, it generally affects large areas of the skin in a discontinuous and symmetrical manner, and being more symptomatic at night.4 The face, chest and extremities are the most commonly reported areas, although it can be generalised in 25–50% of patients who suffer from it.5 Unlike the dermatological causes of chronic pruritus, CKD-aP does not course with primary skin changes, although it can coexist with xerosis in 50%–80% of patients and secondary skin changes, such as excoriations or prurigo nodularis can occur over time as a result of chronic scratching.6,7 Up to 25% of haemodialysis (HD) patients report an increase in the intensity of pruritus during the dialysis session or immediately after.8,9 In addition to the HD session, other triggering factors described are cold, heat, showering, physical activity or stressful situations. Unfortunately, most patients with CKD-aP have it for months or even years.9,10
Prevalence and clinical consequencesCKD-aP is a very common symptom, and its prevalence increases as kidney function worsens. In the pre-dialysis stages, up to 24% prevalence has been observed,11 and this increases to 40%–55% in dialysis patients.12,13 Its negative impact on quality of life is very significant, and is related to the onset of sleep disorders, anxiety, depression, and problems maintaining regular physical and working activity,14–18 as well as with an increase in the mortality of those patients who present with it.19,20 Despite its clinical significance, CKD-aP is an underdiagnosed condition with a poorly standardised and often sub-optimal therapeutic approach. In a DOPPS [Dialysis Outcomes and Practice Patterns Study] analysis, it was found that up to 69% of physicians did not actively investigate the possibility that their patients had pruritus, while almost 20% of patients who did suffer from it did not discuss it with their medical team.12 More recently, a study carried out through the website of the Spanish Society of Nephrology (Sociedad Española de Nefrología) has validated these results.21 After analysing the perception and usual practice of 135 Spanish nephrologists surveyed anonymously, it was confirmed that CKD-aP continues to be an underdiagnosed disorder in our environment, it is not codified, and there is a high degree of ignorance about its prevalence, pathophysiology and therapeutic approach.21
This apparent lack of interest in the diagnosis and management of CKD-aP may be explained, at least in part, by clinicians' lack of knowledge of the exact mechanisms underlying it, which limits their ability to approach it from an aetiopathogenic standpoint to identify and treat many of the patients it affects.12 However, although the precise molecular relationships in the pathophysiology of uraemic pruritus remain unclear, thanks to the promising results of recent clinical trials and the identification of new therapeutic targets, our current understanding of the aetiopathogenesis of CKD-aP has been significantly deepened.22 Although a recent general review of CKD-aP briefly mentions the potential pathophysiological mechanisms of this disease,23 we believe that an in-depth review of these mechanisms is necessary. This article aims to summarise the current knowledge on the pathophysiology of pruritus in patients with CKD, with the aim of guiding the clinician in an adequate aetiopathogenic approach to CKD-aP in their day-to-day practice.
Putting the pieces of the puzzle back togetherThe pathophysiology of CKD-aP is complex and remains somewhat unclear.1,2,10,24 In the absence of convincing experimental models to study pruritus, our understanding of its aetiopathogenesis derives from multiple heterogeneous, sometimes contradictory sources.5 These sources include epidemiological (and not necessarily causal) associations between CKD-aP and other manifestations of CKD, such as secondary hyperparathyroidism or the accumulation of uraemic toxins; studies comparing the immunochemical environment of the skin in CKD patients with and without CKD-aP; and the inferences about the mechanisms underlying the appearance of pruritus obtained ex juvantibus based on the response to the pharmacological action of effective treatments of CKD-aP.5,22
