Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis is characterised by small vessel necrotising inflammatory vasculitis. Prior to immunosupressant therapy availability it usually led to a fatal outcome. Current treatment has changed ANCA-associated vasculitis into a condition with a significant response rate, although with a not negligible relapse occurrence and cumulative organ lesions, mostly due to drug-related toxicities. The use of glucocorticoids, cyclophosphamide and other immunosupressants (such as azathioprine, mychophenolate and methotrexate) was optimised in a series of clinical trials that established the treatment of reference. In recent years, a better knowledge of B lymphocyte function and the role of complement inhibition has transformed the course of this disease while minimising treatment-related adverse effects.
This multidisciplinary document of recommendations is based on the consensus of three scientific societies (Internal Medicine, Nephrology and Rheumatology) and on the best available evidence on diagnosis, treatment and follow-up of patients with ANCA-associated vasculitis, including some special situations. The aim of this document is to provide updated information and well-grounded clinical recommendations to practising physicians as to how to improve the diagnosis and treatment outcome of our patients.
Las vasculitis asociadas a anticuerpos anticitoplasma de neutrófilo (ANCA) se caracterizan por una inflamación necrotizante de vasos pequeños. Antes de la llegada del tratamiento inmunosupresor solían tener un desenlace fatal. El tratamiento ha transformado las vasculitis en una enfermedad con una importante tasa de respuesta, pero con un porcentaje nada despreciable de recidivas y un daño orgánico acumulado, en gran parte debido a las toxicidades relacionadas con los fármacos. El uso de glucocorticoides, ciclofosfamida y otros inmunosupresores (como azatioprina, micofenolato y metotrexato) se optimizó mediante una serie de ensayos clínicos que establecieron un tratamiento de referencia. En los últimos años, la mejora de los conocimientos sobre los linfocitos B y la inhibición del complemento ha revolucionado el curso de la enfermedad y minimizado los efectos adversos del tratamiento.
El presente documento multidisciplinar de recomendaciones se ha basado en un consenso de tres especialidades (Medicina Interna, Nefrología y Reumatología) y en la mejor evidencia disponible acerca del diagnóstico, tratamiento y seguimiento del paciente con vasculitis asociada a ANCA, incluyendo situaciones especiales. El objetivo es brindar a los médicos que manejan habitualmente este tipo de enfermedades, información actualizada y recomendaciones clínicas bien fundamentadas, que mejoren el enfoque diagnóstico y terapéutico de nuestros pacientes.
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis is characterized by necrotizing inflammation of small vessels and, prior to the advent of immunosuppressive therapy, used to have a fatal outcome. New treatments have transformed ANCA vasculitis into a chronic disease with a significant response rate, albeit with a notable recurrence rate and cumulative organ damage largely due to drug toxicities. The use of glucocorticoids (GC), cyclophosphamide (CTX) and other immunosuppressants such as azathioprine (AZA); mycophenolate mofetil (MMF) and methotrexate (MTX) has been optimized based on clinical trials. In recent years, advances in knowledge about B lymphocytes and the role of complement in antibody-associated vasculitis (AAV) have transformed the course of the disease and reduced the adverse effects (AEs) of the treatment.
Recently, we have seen the updating of the recommendations for the management and treatment of ANCA vasculitis according to different scientific societies (American College of Rheumatology [ACR], European League Against Rheumatism [EULAR], Kidney Disease Improving Global Outcomes [KDIGO]), which have proposed important changes in the approach to this disease.
This document of recommendations is based on the best available evidence on the diagnosis, treatment and follow-up of patients with AAV, including specific cases, and with the consensus of three scientific societies (Internal Medicine, Nephrology and Rheumatology). In each section, the recommendations are presented followed by their scientific justification and a summary of the most relevant information in the area.
The main objective is to provide updated information and substantiated clinical recommendations to improve the diagnostic and therapeutic approach of patients with AAV.
MethodsA multidisciplinary panel made up of 15 physicians experts in the diagnosis and treatment of patients with vasculitis, consultants from Nephrology (n = 5), Internal Medicine (n = 5) and Rheumatology (n = 5) participated in these recommendations. To this end, several coordination, discussion and consensus meetings were held between March 2023 and March 2024.
Firstly, a scientific committee was appointed made up of a coordinator from each of the three specialties. This committee was in charge of suggesting the participants, designing a work scheme and assigning their sections to the authors based on their experience and knowledge.
The experts were chosen based on the following criteria: having a specialization experience of more than 10 years, being active in public or private centers, belonging to a scientific society, being the author of publications or communications at conferences on AAV, or have participated in clinical trials in this field.
Each participant was given the task of establishing recommendations in the assigned section, writing a brief justification based on a review of the scientific literature and their own experience. Medline, Embase, Google Scholar and Cochrane Library were used as bibliographic sources. The summaries of the main congresses of the three specialties or specific to AAV were also reviewed. The search was limited to the last 15 years and followed the criteria detailed in Appendix A Table S1. In addition to the selected bibliography, the clinical practice guidelines on AAV in their updated versions were taken into account.
The recommendations proposed by each author were reviewed and critically discussed, initially by the scientific committee and then by the entire group, until the final recommendations were agreed upon. For each recommendation, the supporting literature and the group’s degree of agreement (GA) were collected. To issue a recommendation, a GA equal to or greater than 70% was considered necessary.
The authors sign the document in alphabetical order, alternating the three participating specialties, except for the first two and the last, due to their greatest contribution to the manuscript.
DiagnosisDiagnosis of AAVsRecommendations- •
It is recommended to determine ANCA by indirect immunofluorescence (IIF) and by Enzyme-Linked Immunosorbent Assay (ELISA) as usual practice (GA: 100%).
- •
It is recommended to perform a biopsy that supports the diagnosis of AAV whenever possible (GA: 100%).
- •
It is recommended to maintain a high index of suspicion for AAV in the absence of ANCA in patients with rapidly progressive glomerulonephritis (RPGN), as well as in those with localized inflammatory processes suggestive of AAV (orbital pseudotumor, otomastoiditis, pulmonary nodules, etc.) (GA: 82%).
- •
The determination of other antibodies, such as anti-endothelial antibodies (AECA) or anti-human lysosomal membrane 2 (LAMP-2), is not currently recommended due to their lack of validation and standardization (GA: 83%).
The suspicion of AAV with negative ANCA1 is not justified by the literature.
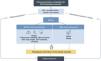
The diagnosis of AAV is challenging given its severity, multisystem nature, and great clinical variability. It must be supported by three aspects: clinical manifestations, serological autoimmunity and histological demonstration (Fig. 1).
Diagnostic strategy in vasculitis associated to anti-neutrophil cytoplasmic antibodies (ANCA). The diagnosis should be based on three aspects: clinical manifestations, serology and histology.
*See Table 2. Clinical features of GPA and MPA.
**If rapidly progressive and ANCA positive, it is not necessary to wait for biopsy results to initiate treatment.
ANCA: anti-neutrophil cytoplasmic antibodies; PR3: proteinase 3; MPO: myeloperoxidase; ENT: otorhinolaryngologic.
Clinical suspicion should be established in patients with general syndrome of, persistent fever and symptoms indicative of organic involvement, with special relevance of renal and pulmonary involvement.2 The main manifestations indicative of AAV are: inflammatory arthralgias or arthritis, myalgias, purpura, livedo reticularis, skin ulcers, nasal scabs/ulcers, purulent rhinorrhea, epistaxis, sinusitis, cough, hemoptysis, dyspnea, glomerular hematuria or proteinuria, arterial hypertension, paresthesias or loss of strength in extremities, diplopia or ocular proptosis, all of them not explained by another cause.
Laboratory and imaging tests should include a complete blood count and a basic biochemical analysis to detect alterations suggestive of systemic inflammation (increased erythrocyte sedimentation rate [ESR]; C-reactive protein [CRP]; alpha-1 and 2 globulins, inflammatory anemia, leukocytosis, thrombocytosis). It is essential to assess kidney function and a urine sediment to rule out the existence of microhematuria or proteinuria.3
The immunological study must include the determination of ANCA using IIF to classify them according to their pattern: perinuclear (pANCA), cytoplasmic (cANCA) or atypical (xANCA); and by ELISA to identify its antigenic specificity: proteinase 3 (PR3), which is usually associated with a cANCA pattern, or myeloperoxidase (MPO), usually pANCA. In granulomatosis with polyangiitis (GPA), 80–90% of patients have cANCA-PR3, 5–20% pANCA-MPO, and up to 20% are negative for ANCA, especially when there is isolated otorhinolaryngological (ENT) involvement. or orbital pseudotumor without renal involvement. 80% of patients with microscopic polyangiitis (MPA) have pANCA-MPO, 5–10% have cANCA-PR3, and 10–15% are negative for ANCA.
It must be remembered that the specificity of ANCA, especially pANCA, is not 100%, and that IFI techniques vary between laboratories, which is why determination of ANCA using IIF and ELISA is recommended as standard practice. Performing a routine thoracic imaging test (preferably chest computed tomography) can be very useful, since a third of patients may present pulmonary involvement despite having no clinical manifestations.4
Finally, it is recommended to obtain a histological sample to confirm the diagnosis of AAV and evaluate the extent and severity of the disease.
When considering a biopsy to made achieve a diagnostic, it should be considered that5:
- -
Cutaneous lesions are very accessible, but usually show leukocytoclastic vasculitis with fibrinoid necrosis not specific for AAV.
- -
Nasal or sinus mucosal biopsies have a low sensitivity (around 20%) despite being performed on ulcerated lesions and by expert surgeons.
- -
Transbronchial biopsies have a low sensitivity (10%).
- -
Lung biopsies are very sensitive (90%) when pulmonary nodules are present and neutrophil granulomas are observed in the granulomatosis with polyangiitis (GPA). In case of pulmonary infiltrates, it is usually observed capillaritis or pulmonary hemorrhage and the presence of granulomas is exceptional, so the differentiation between GPA and microscopic polyangiitis (MPA) should be based on other clinical aspects.
- -
Renal biopsy provides high diagnostic yield (80%). The characteristic pattern is the presence of a necrotizing glomerulonephritis, pauciimmune, with crescents. The presence of granulomas is exceptional, and therefore it is not possible to differentiation between GPA and MPA, but it allows to evaluate the extension, activity and chronicity of the lesions, and has great prognostic value.
- -
Muscle and peripheral nerve biopsy can show vasculitis of the vasa vasorum even in patients with little clinical involvement.6
- •
It is recommended to use the nomenclature of the international Consensus Conference of Chapel-Hill (CCCH), in its 2012 update, when referring to AAVs (GA: 92%).
- •
It is not recommended to use the ACR/EULAR classification criteria proposed for the diagnosis of AAV, although they can be very useful for the differential diagnosis between different vasculitis (GA: 100%).
The CCCH, in its 2012 update, introduces the group of AAVs and includes it within small vessel vasculitis (capillaries, venules, arterioles and small arteries). It defines AAV as necrotizing vasculitis with scarce or absent immune deposits, affecting small vessels with association with ANCA MPO, ANCA PR3, although in some patients ANCA is not detected despite a clinical process typical of this group of vasculitis.7
In the CCCH 2012 the following definitions were established:
- •
GPA, formerly called Wegener’s granulomatosis: necrotizing granulomatous inflammation that generally involves the upper and lower respiratory tract, with necrotizing vasculitis predominantly affecting small and medium-sized vessels, with necrotizing glomerulonephritis being common.
- •
PAM: affects small vessels (arterioles, capillaries and venules), with the possible presence of necrotizing arteritis involving small and medium-sized arteries, with necrotizing glomerulonephritis and pulmonary capillaritis being very common, which is not accompanied by granulomas.
In 1990, recognizing that biopsy and histologic study were not always possible, accessible or conclusive, the ACR published classification criteria for vasculitis.8 These criteria were designed to include, in research studies, patients who shared unequivocal features of the disease, provided they had been diagnosed with vasculitis. In other words, they selected those clinical findings that identify a disease and differentiate it from other diagnoses, although they do not include the spectrum of possible clinical manifestations of each entity, so they do not allow the identification of all cases and, therefore, are not always useful for the diagnosis of individual patients. These 1990 ACR criteria did not yet include PAM as a distinct entity or ANCA as a distinctive of group.9,10
New diagnostic and imaging techniques have contributed to better differentiate between different types of vasculitis, which has led to questioning the sensitivity and specificity of the 1990 classification criteria.11 This made it necessary to revise them using data from the multicenter observational study of diagnosis and classification criteria for systemic vasculitis (DCVAS), resulting in the ACR/EULAR criteria, updated in 2022.12,13 The approach was made based on the size of the affected vessels, and incorporating detailed clinical data, determination of ANCA, biopsy and new imaging tests, which increased its sensitivity.14 These classification criteria should be applied if the diagnosis of vasculitis is already established, and will help to differentiate between different types of vasculitis.
A summary of the development process for the ACR/EULAR 2022 classification criteria for GPA and MPA is set out in Supplementary material.
To define the 2022 ACR/EULAR classification criteria for GPA and MAP, cases and comparators were included.12,15 The final criteria and their weight are shown in Table 1.12,15 After excluding cases that “mimics”, vasculitis a patient with diagnosis a small or medium vessel could be classified as GPA or APM if the cumulative score is ≥ 5 points. As it can be observed, the presence of PR3-ANCA (or cANCA) has a great deal of weight in these criteria, such that its negativity, even in the presence of a compatible clinical picture, would make it difficult (not impossible) to classify as GPA patients who would meet the 2012 CCCH definition or the 2007 EMA algorithm.16
Classification criteria for ANCA vasculitis according to ACR/EULAR 2022.12,15
| Classification criteria | Score |
|---|---|
| Granulomatosis with polyangiitis (GPA) | |
| Nasal involvement: bloody discharge, nasal crusts, congestion or septal defects/perforation | +3 |
| Cartilaginous involvement in ear or nasal cartilage, saddle deformity or endobronchial involvement | +2 |
| Conductive or sensorineural hearing loss | +1 |
| Positive cANCA or antiproteinase 3 (PR3) | +5 |
| Pulmonary nodules, mass or cavitation on chest imaging | +2 |
| Granuloma or giant cells on biopsy | +2 |
| Sinus/paranasal sinus inflammation or consolidation on imaging | +1 |
| Pauciimmune glomerulonephritis | +1 |
| p-ANCA or anti-myeloperoxidase (MPO) ANCA | −1 |
| Eosinophil count ≥1 × 109/l | −4 |
| Microscopic polyangiitis (MPA) | |
| pANCA or ANCA-antimyeloperoxidase | +6 |
| Pauciimmune glomerulonephritis | +3 |
| Pulmonary fibrosis or interstitial lung disease | +3 |
| Sinonasal symptoms or signs | −3 |
| c-ANCA or ANCA- antiPR3 | −1 |
| Eosinophil count ≥ 1 109/L | 4 |
ANCA: anti-neutrophil cytoplasmic antibodies; ACR: American College of Rheumatology; EULAR: European Alliance of Associations for Rheumatology.
- •
Renal biopsy is recommended to confirm the diagnosis of AAV when there is renal involvement, given its high reliability and prognostic value, but its performance should not delay theinitiation of immunosuppressive treatment, especially in forms with rapid progression (GA: 100%).
- •
In case of suspected relapse with kidney involvement, a new kidney biopsy could be considered to confirm the diagnosis and establish the degree of chronicity (GA: 100%).
The presence of ANCA antibodies in a patient with PRGN has a high predictive value for the diagnosis of AAV with renal involvement (98% for PR3 and 90% for MPO),17 so it is not essential to routinely perform a renal biopsy in patients with this form of presentation to establish the diagnosis, especially if the kidney biopsy will delay the start of immunosuppressive treatment or if the risk of bleeding is very high.
It must beconsidered the possible complications of a kidney biopsy, such as the risk of bleeding in patients treated with plasmapheresis, especially if replacement is not performed with plasma,18 in elderly subjects, and of course in anticoagulated patients.19
However, there are forms of AAV, with a negative serological test and whose renal involvement is not rapidly progressive, in which renal biopsy continues to be essential to establish the diagnosis. The presence of vascular inflammation or fibrinoid necrosis in any organ continues to be a diagnostic criterion of the disease. Renal biopsy, when the organ is affected, is the one with the highest diagnostic yield (91.5% in PR3 vasculitis).20 The histological findings of the renal biopsy do not usually allow us to differentiate between types of vasculitis.
Histological findings do not determine the choice of immunosuppressive treatment in patients with AAV, although the Berden classification has been shown to have prognostic value in various populations21,22 and its information can help to decide the intensity or duration of treatment, establishing the degree of activity, or chronicity in the kidney tissue23 (Fig. 2).
Prognostic renal histopathological classification of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis.
*Since it is a systemic disease, despite the different prognosis, it is essential to treat the patient: histology should not modify the intensity of treatment.
ANCA: anti-neutrophil cytoplasmic antibody; MPO: myeloperoxidase; PR3: proteinase 3.
Some recent articles suggest that a predictive risk model that incorporate both clinical and pathological elements could be useful to identify patients with a greater probability of response to plasmapheresis.24
In case of suspected renal relapse, renal biopsy could help to rule out chronic damage or other alternative superimposed pathologies, in cases with clinical doubts on this point, although its routine performance is not recommended.
Role of other biopsiesRecommendations- •
Tissue biopsy of a clinically affected and accessible organ is recommended to confirm the diagnosis of vasculitis, whenever feasible and carefully assessing its potential yield and specificity against possible negative effects (GA: 100%).
- •
Biopsy should not delay treatment in severe cases of AAV (GA: 100%)
In addition to renal biopsies, those performed on other organs affected by vasculitis may be useful for diagnosis.
The 2022 update of the EULAR recommendations for the management of AAV6 establishes, in its first recommendation, that a positive biopsy strongly supports the diagnosis of vasculitis and recommends, whenever possible, biopsies to help establish a new diagnosis and for further evaluation in patients with suspected recurrent vasculitis. It is also highlighted that, given the severity of these entities, treatment should not be delayed while awaiting the result of the histological study.6
Biopsy is especially necessary when clinical, serological and imaging findings do not provide sufficient criteria to classify the vasculitis. For example, there may be patients with atypical or limited symptoms, negative for ANCA or with unusual patterns or specificities, or with rare organ involvement.25 In these situations, a biopsy may be useful to confirm the presence of vasculitis and differentiate AAV from other causes of vascular inflammation.2 Depending on the clinical presentation, biopsies of the skin, nose, airway, lung, muscle or peripheral nerve are indicated, with accessibility and sensitivity that varyies according to the location.3
The most accessible biopsies are cutaneous biopsies. Vascular involvement may affect superficial dermal vessels, but also the middle dermal vasculature. However, in other locations it is not always feasible to obtain a biopsy in every patient with suspected AAV. There may be barriers such as difficulty in accessing the tissue (e.g., retro-orbital tissue in the presence of a mass in GPA), unjustified risk of the procedure (patients on anticoagulant therapy), or low yield. The diagnostic sensitivity of upper airway and transbronchial biopsies has been estimated to be 30% and 12%, respectively, often resulting in nonspecific histological findings.26 In patients with lung lesions that cannot be clearly attributed to active AAV, open thoracoscopic biopsy could be considered, with a high sensitivity (80%–90%), although it is a very invasive test and it is not without risks.
The histopathological findings of neuropathy resulting from AAV are characterized by axonal degeneration of nerve fibers and inflammation of epineurial vessels, accompanied by destruction of vascular structures and/or obstruction of the lumen, with or without fibrinoid necrosis. Both myelinated and unmyelinated fibers are affected.27
Although vascular wall inflammation accompanied by vascular structural damage is necessary for the histopathological diagnosis of vasculitic neuropathy, the sensitivity of this finding is low. Neurological studies have also evaluated the usefulness of muscle biopsy together with neural biopsy in case of single-organ or systemic vasculitis, with a sensitivity of 48–80%.28 In this as in other biopsy locations, in cases of ANCA-negative or atypical AAV, it may be necessary to repeat the biopsy if the first one is negative.
Clinical manifestationsThe spectrum of clinical manifestations of AAV is very varied and ranges from isolated involvement of a single organ to multisystem disease with fulminant evolution and life-threatening.29
The presence of a general syndrome with fever, asthenia, weight loss and arthralgia appears in a high percentage of patients and may precede organic involvement by several weeks, simulating an infectious or neoplastic condition. Occasionally, some patients present an fiery condition with a rapidly progressive course in just few days.2,30
There may be some overlap between the symptoms of GPA and MPA. However, ENT or upper respiratory tract involvement is more characteristic of GPA, while peripheral nervous system injury is more typical of MPA. Pulmonary involvement in GPA may present with cavitated nodules, while in MPA it may be associated with pulmonary fibrosis (generally with a pattern of theusual interstitial pneumonia [UIP]).31
Patients with AAV have a high risk of venous thromboembolic disease, with an estimated frequency of 8%, especially during periods of disease flare. In addition, it has been demonstrated that there is an increased risk of cardiovascular (CV) and cerebrovascular (CVA) events that is respectively increased by three and eight times as compared with the general population.4
The relevance of the connection between specific clinical manifestations and the antibody profile (PR3 or MPO) has been recently highlighted. The presence of these antibodies would have a greater impact on the frequency and type of organ involvement, as compared to the final diagnosis of GPA or MPA. Furthermore, there appears to be an association between certain genetic variants and various clinical manifestations.32 The main clinical characteristics of GPA and MPA are summarized in Table 2.2
Clinical Charateristics Granulomatosis with polyangiitis (GPA) and Microscopic polyangiitis (MPA).2
| Clinical manifestations | Granulomatosis with polyangiitis (GPA | Microscopic polyangiitis (MPA |
|---|---|---|
| General symptoms - | Asthenia, fever, 70–100% weight loss | Fatigue, fever, 55–80% weight loss |
| Ear, nose, and throat symptoms | 70–90% of patients affected | Less characteristic, 19–35% of patients affected, nondestructive lesions |
| Nasal and sinus involvement most common | ||
| Otitis media, nasal septal perforation | ||
| Lower respiratory involvement | Involves lung parenchyma (85%) | Somewhat less frequent pulmonary involvement |
| - Solitary or multiple nodules | Occasional pulmonary fibrosis (NIU) | |
| Alveolar hemorrhage, pulmonary infiltrates | Alveolar hemorrhage due to severe capillaritis in 5% of cases | |
| Ophthalmologic | Up to 52% of cases | Less frequent orbital involvement |
| Orbital involvement (pseudotumor), scleritis, episcleritis | ||
| Renal involvement | Approx. 70% of patients with GPA at some time during the course of the disease. | Nearly 100% of patients with GPA |
| More acute presentation | More indolent and chronic presentation, more glomerular damage | |
| Microscopic hematuria, subnephrotic proteinuria, or rapidly progressive glomerulonephritis | More frequent limited renal involvement | |
| Mucocutaneous manifestations - | Present in 34%. | Affects 28% |
| Purpura on lower limbs, skin ulcers, granulomatous nodules | Purpura and most frequent livedoid lesions | |
| Neurological involvement | Prevalence 24%. | 22% of patients |
| Greater CNS and cranial nerves involvement | Most common peripheral neuropathy | |
| Pachymeningitis | ||
| Other manifestations | Cardiac involvement (up to 44%): pericarditis, ischemia, myocarditis | Less frequent cardiac involvement |
| Gastrointestinal involvement (10–24%) | Gastrointestinal involvement (30–50%): abdominal pain most frequent symptom | |
| Mimics inflammatory bowel disease, mesenteric ischemia |
CNS: central nervous system; UIP: interstitial pneumonia usual.