The classic associations revisitedHistorically, various biochemical abnormalities of bone-mineral metabolism have been correlated with the presence of pruritus in CKD patients. In the first analysis from the DOPPS study, Pisoni et al. observed significant associations between hyperphosphataemia greater than 5.5 mg/dl, or an increased calcium × phosphorus product, with the presence of moderate or severe pruritus.15 These strictly observational findings have traditionally led a large majority of nephrologists to consider dietary phosphorus restriction or chelation therapy as the first therapeutic step in the treatment of CKD-aP.12 Similarly, many nephrologists would intensify treatment with calcimimetics and vitamin D to reduce parathyroid hormone levels in patients with severe pruritus, based on old, uncontrolled studies that reported symptom improvement after parathyroidectomy.25,26 However, subsequent studies have revealed that associations between intensity of itching and elevated phosphorus levels,20,27,28 parathyroid hormone12,28,29 and calcium30 are inconsistent, and there are no intervention studies that demonstrate that better biochemical control of bone-mineral metabolism disorders improves CKD-aP. In addition to its limited utility in improving pruritus, dietary phosphorus restriction in patients with pruritus could lead to an increased risk of malnutrition and unnecessarily blaming patients for their own symptoms.12
Adequate dialysis (estimated by Kt/V) is another of the factors historically related to the presence of CKD-aP.12 In a 1995 study, increasing the Kt/V from 1.05 to 1.24 was associated with an improvement in pruritus.31 However, this association has not been confirmed in more recent studies, where the administered dialysis dose is usually much higher, with a mean Kt/V of 1.5.12,15,27
Finally, histamine has long been considered the main chemical mediator of pruritus, regardless of cause, and the majority of nephrologists all over the world have been using antihistamines as first-line drug treatment, despite the fact that such treatment is not backed by evidence.12 In fact, as we will describe later, the sensation of itching is transmitted through both histaminergic and non-histaminergic nerve fibres.32,33 This explains why antihistamines do not effectively control CKD-aP,10,34,35 and their use is not exempt from relevant side effects such as dizziness and drowsiness.12 In a recent Cochrane review, the use of antihistamines had uncertain effects on the control of uraemic pruritus, with low to moderate quality of evidence. This review also highlighted how most of the clinical trials carried out with other molecules had included patients with no response to antihistamines, which illustrates the limitation of these drugs regarding the control of CKD-aP.36
All these data indicate that we still have a great margin for improvement when it comes to ensuring the well-being of CKD patients through greater knowledge about the pathophysiology of pruritus and greater use of effective treatments. Updating our current understanding of the pathogenesis of CKD-aP will support the clinician in his or her ultimate goal of helping CKD patients improve their quality of life through better control of their symptoms.37
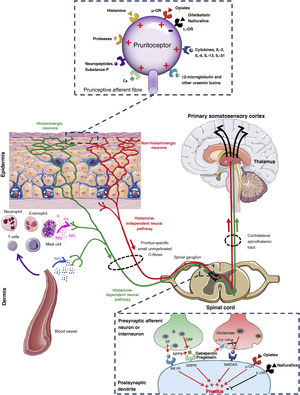
Towards knowledge of the origin and transmission of pruritusFig. 1 shows an overview of the connections and signalling pathways generally involved in the pathophysiology of CKD-aP.5,22,38,39 It originates in the skin due to activation of specific receptors (pruritoceptors) by various substances including prostaglandins, histamine, cytokines, neuropeptides (such as substance P), proteases, and uraemic toxins (such as β2-microglobulin).40–42 These substances, known as pruritogens, are released by keratinocytes, lymphocytes, mast cells, neurons, or other cells present in the epidermis and dermis.41,42 As is also the case at the level of the central nervous system (see below), current studies reveal that peripheral opioid receptors (ORs) also play a key role in modulating pruritus.43 While the μ-OR exerts an inducing role, stimulation of the κ-OR would inhibit the production of CKD-aP, as demonstrated by treatment with difelikefalin, a selective agonist of such peripheral receptor.44 The activity of pruritogens can be modulated by the uraemic environment.5 Thus, for example, in patients with CKD-aP there is an increase in inflammatory and pruritogenic cytokines produced by Th1 lymphocytes, such as interferon-γ, interleukin (IL)-6 and tumour necrosis factor-α45,46 and by Th2 lymphocytes, such as IL-31.47 Similarly, in the skin of such patients there is an increase in the number of mast cells,48 of dermal cells producing proteases (for example, tryptase)49 and of β2-microglobulin,50 which have been shown to have pruritogenic activity per se.40 More recently, a significant decrease in the κ-opioid receptor expression in the skin has been observed in patients with CKD-aP compared to the skin of those with CKD without pruritus.51
Connections and signalling pathways involved in the pathophysiology of chronic kidney disease-associated pruritus.
GRP: gastrin-releasing peptide; H: histamine; ILs: interleukins; NK1: neurokinin-1; NMDA: N-methyl-D-aspartate; NPPB: natriuretic polypeptide B; NPs: neuropeptides; O: opioid; PGs: prostaglandins; PRs: proteases; R: receptor.