- -
In patients with clinical signs suggestive of AAV, it is recommended to determine the presence of both PR3-ANCA and MPO-ANCA (GA: 100%).
- -
The activity status of an AAV and the need for changes in treatment cannot be resulting from the ANCA titers alone (GA: 100%).
ANCAs are useful for the diagnosis, classification, prognosis, and treatment of AAVs. There are two methods to determine ANCAs: IFA and ELISA. In IFA there are two patterns: a cytoplasmic pattern (cANCA) and a perinuclear pattern (pANCA). ELISA differentiates between anti-MPO and anti-PR333 antibodies. Determination by ELISA has a higher specificity and more positive predictive value than IFA,1 although these antibodies can be negative and be found in other diseases.1
It is considered that cANCA (anti-PR3) antibodies are more characteristic of GPA while the pANCA (anti-MPO) antibodies are more characteristic of MPA or eosinophilic granulomatosis with polyangiitis (EGPA).34
Sensitivity and specificity vary between IFA and ELISA Appendix A Table S2.1 The anti-PR3 Ddetermined by ELISA, has a sensitivity of 79.8%–86.6% and a specificity of 96.8%–98.3%.35 The sensitivity for GPA is higher with anti-PR3 (74%) than with anti-MPO (11%). The Anti-MPO has a higher sensitivity for MAP than for GPA (73% vs. 7%) (Appendix A Table S3).36 Up to 30% of AAV do not present ANCA. This is more frequent in localized GPA.37
Receiver Operating Characteristic (ROC) curves have been used to determine the diagnostic value based on ANCA titers (Appendix A Table S4).35 Although relapses of AAV often occur with elevated ANCA levels ascompared to previous levels, this elevation is not necessarily indicative of a relapse,33 as shown in the meta-analysis by Mehta et al.38
Rituximab (RTX) reduce anti-PR3 by less than 50% in 14 months.39 With anti-MPO the relapses are associated with persistent anti-MPO in case of B-lymphocyte recovery, but not if these do not recover (7/12; 58% vs. 0/2; 0%, p = 0.2).39 Anti-PR3 may predict relapses.
Persistent proteinuria is not considered an indicator of active renal disease, but rather a reflection of chronic glomerular damage related to glomerulosclerosis or tubular interstitial damage.40 The significance of persistent microscopic hematuria is unclear: it may reflect glomerular activity or damage,41 although persistent proteinuria and microhematuria are signs of poor renal prognosis.
Activation of the alternative complement pathway is essential in VAA. The products of its degradation are a potential biomarker of renal vasculitis.43
Other biomarkers could be monocyte chemoattractant protein -1 (MCP-1), anti-tissue plasminogen autoantibodies,44 anti-lysosome-associated membrane protein 2 (anti-LAMP2),45 a neutrophil/lymphocyte ratio >5.945 and the neutrophil gelatinase-associated lipocalin (NGAL).46
Recent data indicate that the balance between regulatory T lymphocytes (Tregs) and regulatory B lymphocytes (Bregs), which are critical in tolerance mechanisms, is impaired in AAV.47
Appendix A Table S5 summarizes the serum, peripheral blood and urine biomarkers of AAV.
Non-serological markers of activityRecommendations- -
In the absence of serological markers to assess the activity of AAV, it is recommended to use standardized and validated scales designed for this purpose, specifically the Birminghan Vasculitis Activity Score (BVAS) (GA: 92%).
- -
It is suggested that the Five Factor Score (FFS) be calculated as a severity scale because of its prognostic information regarding mortality, which is relevant for therapeutic decision-making (GA: 92%).
In the absence of robust serological markers to establish the activity of AAV, different instruments/scales have been designed to assess the activity and prognosis of these diseases. The most widely used and validated are the BVAS48,49 and the FFS.50,51
The BVAS has been used in most clinical trials in patients with GPA and MPA to estimate disease activity and assess the response to treatment. In addition, it has been used to stratify treatment intensity and to define the concept of disease remission and relapse. Its first version, published in 1994,48 included 66 clinical and analytical items grouped into nine organs/systems (general, cutaneous; mucous membranes and eyes; ENT manifestations; thorax; CV; abdomen; renal and nervous system), each with a different value and with a maximum score for each organ/system according to its clinical relevance. In 1997 it was modified (BVAS v.2) and in 2001 it was adapted to patients with GPA (BVAS/GW). In 2008 the latest version was modified and validated (BVAS v.3)which includes 56 items grouped into nine organs/systems, with a maximum score of 63 points (Table 3). The BVAS, in addition to its usefulness in assessing disease activity, has a short- to medium-term prognostic value.
Manifestaciones incluidas en el Birmingham Vasculitis Activity Score (BVAS).49
| Score | ||
|---|---|---|
| Persistent manifestations | New or worsening manifestations | |
| General | Maximum = 2 | Maximum = 3 |
| Myalgia | 1 | 1 |
| Arthralgia or arthritis | 1 | 1 |
| Fever ≥ 38 °C | 2 | 2 |
| Weight loss ≥ 2 kg | 2 | 2 |
| Cutaneous | Maximum = 3 | Maximum = 6 |
| Infarction | 1 | 2 |
| Purpura | 1 | 2 |
| Ulcer | 1 | 4 |
| Gangrene | 2 | 6 |
| Others | 1 | 2 |
| Mucous membranes/eyes | Maximum = 3 | Maximum = 6 |
| Oral ulcers/granulomas | 1 | 2 |
| Genital ulcers | 1 | 1 |
| Inflamed glands | 2 | 4 |
| Proptosis | 2 | 4 |
| Scleritis/episcleritis | 1 | 2 |
| Conjunctivitis/blepharitis/1ueratitis | 1 | 1 |
| Blurred vision | 2 | 3 |
| Acute vision loss | – | 6 |
| Uveítis | 2 | 6 |
| Retinal disturbances | 2 | 6 |
| Otorrinolaringológicas (ENT) | Maximum = 3 | Maximum = 6 |
| Nasal secretionBloody nasal discharge/ulcers/granulomas | 2 | 4 |
| Paranasal sinus involvement | 1 | 2 |
| Subglottic stenosis | 3 | 6 |
| Conduction hearing loss | 1 | 3 |
| Sensorineural hearing loss | 2 | 6 |
| Thorax | Maximum = 3 | Maximum = 6 |
| Wheezing | 1 | 2 |
| Nodules or cavities | – | 3 |
| Pleural effusion/pleuritis | 2 | 4 |
| Infiltrate | 2 | 4 |
| Endobronchial involvement | 2 | 4 |
| Hemoptysis/alveolar hemorrhage | 4 | 6 |
| Respiratory failure | 4 | 6 |
| Cardiovascular | Maximum = 3 | Maximum = 6 |
| Absent pulses | 1 | 4 |
| Valvular heart disease | 2 | 4 |
| Pericarditis | 1 | 3 |
| Ischemic cardiac pain | 2 | 4 |
| Cardiomyopathy | 3 | 6 |
| Congestive heart failure | 3 | 6 |
| Abdominal | Maximum = 4 | Maximum = 9 |
| Peritonitis | 3 | 9 |
| Bloody Diarrhea | 3 | 9 |
| Abdominal Ischemia | 2 | 6 |
| Renal | Maximum = 6 | Maximum = 12 |
| Hypertension | 1 | 4 |
| Proteinuria | 2 | 4 |
| Hematuria | 3 | 6 |
| Serum creatinine 125–249 μmol/l* | 2 | 4 |
| Serum creatinine 250–499 μmol/l* | 3 | 6 |
| Serum creatinine ≥ 500 μmol/l | 4 | 8 |
| >30% increase in creatinine | – | 6 |
Scores for persistent manifestations can range from 0 to 33 and scores for new or worsening manifestations can range from 0 to 63.
Serum creatinine 125–249 µmol/l (1.41 mg/dL–2.82 mg/dL): serum creatinine 250–499 µmol/l (2.83 mg/dL–5.64 mg/dL).
The FFS was developed in 199650 by the French Vasculitis Study Group (FVSG) to predict survival in patients with polyarteritis nodosa (PAN), Churg-Strauss syndrome or GEPA and AMP, based on biological and clinical parameters present at the time of diagnosis, regardless of treatment, relapses or other events during the course of the disease. The 1996 FFS included five prognostic factors: proteinuria >1 g; renal insufficiency (peak creatinine 1.40 nmol/L), intestinal involvement, myocardial involvement, and central nervous system involvement, each assessed with 1 point. For a FFS = 0, 1 and > 2, the 5-year survival was 1%, 26% and 46%, respectively. The FFS 1996 has been used to stratify treatment intensity in patients afflicted with GEPA, with high-intensity treatment recommended in patients with an FFS 1996 ≥ 1.
A new revised version of the FFS (FFS 2009) was published in 2011 and also included patients with GEPA.51 The 2009 FFS includes four factors related to poor prognosis (age ≥65 years, myocardial involvement, gastrointestinal involvement, renal failure with peak creatinine of 1.50 nmol/L), each scored 1 point, and one related to good prognosis (ENT involvement), the absence of which scores 1 point. For a FFS = 0, 1 and ≥2, the five-year survival is 9%, 21% and 40%, respectively.
Prognostic markersRecommendations- •
The routine use of emerging biomarkers (B cells, complement, MCP-1, CD125, etc.) is not recommended in routine clinical practice (GA: 100%).
Although in recent years there has been progress in the number and precision of biomarkers, the prognostic value of some of those described lacks sufficient scientific evidence to be recommended in routine clinical practice in terms of prognosis or therapeutic approach.
The diagnostic value of ANCA is well established, but they are discussed as a predictive factor for relapse. Attempts have been made to correlate variations in the ANCA titer as a predictive factor for response or relapse, without enough evidence to be used to restart induction treatment.40 Other antibodies such as anti-LAMP-2 are co-expressed against MPO or PR3 in some cases of AAV and in ANCA-negative RPGN, however methods of detection have not been standardized. Other antibodies against antigens such as moesin, plasminogen or pentraxin-3, which are identified in some subgroups of patients with AAV, have not been validated as prognostic markers in AAV.52
The use of B cells or B cell activating factors such as B cell-activating factor (BAFF) or proliferation inducing ligand (APRIL) as a biomarker of activity or prognosis is limited. Some B cell subgroups (Breg), or total B cells or plasma cells have also been related to ANCA activity and levels.53,54 The T cell subgroups involved in the pathogenesis of AAV have been described in numerous studies as potential biomarkers due to their interaction with B cells. In the future, it will be defined the role of different B and T cell subgroups as markers of activity or recurrence,40 as well as the interaction between them.
The level of C3 at diagnosis has been associated with greater severity and lower renal survival, just as has the deposition of C3d and properdin in renal tissue.55 C3a, C5a or C5b-C9 have also been associated to activity in AAV,43 although they cannot be recommended as validated markers.
Some markers such as the inflammatory mediator CXCL13 (BCA-1), matrix metalloproteinase 3 (MMP-3) and metalloproteinase inhibitory factor 1 (TIMP-1) have been shown to discriminate between active and inactive AAV even better than ESR or CRP in the cohort of 137 patients from the Rituximab versus Cyclophosphamide for ANCA-associated Vasculitis (RAVE) study,56 but they lack robustness as standardized markers.
Among the urinary biomarkers, MCP-1 stands out, which is increased in urine in patients with AAV with renal involvement and has been correlated with disease activity measured by BVAS.57 Soluble urinary CD-163, released by monocytes and macrophages, has also been correlated with activity in AAV compared to AAV in remission58; its value in detecting relapses has even been described combined with the determination of soluble urinary CD125, released by activated T lymphocytes.59 In any case, these are markers with little clinical experience that require further validation studies.
Table 440,52 includes the main biomarkers, both in clinical practice and in AAV research.
Biomarkers in clinical practice and research in ANCA vasculitis.40,52
| Biomarker | Description | Role |
|---|---|---|
| Clinical practice | ||
| Serum | ||
| ANCA | Pathogenic autoantibodies against antigens expressed on neutrophil surface | Diagnostic marker1 |
| B lymphocytes | B lymphocyte dysregulation in AAV leads to inflammatory response and autoantibody production. CD20 + B cells are the target of RTX | CD20 + B lymphocytes can be used as markers of RTX effectiveness11 |
| Urine | ||
| Hematuria | Expression of glomerular inflammation/damage | Renal marker12 |
| Proteinuria | Poor correlation with disease activity | |
| Research | ||
| Serum | ||
| B naïve and regulatory B lymphocytes (Bregs) | Subpopulation of B lymphocytes, involved in self-tolerance | Correlation with disease activity has been described. |
| BAFF | Part of the TNF family. Contributes to the survival and differentiation of B lymphocytes | References related to activity. Contradictory |
| Anti-LAMP2 antibodies | Directed against glycoprotein expressed on neutrophils | Proposed as a diagnostic biomarker in VAA and RPGN-ANCA neg.Drop rapidly after treatment and reappear in relapses |
| Anti-plasminogen antibodies | Autoantibody against plasminogen | Proposed as a diagnostic biomarker in VAA. Associated with increased renal involvement2 |
| Anti-moesin antibodies | Against heparin-binding protein, moesin | Proposed as a diagnostic biomarker in AAV. Associated with increased renal involvement |
| MMP-3 | Matrix metalloproteinase 3 | Could discriminate between active and inactive AAV |
| CXCL13 | Lymphoid production-stimulating chemokine | Could discriminate between active and inactive AAV |
| IL-6 | Proinflammatory cytokine | |
| Calprotectin | Neutrophil-derived protein | Has been associated with activity along with hematuria and sCD163 |
| Extracellular vesicles | Vesicles involved in cell-cell intercommunication and homeostasis | Association with activity |
| Urine | ||
| MCP-1 | Chemokine for monocytes and macrophages | Associated with renal disease |
| CD163 soluble | Cleaved from a glycoprotein expressed on monocytes and macrophages | Associated with renal disease |
| CD25 soluble | Cleaved from activated T lymphocytes | Associated with renal involvement, more effective in association with uCD163s |
ANCA: anti-neutrophil cytoplasmic antibodies; AAV: ANCA-associated vasculitis; RTX: rituximab; TNF: tumor necrosis factor; LAMP-2: lysosome-associated membrane protein 2; RPGN: rapidly progressive glomerulonephritis; MCP-1: monocyte chemoattractant protein -1.
- •
Perform a systematic and structured assessment of disease activity and damage, using indices such as the BVAS and the Vasculitis Damage Index (VDI) (GA: 100%).
- •
Consider a 50% reduction in BVAS or BVAS/WG after six weeks of treatment, and the absence of new manifestations, as a good therapeutic response (GA: 100%).
According to the recommendations of the EULAR,6,60 the ACR/VF61 and the KDIGO62 guidelines, a structured and multisystem clinical assessment should be performed in all patients with AAV. This assessment can be facilitated by the use of indicators of activity and organ damage. The BVAS and BVAS/WG allow a standardised measurement of the degree of disease activity and provide prognostic information.49,63 Furthermore, the VDI damage index helps to distinguish damage from active disease and avoid unnecessary treatments.6
The FFS64 is used to establish the degree of systemic involvement and its potential prognostic value. A strong correlation has been shown between BVAS (Table 3)49 and FFS in AAV.65
All these indices have demonstrated a high correlation and reliability,66 which is why they have been used in most observational studies and clinical trials on AAV. According to the definition proposed by the EULAR,6,60 a response to treatment is considered to occur when there is a 50% reduction in BVAS or BVAS/WG after six weeks of treatment, together with the absence of new manifestations. In contrast, the ACR 2021 guidelines61 do not specify the concept of response, but rather simplify the term and define it as “lack of response or refractoriness to persistent active disease, despite adequate immunosuppressive treatment”. The EULAR and ACR definitions for states of disease are described in Table 5.6,61
EULAR and ACR definitions for disease states in ANCA vasculitis.6,61
| Term | EULAR | ACR |
|---|---|---|
| Active disease | Signs and/or symptoms attributed to ANCA vasculitis | New, persistent, or worsening signs and/or symptoms attributed to ANCA vasculitis and not related to previous damage. |
| Response | 50% reduction in BVAS or BVAS/WG after 6 weeks of treatment and no new manifestations | |
| Remission | Absence of signs and symptoms attributed to ANCA vasculitis with or without immunosuppressive therapy | Absence of signs and symptoms attributed to ANCA vasculitis with or without immunosuppressive therapy |
| Relapse | Recurrence or appearance of a manifestation attributable to ANCA vasculitis activity after remission | Recurrence of active disease after a period of remission. |
| Refractoriness | • Increased or no change in activity after four weeks of standard treatment.• Lack of response• Chronic persistent disease (BVAS with 1 major or 3 minor items after 12 weeks of treatment) actariedad | Persistent active disease despite adequate immunosuppressive therapy |
EULAR: European Alliance of Associations for Rheumatology; ACR: American College of Rheumatology; BVAS: Birminghan Vasculitis Activity Score.
- •
Periodic assessment of disease activity is recommended in patients with AAV due to the risk of relapse (GA: 100%).
- •
Recurrence of signs or symptoms of active vasculitis in any organ after remission is achieved should be considered a relapse. Isolated positive ANCA, should not be considered a criterion for relapse (GA: 100%).
- •
In patients with AAV who experience a relapse, it is recommended to check that suspicious manifestations are attributable to vasculitis and exclude other causes such as organ damage, infection or malignancy (GA: 100%).
Due to the high risk of relapse, AAV patients require close follow-up with disease monitoring, even after achieving remission or a control of disease activity. Most forms of AAV may relapse. Relapses may involve recurrence of active disease or the appearance of new manifestations 67,68, and may occur years after initial presentation or after prolonged remission.69 Relapse rates vary widely from the different studies ranging from 10% to 60%.67,70 This wide range may be due to: differences in the induction or the maintenance of treatment, proportion of patients with different ANCA serotypes, duration of follow-up, as well as criteria used to define relapse. Relapses are more frequent in PR3 vasculitis (25–80%) than in MPO (8–18%).71,72
Therefore, the evaluation of these patients during follow-up should include procedures aimed at checking the proper functioning of vital organs, such as blood pressure or kidney function. In addition, activity indices such as BVAS and BVAS/WG can be used to monitor the disease throughout follow-up.
Relapse of vasculitis is defined as the recurrence or appearance of signs or symptoms of active vasculitis in any organ after achieving remission, provided that these manifestations are attributable to vasculitis and not to other causes.60,69 There is no consensus among the different scientific societies on the definition of relapse or recurrence; the 2023 EULAR guidelines define it as the reappearance of activity after a period of remission6 and the ACR guidelines as recurrence after a period of inactivity.61 The diagnosis of relapse can be complex, since other diagnoses that may cause similar symptoms must be excluded. The consequences of chronic damage, infections, the appearance of malignant lesions or a new type of glomerulonephritis must be included in the differential diagnosis.73 Different risk factors for relapse in vasculitis have been identified (Table 6).
Risk factors of vasculitis relapse.
| Risk Factors |
|---|
| • Seropositivity for PR3-ANCA- Previous history of relapsed disease- Pulmonary involvement prior to remission- Upper respiratory tract involvement prior to remission- Persistence of elevated ANCA titers, particularly PR3-ANCA, and increased ANCA titers |
ANCA: anti-neutrophil cytoplasmic antibodies; PR3: proteinase 3.
Relapses can be classified as severe or mild, depending on the impact on vital organs or the compromised life. They can occur in different situations: relapse during maintenance treatment, after having completed maintenance treatment or patients with multiple relapses. Depending on each case, different therapeutic options can be applied and the treatment is individualized.3
Most relapses of GPA or MPA occur in the first 12–18 months after cessation of immunosuppressive treatment,74 although they can appear after more than 10 years after the initial presentation. Most can be detected early and are limited in patients with close follow-up and who have been educated about their disease.68 In each medical visit, the clinical and analytical biomarkers shown in Table 775,76 should be determined.
Monitoring at each medical visit of the patient with AAV.75,76
| Assessments during AAV monitoring |
|---|
| - Assessment and evaluation of signs or symptoms of active disease- Biochemical analysis including determination of renal function, proteinuria, -urinary sediment and transaminases- Blood count- Inflammatory markers (CRP) - Serological markers (ANCA, PR3 and MPO) |
ANCA-associated vasculitis; CRP: C-reactive protein; PR3: proteinase 3; MPO: myeloperoxidase.
- •
Once remission has been achieved, it is recommended to maintain the treatment for at least 24 months (GA: 100%).
- •
In patients with frequent relapses, with a high risk of relapse or severe organ damage, the maintenance treatment should be extended (three to five years), according to the patient’s preferences and the risk associated with immunosuppression (GA: 100%).
The duration of induction treatment in AAV ranges from three to six months, depending on the form of presentation of the disease, the treatment introduced and the response.6,77,78 Once remission has been achieved, it is advisable to start maintenance treatment for at least 24 months to prevent relapses.60,78,79 After this period, the duration of treatment should be individualized based on patient characteristics, type of ANCA (PR3 or MPO), persistence of ANCA, history of previous relapses, affected organs and their severity, as well as patient preferences.6,77
The randomized trial of prolonged remission-maintenance therapy in systemic vasculitis (REMAIN) demonstrated that prolongation of remission treatment with AZA for an additional 24 month period (up to 48 months) in patients with GPA or MPA reduces the percentage of relapses.80 Likewise, the MAINtenance of Remission Using RITuximab in Systemic ANCA-associated Vasculitis (MAINRITSAN-3) trial demonstrated that, in patients with GPA or MPA, prolongation of treatment with RTX for two more years, up to four years, reduces relapses without a greater number of AEs.81 However, prolonging treatment in all patients with GPA or MPA is questionable, given that 75% of patients treated with RTX for two years are disease-free.2 There is also insufficient evidence to recommend prolonging maintenance treatment based on the persistence of ANCA.6
In Eosinophilic Granulomatosis with Polyangiitis (EGPA), there are not as many studies as in GPA and MPA that compare the impact of the duration of treatment. Induction treatment is recommended to last between three and six months,77 and maintenance treatment between 18 and 24 months.60
The latest recommendations from EULAR6 and KDIGO82 recommend a maintenance treatment in patients with GPA or MPA of 24–48 month duration once remission has been achieved.