Adapted from Makar et al.,5 Verduzco and Shirazian,24 Ikoma et al.,38 and Yosipovitch and Bernhard.39
Pruritogens activate primary sensory neurons, which may be histamine-dependent or histamine-independent,38 which would explain the limited response to antihistamines observed in patients with CKD-aP. Both histamine-mediated and non-histamine-mediated pathways of pruritus overlap with pain pathways.1,52 Therefore, the transmission of itching and pain are closely related.53 Itching (and pain) are transmitted through unmyelinated fibres (C-type), which are considered polymodal because they can react to various stimuli of a thermal, mechanical, or chemical nature (such as hypoxia, hypoosmolarity, or the accumulation of substances), both at the local level (skin) and systemically.54 Primary sensory neurons are capable of transmitting the sensation of itching by synapsing with secondary sensory neurons in the dorsal ganglia using itch-specific neurotransmitters. For example, glutamate, released by presynaptic neurons, exerts its pruritogenic function through N-methyl-D-aspartate receptors. Such glutamate release can be blocked by decreased Ca+2 influx induced by gabapentin or pregabalin, leading to less activation of N-methyl-D-aspartate receptors and therefore a reduced sensation of itching.55 Other neurotransmitters, such as substance P and gastrin-releasing peptide, are released by presynaptic neurons and transmit the sensation of itching through NK1 and BB2 receptors, respectively.38 More recently, B-type natriuretic polypeptide, also known as brain natriuretic peptide, has been implicated as one of the major neurotransmitters between itch-sensitive nerve fibres and dorsal horn neurons in the spinal cord via the gastrin-releasing peptide-dependent pathway,56,57 its blood levels being correlated with the degree of CKD-aP in HD patients.58 As at the peripheral level, opioid receptors in the spinal cord also play a fundamental role in the control of pruritus. Morphine administration induces pruritus by binding to μ-OR, while pruritus can be reduced both with μ-OR antagonists such as naloxone and naltrexone59 as with κ-OR central agonists like nalfurafine,60 without modifying the antinociceptive effects of morphine.38
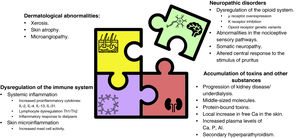
Factors involved in CKD-associated pruritusThe understanding of the previously simplified model of pathophysiology has become much more complex. The aetiopathogenic mechanisms underlying pruritus are considered to be multifactorial (Fig. 2).1,12,53 Such mechanisms include dermatological factors such as xerosis or skin barrier dysfunction; systemic factors such as dysfunction of immune system and the proinflammatory state inherent in CKD; neurological factors, including uraemic neuropathy and dysregulation of the endogenous opioid system, with overexpression of μ opioid receptors and downregulation of κ opioid receptors; the accumulation of toxins and other metabolic substances; and factors associated with the type of renal replacement therapy that promote or exacerbate the appearance of pruritus.1,2,10,53,61,62 Although the treatment of CKD-aP is beyond the scope of this review, Table 1 describes the main interventions used in its management along with their mechanisms of action, to help the nephrologist to link the pathophysiological elements of pruritus with its clinical management.5,36,44,50,63–81
Interventions and mechanism of action of the main therapies used in the management of CKD-aP.