In patients with frequent relapses or at high risk of relapse, its prolongation should be considered according to the patient’s preferences and the risk associated with immunosuppression. There is no specific recommendation regarding the duration of maintenance treatment in EGPA.
Organ damage, quality of life and its assessmentRecommendations- •
It is recommended to measure organ damage periodically using a standardized scale such as the VDI (GA: 100%).
- •
Reducing the risk of organ damage is a therapeutic objective to be taken into account in all patients with AAV in order to improve their quality of life and prognosis (GA: 100%).
Patients with AAV should have access to medical specialists with experience in this disorder, ideally within a multidisciplinary context. A holistic view of the disease improves the survival and quality of life of these patients. The evaluation of the disease should consider the following domains: activity, organ damage, prognosis and quality of life.49,83
The VDI index is used to evaluate the chronic organ damage produced by activity of the disease and the toxicity of immunosuppressive drugs (cumulative dose of GC). Thisnindex allows the prediction of mortality.6,51
The deterioration of quality of life is the result of multiple factors, not only from the active inflammatory disease, but also from the sequelae of the disease, which affect psychosocial aspects such as fatigue, dysfunctionality and the musculoskeletal system.84
The improvement of immunosuppressive treatment has transformed vasculitis into a chronic disease and, consequently, the priorities of patients have been readjusted to this reality. Instead of focusing on the consequences of organic damage, patients consider fatigue and chronic pain as the main factors of the disease that impair their quality of life.
Despite the advances of new induction drugs in vasculitis, the benefits in quality of life are modest and rarely become normal. This may be explained by different reasons, such as the use of high doses of GC, which are very often accompanied by AE. In fact, the latest clinical trials have incorporated the GC toxicity index with the aim of improving its measurement, which would facilitate the reduction of GC doses, thus decreasing the organic damage derived from its use.85 In addition, generic quality of life questionnaires have been applied to patients with vasculitis that do not include specific aspects of vasculitis. Therefore, we have new tools to evaluate more accurately the quality of life of these patients.71 The OMERACT Vasculitis Working Group developed the ANCA-associated vasculitis patient-reported outcome (AAV-PRO) questionnaire, with 29 items covering six domains (organ-specific symptoms, systemic symptoms, treatment AEs, emotional AEs, worries about the future and physical function). This questionnaire is being used in clinical trials. Finally, the Patient-Reported Outcomes Measurement Information System (PROMIS) allows the measurement of fatigue, physical condition and the role of pain in daily life.86,87
Monitoring and control of activityRecommendations- •
It is not recommended to use ANCA titers as the only marker of disease activity (GA: 100%).
- •
Patients should be followed monthly until they achieve clinical remission. Thereafter follow-up visits should be scheduled every one to three months during the next year, and every three to six months afterwards (GA: 100%).
ANCA plays a fundamental role in the pathophysiology of AAV and there is no doubt about its value in establishing the diagnosis in patients with compatible symptoms. Table 888 summarizes the clinical indications that advise the determination of ANCA according to an international consensus document.88 ANCA have also been associated with other autoimmune pathologies other than those described in the table (systemic lupus erythematosus, rheumatoid arthritis, Crohn’s disease, etc.), although their diagnostic interpretation and possible prognostic value are unclear, so their routine measurement is not recommended.89
Clinical situations in which it is recommended to measure ANCA to establish the diagnosis.88
| Clinical situations in which ANCA measurement is advisable |
|---|
| - Glomerulonephritis, especially rapidly progressive forms- Pulmonary hemorrhage- Cutaneous vasculitis associated with systemic clinic- Multiple pulmonary nodules- Chronic and aggressive upper respiratory tract damage- Long-standing otitis/sinusitis- Subglottic tracheal stenosis- Multiple mononeuritis multiplex or other peripheral neuropathic damage- Retroorbital mass- Scleritis |
ANCA: anti-neutrophil cytoplasmic antibodies.
The value of ANCA as indicative of activity is controversial. It is well established that the magnitude of the titer does not correlate with the extent or intensity the damage induce by vasculitis. Nonetheless, the possible usefulness of ANCA in predicting relapses has been hypothesized. The main conclusion of a meta-analysis that included studies which evaluated the risk of relapse in cohorts with MPO and PR3 vasculitis was that an increase or persistently elevated levels of ANCA after achieving clinical remission is only a modest predictor of future relapses.90 A pragmatic approach would be to recommend monitoring the ANCA levels regularly and, if there is an increase compared to the previous situation, perform a more frequent clinical follow-up looking for the appearance of symptoms/signs of activity, but in no case would it justify a change in the therapeutic strategy.
The diagnosis of relapse should always be based on the appearance of signs or symptoms of active vasculitis in any organ. The appearance of microhematuria, especially if accompanied by an increase in creatinine or the persistece of high serum Cr values, has been recognized as a predictive sign of relapses.42,75
During follow-up visits, a close screening of the signs or symptoms of activity should be performed, as well as an analytical control that should include renal function, electrolytes, transaminases, blood count, urine protein/creatinine ratio and study of the urinary sediment (Fig. 3).
Targets and follow-up of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides with predominantly renal involvement
CRP: C-reactive protein; ANCA: anti-neutrophil cytoplasmic antibodies; BVAS: Birmingham Vasculitis Activity Score; Crs: serum creatinine; GRF: renal glomerular filtration rate; Igs: immunoglobulins; RTX: rituximab; AE: adverse effect; BP: blood pressure.
- •
In patients without severe organ dysfunction or renal involvement, it is recommended to consider the use of MTX or RTX (GA: 100%).
In cases of GPA limited to the upper respiratory tract, as well as in systemic forms without major organ involvement or life-threatening disease, less potent immunosuppressants than CFM should generally be used in the induction phase. In the ACR 2021 recommendations and according to the results of the Nonrenal Wegener’s Granulomatosis Treated Alternatively with Methotrexate (NORAM)91 randomized clinical trial, the drug of choice in these situations is MTX. However, in the 2022 update of the EULAR6 and KDIGO82 guidelines, the use of RTX is also recommended, although MTX91 or MMF92 are considered as alternatives (Figs. 4 and 5).
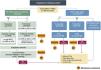
Induction therapy for anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis.
GPA: granulomatosis with polyangiitis; PAM: microscopic polyangiitis; MTX: methotrexate; GC: glucocorticoids; RTX: rituximab; PR3: proteinase 3; CFM: cyclophosphamide; AZA: azathioprine; LFN: leflunomide; Crs: serum creatinine; FGR: renal glomerular filtration rate; BR: renal biopsy; DAH: diffuse alveolar hemorrhage.
*Depending on severity, experience of use and patient tolerance.
a See Table 9- Severity of clinical manifestations, according to EULAR 2022;
b GC pulses: between 250–500 mg x 2–3;
c See Table 6- Risk factors for vasculitis relapse; d diffuse alveolar hemorrhage with ventilatory support.
To induce remission in patients with recent-onset or recurrent GPA or MPA, with disease that is not life-threatening or associated with severe organ damage, treatment with a combination of GC and RTX is recommended. MTX or MMF can be considered as alternatives.6
Rituximab- -
RTX should be prioritized over MTX or MMF in patients with GPA andM PA, even without severe manifestations, as RTX induction and remission regimens are associated with higher rates of sustained remission and lower exposure to GC. CFM is associated with long-term complications and should be avoided as a first-line option in non-severe vasculitis.6
The efficacy of MTX as a treatment to induce remission in cases without major organ or life-threatening involvement is mainly supported by data from the randomized controlled trial (RCT) NORAM.91 This trial compared the safety and efficacy of treatment with MTX 25 mg weekly with the classical oral CFM regimen at a dose of 2 mg/kg, both combined with GC in 100 patients with GPA, the vast majority of whom (94%) did not have severe involvement. The 6-month remission rate in the MTX group was comparable to the classical regimen: 89.8% vs. 93.5%. However, the response to MTX was slower in patients with more extensive disease or with significant pulmonary involvement, and remission was achieved later than with CFM. Furthermore, that comparison of MTX vs. CFM showed that the relapse rate at 18 months was notably higher (69.5% vs. 46.5%) and the time from remission to relapse shorter (13 vs. 15 months) with MTX than with CFM.91
Other treatmentsTreatment of tracheobronchial stenosisA 10–20% of patients with GPA present with life-threatening tracheobronchial strictures (TBS).93–96 TBS is a granulomatous and stenosing condition that can occur isolated or not, at the onset or during the course of the disease. The most frequent is subglottic tracheal stenosis, which usually causes dysphonia, cough, dyspnea, and stridor if it is severe. It is usually associated with other ENT manifestations or with bronchial stenotic involvement, and in 50% of cases it appears while the patient is receiving immunosuppressive treatment for other causes. Bronchial stenoses are less frequent, can affect bronchi of any size, be single or multiple, isolated or associated with pulmonary nodules.93–96 Both are usually present in cases with frequent relapses. Glottic or supraglottic tracheal involvement is less frequent and it is associated with a risk of bronchoaspiration due to immobility of the vocal cords.95–97
The treatment of ETB is complex. It includes a combination of medical treatment and endoscopic interventions is usually required to maintain airway flow. The granulomatous tissue can be resected by surgery or laser; mechanical dilatation of the stenosis can be performed using a rigid bronchoscope or balloons; local injection of steroids or mitomycin C can be administered, an endobronchial or endotracheal prosthesis can be placed, or resection of the tracheal stenotic area and end-terminal reanastomosis can be performed.93–98 There are no standardized recommendations on the procedure to be followed.
Mechanical tracheal dilatation is the most commonly used technique, and the results are favorable.93,94,97,99 Periodic dilatations are usually necessary due to relapses of the disease and the interval between them is longer if immunosuppressive treatment (MTX, leflunomide [LFN] or RTX) is administered. However, these treatments are not usually indicated when ETB occurs in isolation. In these cases, oral corticosteroids (0.5 mg/kg bw/day) and tracheal dilatation with local steroid infiltration can be administered.93–97 RTX administration reduces the risk of relapse 95–98,100 (Fig. 6).
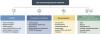
Induction treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Special situations
GC: glucocorticoids; MTX: methotrexate; RTX: rituximab; CFM: cyclophosphamide; AVA: avacopan; ANCA: anti-neutrophil cytoplasm antibodies; AMBG: anti-glomerular basement membrane antibodies.
In case of long (> 1.5 cm) and severe (> 70%) tracheal stenosis, surgical treatment is advised. In case of a critical stenosis (> 70%) of the bronchial lumen and it is not possible to resect the granulomatous tissue and dilate the stenotic area, an endoprosthesis can be placed. Some patients develop tracheomalacia and require a permanent endotracheal prosthesis. Sometimes a tracheotomy is necessary to ensure airway flow.
Other surgical proceduresIn patients with nasal cartilage collapse, may beneft from reconstruction by performing costal cartilage implantation. It is important that the disease is in complete remission for at least six months and under immunosuppressive therapy, and if therapy has been discontinued, allow at least six to 12 months prior to surgery.101 Repair of nasal septal perforation is only recommended when it is less than 2 cm and the disease is in prolonged remission, given its high tendency to relapse.100,101
In patients with orbital pseudotumor, surgical decompression should be considered in the cases of uncontrollable pain, proptosis or optic nerve compression with no response to intensive immunosuppressive therapy.101,102
In patients with persistent epiphora or recurrent dacryocystitis due to involvement of the lacrimal sac or nasolacrimal duct, surgical or endoscopic dacryocystostomy can be performed.101,102 Endoscopic management allows simultaneous treatment of nasosinusal disease, which usually coexists, and avoids the risk of nasocutaneous fistula or endonasal cyst, as complications of surgical management, due to wound necrosis.101,102
Induction therapy with severe organ dysfunctionRecommendations- -
For remission induction therapy in generalized forms of VAA, it is recommended the use of RTX or CFM, together with GC. In relapsed cases, PR3 vasculitis or for long-term safety reasons (fertility or oncologic risk) RTX should be prioritized. (GA: 100%)
- -
CFM administration should be considered to induce remission in severe cases, situations in which it can be used as monotherapy or associated with RTX, and avoid to exceed 10 g of cumulative dose or more than six months of exposure (GA: 100%).
- -
CFM should preferably be used as intravenous (iv) pulses, the opposed to the oral modality whch is associated with greater toxicity (GA: 100%).
- -
It is recommended to administer two to three pulses of GC (250–500 mg) followed by an oral administration (0.5–1 mg/kg/weight according to severity and form of presentation) and proceed to decrease the dose trying to reach a dose of 5 mg/day at five months, provided that the clinical situation allows it (GA: 83%).
- -
In patients with reduced GFR or high risk of presenting AEs associated with corticosteroid therapy, the use of avacopan can be considered, always administered in combination with standard treatment (RTX or CFM) (GA: 83%).
- -
In cases of severe renal involvement or alveolar hemorrhage, it is recommendedto consider the combination of RTX and CFM (GA: 100%).
- -
In patients with severe diffuse alveolar hemorrhage (DAH) or rapidly progressive renal failure it is recommended to prudently consider plasma exchange (GA: 69%).
- -
Mycophenolic acid derivatives can be considered as alternative induction drugs in patients with MAP without severe renal involvement (GA: 83%).
Due to their clinical similarities, the same treatment scheme is used in GPA and PAM. According to the recommendations for the treatment of AAV developed in 2022 by EULAR, the European Renal Association-European Dialysis and Transplant Association60 (ERA-EDTA) and KDIGO in 2024, the treatment is stratified according to disease severity (Table 9) and is divided into a remission induction phase and a remission maintenance phase.6
Severity of the clinical manifestations, according to EULAR 2022.6
| Potentially life-threatening or with serious organ damage* | Initially not life-threatening or with serious organ damage*,a |
|---|---|
| GlomerulonephritisAlveolar hemorrhage | Nasal or paranasal disease without bone lesion, cartilage destruction, olfactory dysfunction or hearing loss |
| Meningeal involvement | Cutaneous vasculitis without ulceration |
| Central nervous system lesion | Myositis (isolated skeletal muscle) |
| Retro-orbital disease | Non-cavitated pulmonary nodules |
| Cardiac disease | Episcleritis |
| Intestinal or mesenteric vasculitis | |
| Mononeuritis multiple |
ANCA: anti-neutrophil cytoplasmic antibodies; AAV: ANCA-associated vasculitis.
According to the European Group for the Study of Vasculitis (EUVAS), generalized disease is defined as renal involvement with serum creatinine values ≤ 500 mmol/l (5.6 mg/dL)103 or of any other organ that threatens the patient’s life.
Two therapeutic schemes can be used to induce disease remission: CFM plus GC or, alternatively, RTX plus GC.
When CFM is used, it is preferable its administration in the form of monthly iv pulses, although classically it was administered orally in doses of 2 mg/kg/day (in severe cases 5 mg/kg was administered in the first three days of treatment and maintained for a maximum of three months after induction of remission). The Cyclophosphamide Daily Oral versus Pulsed (CYCLOPS) randomized controlled trial demonstrated similar remission rates with both routes of administration.104 With oral CFM there were more AEs, but lower relapse rates.72
The most commonly used IV CFM protocols are two: a) that of the French group,105 which adjusts the dose to body surface area by administering three biweekly boluses of 0.6 g/m2 (days 1, 15 and 29) and, subsequently, pulses at a dose of 0.7 g/m2 every 21 days, up to a total of six boluses; and b) the British Rheumatology Society scheme106 which adjusts the dose to body weight at a rate of 15 mg/kg (maximum 1. 500 mg) and also advises administering initially three biweekly pulses and, subsequently every three weeks, also recommending a total of 6. The dose of CFM should be adjusted according to creatinine and should be decreased by 25% in patients older than 65 years.
RTX has been shown to be as effective as CFM in inducing disease remission in two randomized clinical trials (RITUXVAS and RAVE),107,108 being even superior in relapsed cases (RAVE),107 and with a better safety profile. In the recommendations for the treatment of AAV published by ACR/VF in 2021, the use of RTX was prioritized over CFM to induce remission.61 In contrast, the 2022 update of the EULAR recommendations only prioritizes the use of RTX in relapsed cases. With respect to dosage, observational studies suggest that a schedule of 1 g of RTX on days one and 15 achieves the same results as a schedule of four weekly infusions of 375 mg/m2.109
Both CFM and RTX should be administered in combination with GC. Classically, the initial doses recommended of prednisone (PDN) is 1 mg/kg/day and progressively reduce it from the first month trying to reach a dose of 7.5–10 mg at three to six months. Several recent randomized clinical trials (Plasma Exchange and Glucocorticoids in Severe Antineutrophil Cytoplasmic Antibody-Associated Vasculitis [PEXIVAS], the Low-Dose Glucocorticoid Vasculitis Induction Study [LoVAS] and the Rituximab With Azathioprine as Maintenance Therapy in Relapsing ANCA-associated Vasculitis [RITAZAREM])110–112 have shown that induction regimens with reduced doses of PDN achieve the same efficacy as high doses of PDN, with a significant decrease in severe infections and AEs.
Based on this evidence, the ACR/VF recommendations recommend the use of reduced doses of GC to induce remission.61 In the 2022 update of the EULAR guidelines, as in the KDIGO guidelines, it is still recommended a PDN dose of 50 to 75 mg/day in the induction phase, but with a rapid tapering, following the protocol of the PEXIVAS randomized clinical trial, so that, at one week, the GC dose should be reduced by half, with a further progressive tapering until reaching doses of 5 mg/day at four to five months (Fig. 7).6
Avacopan, a selective oral inhibitor of the complement C5a receptor, has been approved to induce remission in adult patients with active and severe GPA or MAP, always administered in combination with standard treatment (RTX or CFM).113 In United States its use has not been authorized as an alternative to GCs; in Europe this possibility is left open. The dose that has been approved is 30 mg every 12 hours orally. The addition of avacopan to standard therapy for 52 weeks results in a better control of disease activity with a marked GC-sparing effect114 (Fig. 4).
Extremely severe expression of the diseaseSevere generalized disease is defined as renal involvement with serum creatinine values > 500 mmol/L (5.6 mg/dL) or the presence of DAH. DAH is an emergency that requires early diagnosis and aggressive treatment. It refers to different forms of hemorrhage that originate in the pulmonary microcirculation (alveolar capillaries, arterioles and venules) and can affect various areas of the pulmonary parenchyma.115
In these severe generalized forms, there are two possible therapeutic strategies: 1) boluses of methylprednisolone (500 mg or 1 g for three days) prior to the start of treatment with PDN; and 2) as proposed in several clinical studies, a combination of CFM and RTX, with acceptable efficacy results and a good safety profile (two doses of CFM associated with four doses of RTX).116
Until recently, plasmapheresis (seven sessions in 15 days according to data from the MEPEX randomized clinical trial) was recommended to prevent or reduce progression to end-stage renal disease (ESRD). However, according to recent evidence provided by the randomized PEXIVAS trial110 and several meta-analyses,117 in the ACR/VF 2021 recommendations and in the 2022 update of the EULAR guidelines and KDIGO 2024 guidelines, the use of plasmapheresis in patients with pulmonary hemorrhage is discouraged. The reasons are that it does not provide any additional benefit and increases the risk of severe infection.6,61 Its systematic use is not recomended in all patients with severe renal compromise, although the possibility is left open in the subgroup of patients with a higher risk of progression to ESRD (creatinine >300 micromol/L according to the latest update of the EULAR guidelines), and the decision must be individualized in each case, taking into account the benefit-risk ratio. Plasmapheresis continues to be indicated in patients with GPA or MPA who also present anti-GBM antibody positivity (Fig. 4).
The available evidence for specific treatments is specified below.
RituximabIt is a glycosylated immunoglobulin (Ig) containing the constant regions of human IgG1 and variable region sequences of murine light and heavy chains that binds specifically to the CD20 antigen. Possible mechanisms of effector-mediated cell lysis include complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity. The various recommendations, especially those of the ACR, EULAR and KDIGO, often consider PAM and GPA together.
To induce remission in patients with recent-onset or recurrent GPA or GAM with life-threatening disease or potentially serious organ damage, it is recommended treatment with a combination of GC and RTX or CFM. RTX is preferred in recurrent disease. RTX is gaining supprt over CFM, mainly because of the latter’s lower long-term safety. CFM increases the risk of premature ovarian failure and male infertility118,119 and it is associated with the development of bladder cancer, as well as bone marrow failure, myelodysplastic syndrome, lymphoma and other malignancies.120
In AAV patients receiving RTX, it is recommended that serum Ig concentrations be measured before each RTX cycle to detect possible a secondary immunodeficiency.
There are currently four studies analyzing the use of RTX for treatement of induction in vasculitis (three randomized controlled clinical trials and one meta-analysis). All three trials use one cycle of RTX with the oncology guideline (375 mg/m2/week for four doses) compared to CFM. The meta-analysis compares this RTX guideline with the rheumatoid arthritis guideline (two doses of 1 g separated two weeks). The RAVE107 study is a non-inferiority study comparing the use of RTX with CFM (2 mg/kg/day orally) to induce remission. Follow-up time was six months. Remission was defined as a BVAS/WG score of zero and a successful decrease in PDN at six months. Sixty-four percent of patients treated with RTX achieved remission as compared to 53% with CFM (p < 0.001), demonstrating the non-inferiority of RTX versus CFM. The RITUXVAS study108 included 44 patients with newly diagnosed AAV, all with renal involvement. Of these, 33 were treated with RTX and two pulses of iv CFM (15 mg/kg) and 11 patients with iv CFM for three to six months. Both groups received the same GC regimen (1 mg/kg/day initially, with reduction to 5 mg/day at six months). Remission was defined as a BVAS score of 0 maintained for two months, and sustained remission if maintained for at least six months. After 12 months of follow-up, 76% of patients treated with RTX vs. 82% of treated with CFM met the goal of sustained remission. In the RITAZAREM112 study, there is a four-month induction phase in which patients received RTX according to the oncology guideline. In this study, remission was defined as a BVAS/WG score <1 or a dose of PDN or equivalent <10 mg/day. Ninety percent of patients achieved remission after four months of treatment. Finally, a meta-analysis of observational studies109 compared the oncology regimen with the rheumatoid arthritis regimen without finding differences in efficacy or safety.
CyclophosphamideCFM, an alkylating agent of unquestionable efficacy in VAA, continues to have a role in the induction of remission, reserved for severe cases that may compromise the survival of the patient or a vital organ, as stated in the recent update of the EULAR recommendations.6 Given its high toxicity associated with the accumulated dose, it is not currently recommended in the maintenance of remission.
Its mechanism of action is not well understood. CFM has a significant effect on B lymphocyte and plasma cells. In addition, it reduces the synthesis of adhesion molecules and proinflammatory cytokines, which could explain its relatively rapid effect on VAA.121
In an attempt to reduce the toxicity of CFM while maintaining its efficacy, different optimization strategies have been carried out. A meta-analysis showed the superiority, in terms of AE, of pulsed versus oral MFC,122 confirmed in the randomized clinical trial CYCLOPS.104 Although some recommendations, such as those of the ACR/VF, continue to contemplate the use of oral MFC,61 its use by this route is marginal.