| Intervention/drug | Known or presumed mechanism of action | Most relevant clinical trials |
|---|---|---|
| Neuropathic disorders | ||
| Gabapentinoids (gabapentin and pregabalin) | Derived from the GABA neurotransmitter, the mechanism of action is unclear. Despite its name, it does not act on GABA receptors, but probably inhibits the α2δ subunit of calcium channels in the dorsal horn, decreasing Ca+2 influx and glutamate release in the presynaptic neuron5,50 | In a meta-analysis that included 297 patients from five RCTs, gabapentin and pregabalin significantly reduced CKD-aP (4.95 cm reduction on the VAS, 95% CI: 5.46 to 4.44) compared to placebo (high certainty evidence)36 |
| κ-OR agonists (nalfurafine, difelikefalin) | Stimulation of κ opioid receptors inhibits the neural pathway of pruritus both peripherally (difelikefalin) and centrally (nalfurafine). At the peripheral level, receptors are located on the surface of cutaneous mast cells and keratinocytes. At the central level, they are located in the dorsal horn of the spinal cord. The efficacy of peripheral κ-OR agonists indicates that the main mechanism of action on CKD-aP is peripheral63 | In a meta-analysis that included 661 patients from six RCTs, (four with nalfurafine and two with difelikefalin), κ-OR agonists significantly reduced CKD-aP (1.05 cm reduction on VAS; 95% CI: 0.71–1.4) compared to placebo (high certainty evidence).36 In two RCTs published subsequently, which included 174 and 378 HD patients, respectively, difelikefalin, compared with placebo, significantly reduced the intensity of pruritus and improved CKD-aP related quality of life44,64 |
| Topical capsaicin | It acts on TRPV1 channels in peripheral sensory neurons, depleting and preventing the accumulation of substance P.5 | In a meta-analysis including two RCTs with 116 patients, four times daily application of topical capsaicin versus cream (placebo) reduced symptoms of uraemic pruritus (SMD − 0.84, 95% CI − 1.22 to −0.45; moderate-certainty evidence)36 |
| Dermatological abnormalities: | ||
| Emollients | Restore the permeability of the skin barrier5 | An intraindividual comparison RCT (left versus right leg comparison) involving 100 patients with moderate to severe uraemic xerosis, with application two times a day for one week of an emulsion containing 15% glycerol and 10% paraffin on one leg compared with emulsion alone on the other leg, followed by open use of the test product on all xerotic areas, demonstrated that the use of emulsion containing glycerol and paraffin was highly effective in CKD-aP (improvement in 75% of patients) and quality of life at the end of the study65 |
| Dysregulation of the immune system | ||
| Turmeric | Turmeric, commonly used as a spice, is a powder from the rhizomes of Curcuma longa L. which is used in Asian medicine for the treatment of inflammation and skin wounds. Curcumin (diferuloylmethane), the most active and non-toxic component of turmeric, is a polyphenol with anti-inflammatory activity66 | An RCT of 100 HD patients demonstrated how the administration of 500 mg of turmeric (22.1 mg of curcumin) every eight hours compared to placebo decreased the intensity of CKD-aP and hsCRP levels67 |
| Polyunsaturated fatty acids (evening primrose oil) | Formed mainly by linoleic acid that has Ω-6 fatty acids, which reduce the production of arachidonic acid and, therefore, the synthesis of proinflammatory cytokines such as leukotriene B4 and prostaglandin E25 | A small RCT with 16 HD patients demonstrated that evening primrose oil rich in polyunsaturated fatty acids significantly improved general skin symptoms, including pruritus, compared to those receiving linolenic acid alone68 |
| Sodium cromoglycate | Sodium cromoglycate is a drug that blocks mast cell degranulation in response to antigens, resulting in decreased release of histamine, leukotrienes, and other inflammatory products from mast cells69 | An RCT of 62 patients on HD demonstrated how the administration of 135 mg of oral sodium cromoglycate every eight hours compared to placebo decreased the intensity of CKD-aP70 |
| Zn sulphate | Zn deficiency favours excessive mast cell degranulation, while Zn supplementation prevents degranulation and histamine release71 | In a meta-analysis that included 76 patients from two RCTs, supplementation with 220−400 mg daily of Zn sulphate significantly reduced CKD-aP (1.77 cm reduction on the VAS; 95% CI: 0.66–1.77) vs. placebo (moderate-certainty evidence)36 |
| Montelukast | Leukotriene receptor antagonists block leukotrienes from maintaining the inflammatory response after degranulation | In a meta-analysis that included two RCTs with 87 patients, the administration of montelukast compared to placebo reduced CKD-aP symptoms (SMD − 1.40, 95% CI − 1.87 to −0.92; moderate-certainty evidence)36 |
| UVB phototherapy | Apoptosis of cutaneous mast cells and keratinocytes. Decreases the expression of Th1 lymphocytes72 | In a meta-analysis that included four RCTs with 86 patients, UVB phototherapy reduced CKD-aP symptoms (SMD − 2.49, 95% CI − 4.62 to −0.36; low-certainty evidence)36 |
| Accumulation of toxins and other substances | ||
| High-flow haemodialysis | Improves the clearance of uraemic toxins73 | In a meta-analysis that included three RCTs with 202 HD patients, high-flow HD significantly reduced CKD-aP (2.60 cm reduction on the VAS; 95% CI: 1.97–3.22) compared to low-flow HD (low-certainty evidence)36 |
| Online haemodiafiltration | Improves the clearance of uraemic toxins and the inflammatory state74–76 | In an RCT with 51 HD patients with CKD-aP, the use of high-flow HD and online haemodiafiltration was associated with a significant decrease in CKD-aP77 |
| Adsorptive haemodialysis | Decreases the levels of protein-bound toxins and proinflammatory cytokines such as IL-6, difficult to clear by other dialysis techniques78,79 | An RCT of 90 HD patients showed how the use of HD with resin haemoperfusion significantly reduced CKD-aP (2.37 cm reduction on the VAS; 95% CI: 1.97–3.22) compared to standard HD.80 In a small crossover trial of 19 HD patients with severe pruritus, the use of a pomethylmethacrylate adsorptive membrane was associated with a significant decrease in CKD-aP81 |
CKD-aP: chronic kidney disease-associated pruritus; GABA: gamma-aminobutyric acid; HD: haemodialysis; hsCRP: high-sensitivity C-reactive protein; IL: interleukin; RCT: randomised clinical trial; OR: opioid receptor; SMD: standardised mean difference; TNF-〈: tumour necrosis factor-alpha; TRPV1: transient receptor potential vanilloid 1; UVB: ultraviolet B; VAS: visual analogue scale.