Current protocols for the administration of CFM in pulses coincide in the aministration of three biweekly doses of 15 mg/kg or 600 mg/m2, continuing with pulses every three to four weeks until remission is achieved (maximum six months of exposure). In total, one does not come to accumulate more than 8–10 g of CFM, a dose considered safe in terms of risk of non-cutaneous neoplasia.123
It has been suggested a dose adjustment of CFM pulses according to age and GFR (Appendix A, Table S6).60 Pagnoux et al. compared a low-dose regimen (maximum six pulses of 500 mg iv every two to three weeks, in induction and maintenance with AZA or MTX) versus standard CFM doses in elderly patients in a randomized clinical trial finding no difference in remission rate and a lower incidence of AEs.124
As a consequence of the randomized clinical trials that have shown non-inferiority of RTX with respect to CFM and considering its greater safety, 107,108 we have witnessed a progressive substitution of CFM in favor of RTX.6
In extremely severe situations, some authors propose the combination of CFM and RTX, to induce remission presuming a faster effect a more aggressive treatment. This strategy is supported by the fact that RTX has no effect on B lymphocytes that do not express CD20, such as memory B lymphocytes or plasma cells. Although no randomized clinical trials are available, uncontrolled retrospective studies suggest its usefulness. These are mostly small series of patients with heterogeneous patterns.116,125,126 However, one of them included 66 patients and compared the combination with a historical control group, extracted from the EUVAS randomized clinical trials and adjusted by a propensity score for severity. Patients treated with the combination had lower mortality, lower risk of progression to advanced chronic kidney disease and relapse, without relevant safety events.127 However, the association has not always been successful or safe and causes a greater decrease in Ig.128 An ongoing randomized clinical trial, ENDURRANCE l, will attempt to evaluate the presumed superiority and safety of the association over each therapy separately.129
The new protocols for the use of CFM in induction, with less exposure time and a markedly lower cumulative dose, have reduced, but not eliminated, the risks of infection, neoplasia and infertility.130
The current increase in the risk of cancer associated with the use of CFM seems to depend on the increase in non-melanoma skin cancer, without a clear increase in bladder cancer.130,131 However, at present, bladder surveillance after exposure to CFM is still recommended, regardless of the regimen used6,132 as well as adequate photoprotection, also avoiding smoking.131 Other neoplasms, such as lymphoproliferative neoplasms, have also been associated with the use of CFM. In contrast, RTX has not been associated with an increased incidence of cancer as compared to the general population.120
In terms of fertility protection, the safest option with the most evidence is the use of gonadotropin-releasing hormone (GnRH) agonists such as leuprorelin, evidence extrapolated from its use in patients with SLE.133,134
CorticosteroidsCorticosteroids continue to be essential in the remission induction therapy of AAV. They are necessary to rapidly reduce inflammation until the biological effect of other immunosuppressive agents have their effect.3,6
Classically, high initial doses have been used, followed by progressive tapering. The most common therapeutic schemes have been iv pulses of methylprednisolone (500–1,000 mg/day, three to five days) or oral PDN (1 mg/kg wt/day) or its equivalent. In patients with RPGN or DAH, methylprednisolone pulses have been used more than upfront oral PDN, although there are no randomized studies comparing both guidelines.3,6 Recently, a multicenter, observational study has been published including 114 patients from five centers in Europe, in which it was observed that the administration of pulses has no greater benefit in inducing remission and is associated with a greater risk of infections and a higher incidence of diabetes.135 In this regard, the latest EULAR recommendations advise the administration of pulses only in patients with renal involvement with a renal glomerular filtration rate (GFR) <50 mL/min/1.73 m2 or DAH.6
Similarly, the oral guideline of 1 mg/kg bw/day is controversial, since the LoVAS136 and RITAZAREM137 studies showed that in both newly diagnosed and relapsed AAV patients, a dose of 0.5 mg/kg/day of GC was sufficient to induce remission. Likewise, the PEXIVAS study110 demonstrated that the classic GC reduction scheme could be performed more rapidly, reaching doses of 20 mg/day at 7 weeks and 5 mg/day at 19 weeks. This guideline reduced the total dose of GC by 40% during the first six months, thus reducing severe infections during the first year without decreasing the efficacy in obtaining remission. In the latest EULAR6 recommendations, the titration and tapering regimen used in this study is recommended (Fig. 7).
The use of different methylprednisolone i.v. pulse regimens in the induction phase is based on clinical judgment. Given that the evidence is only indirect through studies such as PEXIVAS or ADVOCATE, the different regimens should be evaluated in future prospective randomized studies.138
Complement blockersThe role of complement in AAV has always been a matter of controversy139; however, experimental models have shown that ANCA-induced activation of neutrophils resulted in the release of alternative complement pathway factors and reduced the activity of regulatory factors such as factor H, favoring the severe necrotizing leukocytoclastic inflammation characteristic of acute AAV.140–143 Activation of the alternative complement pathway leads to the formation of C5a, a potent anaphyllotoxin that, after binding to its receptor (C5aR/CD88), activates neutrophils and the endothelium, increasing vascular permeability.144,145 This complex is involved in the formation of crescents. In experimental models with mice expressing this receptor, the administration of avacopan (CCX168), a C5aR antagonist molecule, improved necrotizing extracapillary glomerulonephritis induced by anti-MPO antibodies, since it dose-dependently blocks neutrophil tissue migration, reduced proteinuria, hematuria and leukocyturia, and also the percentage of crescents.140,146,147
The positive results in experimental studies with avacopan prompted clinical trials to demonstrate this benefit. The first of these was the Phase 2 trial (CLEAR), which analyzed 67 patients with newly diagnosed or recurrent AAV with GFR >20 mL/min/m2 (without RPGN or DAH with hypoxia), and compared the effect at 12 weeks of treatment with oral PDN 60 mg/d vs. avacopan 30 mg twice daily + PDN 20 mg, and only avacopan 30 mg twice daily. It was observed a greater reduction in BVAS and albuminuria in the two avacopan groups. In the group that did not receive PDN, patients had better quality of life and a reduction in steroid-associated AEs.148 The CLASSIC study analyzed the safety of avacopan in 42 patients with newly diagnosed VA. No differences in the rate AE were observed among the three groups, but standard immunosuppression plus avacopan 30 mg was superior to the other patterns in inducing early remission, increasing GFR and improving quality of life.149
The ADVOCATE study included 331 patients with newly diagnosed or recurrent AAV and severe AAV requiring conventional immunosuppression.114 The study evaluated the effect of avacopan (30 mg/12 hours for one year) versus PDN (starting 1 mg/kg/d with progressive reduction until withdrawal at month 6). The primary endpoint was remission induction at week 26 (BVAS = 0) and maintenance at 52 weeks. Avacopan demonstrated non-inferiority in inducing remission at 26 weeks, and superiority at 52 weeks, with no increase in AEs. The relapse rate was lower in the avacopan group (10 vs. 21%). The increase in GFR was greater in the avacopan group (7.3 vs. 4 mL/min/1.73 m2; p = 0.026). A subanalysis demonstrated better outcomes in avacopan patients treated with RTX, ANCA-positive and recurrent MPO. Importantly, in patients with a GFR <20 mL/min/1.73 m2, avacopan had a greater increase in GFR over the group treated only with GC (16 vs. 7.7 mL/min/1.73 m2).150
Other complement blockers have been used for the treatment of refractory AAV(eculizumab)151 or are being evaluated (vilobelimab), showing promising results.152
PlasmapheresisPlasmapheresis eliminates circulating ANCA, which play a key role in the pathogenesis of AAV.153 Despite the reduction of ANCA achieved with plasmapheresis, in patients who do not present significant deterioration of renal function or DAH, its use is not recommended.154 This is due to the fact that the creatinine level at the time of diagnosis is one of the main predictors of ESRD in the medium to long term154 and that the detrimental effects related to the use of plasmapheresis (increased risk of infections, catheter-related problems, hypocalcemia, etc.) are greater than the benefit obtained in patients with acceptable initial renal function.155,156
Plasmapheresis has been used during several decades for the treatment of AAV with severe renal involvement (Cr >5.7 mg/dL). The most commonly used therapeutic scheme is 7 sessions over 14 days. The recommendation was based on the results of the MEPEX clinical trial, which showed that, in this type of patient, the addition of plasmapheresis to standard immunosuppression reduced the risk of progression to ESRD by 24% at month 12 (p < 0.03), with no differences observed in AEs.157 However, in a subsequent analysis of the same group of patients carried out over the long term, no difference was observed in the rate of ESRD or mortality between the two groups.110 In 2020 it was published the PEXIVAS trial,158 this study compared the efficacy of plasmapheresis associated with usual immunosuppression in more than 700 patients with AAV and renal involvement (GFR <50 mL/min/1.73 m2) or DAH. There were no significant differences in achieving the composite primary endpoint (death-ERT) at the end of follow-up between the two groups, in addition there were no differences in the probability of maintaining long-term remission, and in the rate of serious AEs and infections at 1 year. In patients with severe renal involvement, no significant difference was observed in achieving ESRD; however, the wide confidence intervals obtained suggest that the study may not have sufficient power to detect differences between the subgroups.158 Therefore, the recommendation to perform plasmapheresis in patients with severe renal involvement is not clear, and it is necessary to assess the benefits and risks according to the type of patient (with more marked risk in older and very immunosuppressed patients, as well as in patients with worse renal function at the time of diagnosis) (Table 10).2,24,159 Histology can help in decision making, since in patients with a great deal of chronic damage in the renal biopsy, plasmapheresis may be more harmful than beneficial.23,24,159
Recommendation on the use of plasmapheresis in patients with VAA.2,24,159
| Use of plasmapheresis in ANCA vasculitis | |
|---|---|
| Against | For |
| Patients with mild-moderate renal involvement (creatinine < 5.7 mg/dl) | Patients with severe renal involvement (creatinine > 5.7 mg/dl or on dialysis) |
| Patients with isolated DAH without hypoxemia and no need for mechanical ventilation) | Patients with DAH severe (hipoxemia sO2 < 85%, need for mechanical ventilation) |
| Patients with high risk of infections | |
ANCA: anti-neutrophil cytoplasmic antibodies; DAH: diffuse alveolar hemorrhage.
In patients with severe DAH (oxygen saturation <88%) no benefit of plasmapheresis was observed in the PEXIVAS trial, but the number of patients included was very small (31 vs. 30), and the confidence interval very wide (RR 0.67; 0.28–1.64), again indicating lack of statistical power to detect differences between groups.110 In another study that included 73 patients, 34 of whom required mechanical ventilation, plasmapheresis did not reduce mortality, but the group of patients who received plasma exchange showed signs of greater severity at the time of diagnosis.160 The recent KDIGO guidelines82 recommend that, given the severity of this condition and the lack of alternative therapeutic options, the benefit/risk of plasmapheresis should be carefully evaluated.161,162
Mycophenolic acid derivativesMycophenolic acid and its derivatives (MMF and sodium mycophenolate) inhibit T and B lymphocyte proliferation through inhibition of inosin-5-monophosphate dehydrogenase, which suppresses cellular immune response and antibody formation. Unlike CFM, they are not associated with urothelial neoplasia or infertility. Initial trials in phase II and small studies suggested their efficacy in inducing remission in VAA, especially in ANCA-MPO AAV with renal involvement.163,164 To test this hypothesis, the MYCYC trial was conducted. This open-label, randomized, controlled, non-inferiority trial that compares MMF (2–3 g/day) versus CFM, followed by AZA in both groups, for the treatment of AAV in patients without life compromise or severe renal involvement (no rapidly progressive forms or GFR < 15). There were 140 patients with newly diagnosed AAV. 92 MMF was non-inferior to CFM in inducing remission (67% vs. 61%). However, the relapse rate was higher in the MMF group (33% vs. 19%, p = 0.049), especially in patients with PR3-ANCA (48 vs. 24%). There was no difference in the rate of severe infections (26% vs. 17%), nor in other AEs (including ESRD and death).