Dry skin or xerosis, characterised by a rough, cracked and scaly skin surface, is closely associated with the sensation of chronic pruritus, a common skin manifestation in patients with CKD, reaching a prevalence of up to 85% in HD patients.82–84 Numerous factors may contribute to the development of xerosis in this population, including atrophy of the secretory glands, thickening of the basal zone, alteration of the lipid composition of the stratum corneum, and decrease in the degree of moisture in the epidermis, making it more sensitive to external damaging factors.83–85 The reasons why this dehydration of the epidermis occurs in patients with CKD have not yet been sufficiently clarified; the main factors proposed are the displacement of osmotic fluids through cell membranes, as well as the gains and losses of water in relation to the dialysis sessions and, more recently, the presence of microangiopathy of the dermal blood vessels.22,84–86 This microangiopathy, present from pre-dialysis stages of CKD, is produced by the direct effect of endocrine, metabolic and immunological alterations associated with the uraemic environment,87,88 generating endothelial dysfunction at the level of the vessels distal to the smallest arterioles, which results in hypoperfusion with tissue hypoxia at the cutaneous level,89 among other tissues.86,87,90 The improvement in microangiopathy observed after transplantation reinforces the aetiopathogenic role of uraemia in this disorder.89 Additionally, continuous scratching by patients with pruritus can result in further damage and inflammation of the skin that promotes CKD-aP which becomes chronic, as it establishes a vicious cycle between itching and scratching, producing secondary skin lesions.6,7,61
All these findings show that dry skin can aggravate CKD-aP, as in many other pruritic conditions91 and it is reasonable to recommend the use of emollients in patients with CKD-aP, since measures as simple as moisturising the skin can at least partially improve said symptom.5,92 However, the fact that patients with CKD-aP and dry skin improve when moisturising the skin (Table 1),65 but that many patients with marked xerosis do not necessarily suffer from pruritus,93 suggests that dry skin is probably a factor that enhances the sensation of itching rather than an aetiological factor per se.84,94
Inflammation causes itchingMicroinflammation, both at the cutaneous and systemic levels, has been proposed in recent years as one of the main factors responsible for CKD-aP.5,10,22,24 At the local level, proteases, such as trypsin, tryptase, cathepsins and kallikreins, also function as pruritogens in situations of skin inflammation.41,42 At the systemic level, various observational studies have shown how dialysis patients with pruritus have a significantly higher proportion of various inflammatory markers, including Th1 cells, C-reactive protein, IL-6 and IL-2 levels, compared to patients without pruritus.45,95 Other inflammatory markers such as leukocytosis, elevated ferritin, or decreased albumin have also been associated with the appearance of pruritus.6,96
The interaction between the nervous system and the immune system occurs through small molecules and cytokines that are released by inflammatory cells that activate pruritoceptors in sensory neurons that subsequently release neuropeptides such as substance P and calcitonin gene-related peptide, which activate immune and non-neuronal cells.1 Among all these mediators, histamine is historically one of the best-studied substances, being involved in the maturation, activation, and chemotaxis of immune cells such as monocytes, T cells, macrophages, and others.97 However, the role played by histamine in CKD-aP, as in other forms of pruritus beyond urticaria and allergic reactions, is considered very limited.24 Although the allergic response may also be dysregulated in CKD patients, in whom elevated histamine and mast cell levels have been reported, the classic histamine-specific skin changes, such as hives, are absent in CKD-aP patients,10 in whom, most of the clinical trials designed to decrease the release of histamine have obtained negative results. Although it is known that serotonin is an activator of the neuronal receptors of the spinothalamic tract pathway and has been proposed as another of the mediators responsible for pruritus,24 the use of serotonin receptor antagonists such as ondansetron has not been shown to be effective in the control of CKD-aP.36,98
Along with histamine and serotonin, a series of key cytokines involved in pruritus (and pain) have been described in recent years, the best known are: IL-2, IL-4, IL-13 and IL-31.22 Consistent with this hypothesis, intradermal administration of IL-2 produces a sensation of itching,99 while antibodies that block IL-31 or its receptor, such as nemolizumab, have been shown to reduce this sensation.100,101 Although a metabolic cascade known as pruritus induced by IL-6/pBRK/p-ERK signalling has recently been described, whereby IL-6 (and calcium) trigger the sensation of itching. However other molecular mechanisms that relate to the regulation of the inflammatory state with the presence of pruritus are still poorly understood.102 The fact that pruritus experienced by CKD patients improves when applying immunomodulatory therapies such as ultraviolet B phototherapy36,103,104 or when using dialysis techniques capable of reducing the inflammatory state (Table 1)74–81 undoubtedly supports the immunological hypothesis in the aetiopathogenesis of CKD-aP. Additionally, there is increasing evidence that inflammation is also involved in the modulation of the opioid system and therefore could be also potentially involved in the development of pruritus (see below).24
Dysregulation of the endogenous opioid system and other neuropathic changesThe ORs, distributed in the central and peripheral nervous systems, as well as in other cells such as keratinocytes, melanocytes, and immune cells, appear to play an important role in modulating pruritus,1,105 including CKD-aP.1,10,22,24,43 Recent data demonstrate that dysregulation of the endogenous opioid system may participate in the appearance and transmission of CKD-aP, either by overstimulation of μ-ORs, peripheral κ-OR antagonism or an imbalance between the stimulation and inhibition of μ-ORs and κ-ORs, respectively.106,107 This hypothesis is based on the observation that opioids used for pain, such as morphine (μ-OR agonists), can, however, trigger pruritus, while the μ-OR antagonists, such as naloxone,59 as well as κ-OR agonists both at the level of the central nervous system (such as nalfurafine) and peripherally (such as difelikefalin), can decrease it (Table 1).36,43,44,60 Additionally, an association between the activity of the κ-ORs and the intensity of the pruritus has been demonstrated in CKD-aP patients.51
Beyond its direct dependence on pruritus and pain, there is increasing evidence of the interaction between the endogenous opioid system and inflammation, which highlights the complex interplay between keratinocytes, immune cells, and nerve fibres in the aetiopathogenesis of CKD-aP.10,24 The discovery that the inflammatory state modifies the expression of peripheral ORs and the fact that opioids have a greater analgesic effect in inflamed tissue led to the discovery of opioid peptides in lymphocytes, polymorphonuclear cells, and monocytes/macrophages.108–111 The activation of κ-ORs in immune cells, including monocytes and T lymphocytes, decreases the release of pro-inflammatory chemicals, such as prostaglandins, which in turn can also be pruritogenic.112,113 Interestingly, IL-6 and tumour necrosis factor-α produce opioid-mediated analgesia in inflamed tissue,114 while those same cytokines and others like IL-1α or IL-1β induce hyperalgesia in non-inflamed tissue.115 There are also genetic variants of the ORs that could explain the differences that we observed in our patients in their susceptibility to pruritus or in the responses to its treatments.116
In addition to dysregulation of the opioid system, other mechanisms of central and peripheral neuropathy associated with CKD could contribute to the development of CKD-aP.62 In this sense, an abnormal central response to the stimulus of pruritus has been described in dialysis patients,117 as well as an association between the intensity of the CKD-aP and the degree of somatic neuropathy in the form of paresthaesias.118 Evidence that treatments for uraemic neuropathy such as topical capsaicin or oral gabapentinoids may also alleviate pruritus (Table 1)36 demonstrate how neuropathic disorders are at least partially responsible for the manifestation of CKD-aP.
The accumulation of toxins and other substancesThe accumulation of uraemic and other substances due to the decrease in glomerular filtration rate in CKD patients has been postulated as one of the mechanisms involved in the development of CKD-aP. Among these substances, have been included classically parathyroid hormone, calcium, phosphorus, and aluminium. A higher prevalence of CKD-aP has been observed in patients with secondary hyperparathyroidism, although not all patients with secondary hyperparathyroidism have pruritus and in those who do, treatment of hyperparathyroidism is not always associated with better control of the pruritus.119 In patients with CKD-aP, there is an increase in extracellular free calcium ions in the basal layer of the epidermis compared to CKD patients without pruritus120; the hyperphosphataemia can foster the formation and deposition of calcium phosphate salts in the skin and other tissues, which can activate local nerve fibres and favor the appearance of pruritus.10,121 In a recent study, it was found that patients with higher levels of aluminium in their blood had a higher prevalence of pruritus,122 which highlights the importance of periodically determining aluminium levels in HD patients and keeping them in range.123 Regarding other substances that have been associated with the appearance of CKD-aP, in a recent study that assessed the metabolic profile of HD patients with pruritus using liquid chromatography and mass spectrometry, there were up to nine biomarkers proposed that could be related to this condition, including protein-bound toxins, phospholipids and steroids: LysoPE (20:3(5Z,8Z,11Z)/0:0), p-Cresol glucuronide, LysoPC(20:2(11Z,14Z)), hypotaurine, 4-aminohippuric acid, LysoPC(16:0), phenylacetic acid, kynurenic acid and androstenedione.124 These metabolites appear to be increased to a greater degree in patients with severe CKD-aP, so that dialysis treatments or techniques aimed at lowering their blood levels may improve the symptoms related to uraemic pruritus (Table 1).36,74–81 Describing these metabolites, studying them and knowledge about their characteristics can help us understand why some clearance techniques can be more effective than others in the treatment of this disease (see below).125
The type of dialysis is importantTo date, none of the dialysis treatments available have been shown to be fully effective in reversing CKD-aP. Although peritoneal dialysis was classically believed to be more effective (because of the better clearance of middle-sized molecular weight molecules in those patients who maintain residual renal function),126 other more recent works find just the opposite.127 In a recent meta-analysis of 42 cross-sectional studies, the prevalence of CKD-aP according to the technique was similar between patients on HD and peritoneal dialysis (55% vs. 56%).13 Therefore, there is no clear consensus on which of the dialysis options (HD or peritoneal dialysis) is better to avoid the appearance of pruritus. Thus, when choosing the best option for a patient with CKD-aP, both techniques seem to have a similar therapeutic profile. It is known that the dialysis dose, estimated by Kt/V, has an inverse relationship with the intensity of pruritus, so it is therefore essential to optimise the prescription of dialysis in this population.31 Studies have been published in which the adequacy of the dose and the prescribed technique can at least partially improve the symptoms of CKD-aP.128,129 What is clear is that receiving a kidney transplant that restores kidney function in patients with CKD significantly improves the symptoms, which undoubtedly supports the hypothesis of the accumulation of toxins in the pathophysiology of CKD-aP.130
Among the HD techniques, considering that protein-bound toxins and middle-sized molecular weight toxins may play a role in the pathophysiology of CKD-aP,125 those techniques that help eliminate these uraemic toxins, such as techniques that combine diffusive transport with convective or adsorptive transport, can improve CKD-aP, including high-flow HD,36 online haemodiafiltration77 or adsorptive HD techniques (Table 1).80,81 Within this last group, haemoperfusion with neutral resins,80 the use of adsorptive polymethylmethacrylate membranes81,131,132 and haemodiafiltration with ultrafiltrate regeneration (HFR-Supra)133 have shown promising results on alleviating CKD-aP and the antipruritic effect is being attributed to a greater clearance of protein-bound toxins and proinflammatory cytokines such as IL-6, that are difficult to clear with other dialysis techniques.78,79,134 Pursuing this purification mechanism applied to the control of CKD-aP, we must comment on two other therapeutic options with very few results obtained; the use of oral activated charcoal with low selectivity for adsorbing substances which has hampered its general use135; and expanded HD, which seems to improve clearance of middle-sized molecules even in patients in whom convection techniques is not advantageous due to problems of vascular access flow, and which could be a good option to improve patient symptomshowever we still do not have any studies that demonstrate its efficacy in the control of CKD-aP beyond isolated clinical cases.136,137 The type of vascular access for HD could also play a role in the appearance of CKD-aP, it being less common in patients with arteriovenous fistula than with a catheter. Possible mechanisms involved could be a better clearance of toxins and a lower inflammatory state associated with the use of a fistula compared to a catheter.138
Causes of pruritus not associated with CKDThe presentation of pruritus in patients with CKD can be most varied, and it may be difficult to make a differential diagnosis with other causes of pruritus. The possibility that pruritus in CKD patients may not be due to their kidney disease should always be considered, and other causes of pruritus should be ruled out.7,9 We must consider the possibility that the patient's pruritus is not uremic in origin if it does not respond to a reasonable attempt at treatment, if the symptoms are asymmetric and accompanied by bullous or ulcerative lesions, or if they occur together with other clinical symptoms consistent with other systemic diseases. Sometimes pruritus is related to the start of the administration of a new drug, or even to medications that the patient has been on for some time.4 The causes of pruritus not related to uraemia that should be ruled out in patients with pruritus are listed in Table 2.
Causes of non-uraemic pruritus.
| Dermatological diseases | Systemic diseases |
|---|---|
| Drug hypersensitivity and other allergies | Hypercalcaemia |
| Contact dermatitis | Cholestasis |
| Primary biliary cirrhosis | |
| Psoriasis | Viral hepatitis |
| Bullous pemphigoid | Tumour haematological diseases |
| Hodgkin's lymphoma | |
| Dermatophytosis | Cutaneous T-cell lymphoma |
| Tinea cruris | Polycythemia vera |
| Tinea pedis | |
| Tinea corporis | Human immunodeficiency virus |
| Other non-fungal infections | |
| Scabies | |
| Mites | |
| Lice |
Although pruritus is the most common cutaneous symptom in our CKD patients, we have not been equipped to provide adequate treatment, due, among other causes, to a lack of knowledge about its pathophysiology, which has greatly limited the use of effective treatments. The aetiopathogenesis of CKD-aP is complex and remains unclear, although mechanisms potentially involved in its appearance and transmission have recently been described, including: (1) the abnormalities in the structure and function of the skin present in CKD; (2) the abnormal immune response and the chronic inflammatory state associated with uraemia; (3) dysregulation of the endogenous opioid system and uraemic neuropathy, and (4) accumulation of uraemic toxins in the skin and subcutaneous tissue. A deeper understanding of the pathophysiology of CKD-aP will undoubtedly help us overcome its treatment challenges, providing us with a valuable basis for the future development of treatments for this condition, which will ultimately result in a better quality of life for patients with CKD.
Conflicts of interestESA: consulting fees from Vifor Pharma.
GA: consulting fees from Vifor Pharma.
JMB: conference fees from Fresenius, Baxter and Vifor Pharma; consulting fees from Vifor Pharma.
MP: conference fees from Fresenius, Baxter, Astellas and AstraZeneca; consulting fees from Vifor Pharma and Baxter.
NA: conference fees from Vifor Pharma, Baxter, Amgen, AstraZeneca and Rovi; consulting fees from AstraZeneca and Novo Nordisk.
PM: conference fees from Abbott, Amgen, Fresenius-Kabi, Nutricia, Palex, Sanofi and Vifor Pharma; consulting fees from Fresenius-Kabi, Palex and Vifor Pharma; and travel grants from Amgen and Fresenius Medical Care.
RSV: conference fees from Amgen, Fresenius-Kabi, AstraZeneca and Baxter; consulting fees from Baxter and Vifor Pharma.
VES: consulting fees from Amgen, Novo Nordisk and Vifor Pharma, and travel grants from Amgen and Baxter.
The rest of the authors confirm that they have no conflicts of interest.
The authors would like to thank Roser Peiró, PhD, for her encouraging comments and suggestions throughout the drafting of the manuscript; and Marta Maojo, for her help in drafting the abstract.
We would also like to thank Servier Medical Art (https://smart.servier.com/) for the free distribution of medical images, used in part for creating the figures in the article.