Two recent meta-analyses165,166 confirm that the remission rate with MMF is similar to that with CFM, with no reduction in AEs (leukopenia and infections) in the short term. A detailed analysis of the data reveals a great deal of heterogeneity in the patients included in the studies. A higher remission rate is observed in the trials that include only patients with renal involvement than in those with and without renal involvement (92 vs. 56%).92,163,164,167–171 The patients most responsive to MMF are MPO-ANCA-positive, with mild-moderate renal involvement and without vital organ involvement.
The IMPROVE trial172 compared maintenance treatment in AAV (GPA, PAM) with AZA versus MMF in 156 patients after induction of remission with GC and CFM. After a 39-month follow-up, an increased relapse rate was observed in the MMF group (55% vs. 37.5%, p = 0.02). There were no differences in secondary endpoints (GFR or incidence of AE at the end of follow-up), although a trend toward a greater reduction in proteinuria was observed in the MMF group, which was not statistically significant.
Another retrospective study with 67 mostly MPO patients (>90%) analyzed maintenance treatment with MMF after induction with MMF or CFM, and showed that the relapse rate was low (9%), and that in patients initially treated with CFM there were more infections, with neutropenia and neoplasms only in patients in this group.173 A systematic review showed that relapses were higher in patients treated with MMF (45%) compared to a previous cohort treated with CFM (14.5%).165 A meta-analysis showed that there were no differences in the maintenance of remission between MMF and other treatments, but in studies that included patients with renal involvement, remission was superior with MMF.104 Finally, the IMPROVE trial, in which AZA was superior to MMF, included patients with and without renal involvement, which has conditioned the result.
No trial has been performed comparing the maintenance of remission between MMF and RTX in patients with renal involvement.
AzathioprineAZA plays an important role in the treatment of AAV. Historically, it has been positioned as the preferred treatment to maintain remission after induction with CFM (CFM), being characterized by its excellent safety and tolerability profile, managing to reduce relapses to 14% per year after remission. It is advisable to measure thiopurine methyltransferase enzyme activity for better dose adjustment, thus avoiding toxicities such as cytopenias, hepatitis, pancreatitis and diarrhea. Although AZA generally has a good safety profile, an increased risk of lymphoid and cutaneous neoplasms has been reported.6
As for biologic therapy, the MAINRISTAN and RITAZAREM studies demonstrated the superiority of RTX vs. AZA in relapse prevention, presenting a similar safety profile and even a lower mortality rate. The MAINRISTAN study showed that in patients with newly diagnosed or relapsed GPA or PAM, RTX was superior to AZA at 28 months, this difference was maintained even at 60 months (recurrence-free survival of 72% vs. 49%), even after discontinuation of RTX treatment. The RITAZAREM study compared RTX 1,000 mg every four months for two years vs. AZA, and showed at 24 months a greater efficacy of RTX in preventing recurrences (18% vs. 38%).6,174,175
Maintenance treatmentMaintenance treatment without major organ dysfunctionRecommendations- -
RTX or MTX should be considered as first-line options for maintenance of remission without major organ involvement (GA: 100%).
- -
AZA, LFN or mycophenolic acid derivatives could be useful as second-line drugs in maintenance in non-severe forms of AAV (GA: 77%).
The efficacy of MTX for maintenance of remission appears similar to that of AZA. A randomized clinical trial compared the efficacy and safety of AZA at doses of 2 mg/kg vs. MTX 25 mg/weekly for 12 months in 126 patients after induction with CFM and GC.176 There was no significant difference in the relapse rate (36.5% in the AZA-treated group and 33% in the MTX-treated group), but the frequency of AE was higher in the MTX group (55.5% vs. 46%).176,177 In vasculitis with ENT involvement, the use of MTX is preferable.
The efficacy and safety of LFN at a dose of 30 mg/day for maintenance of remission only has been compared with MTX in a randomized clinical trial conducted in 54 patients after induction with CFM and GC. The frequency of relapses was lower in the LFN-treated group (23% vs. 46.4%) and more severe relapses also occurred in the MTX-treated group, which prompted premature cessation of the study. However, the frequency of AE was higher with LFN. The poor results obtained with MTX could be explained by the dose used in the study (starting with 7.5 mg weekly, with a progressive increase until reaching 20 mg from the eighth week onwards).178,179
AZA, in comparison with other immunosuppressants, is considered similar to MTX for the maintenance of remission, as demonstrated by the WEGENT study, and superior to MMF according to the IMPROVE study, and MMF should be used only in those cases in which it is not possible to use AZA or MTX.172,176
Maintenance treatment in cases with important organ dysfunctionRecommendations- -
It is recommended to maintain a dose of PDN or equivalent ≤ 5 mg/day for up to 18 months, depending on severity and adjuvant treatment. In selected patients, longer use as an adjuvant to maintain remission may be warranted, provided that severe infections or vertebral fractures do not occur (GA: 85%).
- -
RTX is recommended as the drug of choice for maintenance of remission (GA: 92%).
- -
Maintenance with RTX can be done following a fixed dosing schedule or on demand (GA: 83%).
- -
If RTX is not chosen or in case of intolerance or toxicity to RTX, it is recommended to administer AZA or mycophenolic acid derivatives (GA: 100%).
- -
Avacopan can be used as adjuvant in maintenance of remission until completion on one year of treatment (GA: 92%).
Figs. 8 and 9 show the treatment for maintenance of remission, with and without major organ involvement, and the recommended doses for each drug.
Maintenance immunosuppressive treatment of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis.
GC: glucocorticoids; MTX: methotrexate; RTX: rituximab; CFM: cyclophosphamide; AZA: azathioprine; LFN: leflunomide; MMF: mycophenolate mofetil; AMF: mycophenolic acid; AVA: avacopan; IgG: immunoglobulin G; GPA: granulomatosis with polyangiitis; IS: immunosuppressants; ANCA: anti-neutrophil cytoplasmic antibodies; PR3: proteinase 3; ENT: otorhinolaryngology.
The available evidence for specific treatments is included below.
GlucocorticoidsAlthough it is not available high-quality evidence to determine the optimal dose and duration of GCs for maintenance of remission, it could be recommended to maintain a dose of PDN or equivalent 5 mg/day for up to 18 months, depending on severity and adjuvant treatment. A meta-analysis180 demonstrated that prolonged GC utilization during remission was associated with a lower frequency of relapse. However, the designs of the clinical trials performed do not allow to draw definitive conclusions. Therefore, it is not possible to establish general recommendations on the use of GC to maintain remission. The emergence of new GC-sparing treatments, such as avacopan among others (Fig. 7),181 will probably modify traditional treatment regimens.
In selected patients, more prolonged use as an adjuvant to maintain remission may be justified, provided that severe infections or vertebral fractures do not occur.
RituximabRTX is recommended as the drug of choice for maintenance of remission (Fig. 8).
The pivotal studies of maintenance treatment with RTX are the MAINRITSAN 1 and 2 studies and RITAZAREM. The MAINRITSAN trial174 compared AZA vs. a RTX regimen of 500 mg iv every six months (first cycle two 500 mg boluses) for 18 months. RTX was significantly superior to AZA in maintaining remission at month 28 (flare-free period), a difference that was maintainedduring the prolongation of the study to a further 18 months (MAINRITSAN 3).81 AEs were similar in both groups.
In the MAINRITSAN 2 study,182 two RTX regimens were evaluated for maintenance of remission: a fixed RTX regimen of 500 mg every six months vs. another of 500 mg individualized on demand, when ANCAs became positive or the if the titer increased or the CD19+ B-lymphocyte counts exceeded 0/mm3. The duration of treatment was 18 months. After 28 months of follow-up 9.9% of patients receiving the fixed regimen relapsed, compared to 17.3% of patients receiving the on-demand regimen, although this difference was not significant (p = 0.22). However, it was achieved a reduction in the cumulative dose of RTX.
In the RITAZAREM study, after a four-month remission induction phase with RTX, the RTX maintenance regimen of 1 g/4 months for 20 months was superior to AZA (2 mg/kg/day)137 (Fig. 9).
AzatioprinaIn the different European and American guidelines and recommendations, AZA is placed as second line if RTX is not an option.
Mycophenolic acid derivativesIn the case of maintenance with MMF (Fig. 9), a retrospective study with 67 patients, mostly with MPO phenotype (>90%), analyzed maintenance with MMF after induction with MMF or CFM. It was shown that the relapse rate was low (9%), and that in patients initially treated with CFM there were more infections and only patients in this group presented neutropenia and neoplasms.173 In a systematic review it was observed that relapses were higher in patients treated with MMF (45%) compared to a previous cohort treated with CFM (14.5%).165 In a meta-analysis there were no differences in the maintenance of remission between MMF and other treatments, but in studies that included patients with renal involvement, remission was superior with MMF.104 No clinical trial has been conducted comparing the maintenance of remission with MMF vs. RTX in patients with renal involvement.
AvacopanFinally, avacopan can be used as an adjuvant in maintenance of remission until completion of one year of treatment. However, the optimal duration of treatment with avacopan and its long-term safety have not yet been established183 (Fig. 9).
Treatment of relapsesRecommendations- -
In patients with AAV relapses it is preferably recommended reinduction and maintenance treatment with RTX (GA: 100%).
It is estimated that 25% of patients with GPA will relapse in the first two years after diagnosis and more than 50% in the first five years. Most will relapse with the removal of maintenance therapy.184
Before assuming a relapse, infection, treatment toxicity or irreversible damage without overt activity must be ruled out. Patients with ANCA-PR3, previous recurrence, pulmonary or upper respiratory tract involvement, persistence of ANCA despite clinical remission, ANCA positivity or significant increase in ANCA titer, as well as nasal carriers of Staphylococcus aureus are at higher risk of relapse. These parameters may be useful in decide the duration of maintenance treatment after a first relapse (whether 24–48 months or longer).6
According to current recommendations,6,61 the therapeutic scheme will be conditioned by the severity of the relapse, depending on the impact on vital organs or vital compromise (Table 9).
Regarding reinduction therapy in relapses with life-threatening risk or severe organ damage, in patients with GPA or MPA who relapse after induction therapy with CFM or RTX, reinduction is preferably recommended with RTX, since in two clinical trials the complete response rate at six months was higher than that achieved with CFM.70,107 In the RITAZAREM112 trial, which included 188 relapsed patients receiving RTX plus corticosteroids, 90% achieved remission at four months regardless of the immunosuppressant used in the previous induction therapy. The results of this study were not available when the ACR/VF 202161 recommendations were developed.
Patients who relapsed during maintenance treatment with RTX and had received the last dose <6 months earlier should undergo reinduction with CFM. If the last dose of RTX had been administered > 6 months earlier, reinduction with RTX61 may be tried. In cases of relapse while on RTX 500 mg/6 months, it may be considered to increase the dose to 1 gram, or increase the frequency to every four months, or both.6,112
In relapses with ADH or severe renal failure, some authors prefer MFC over RTX.6 With the combined administration of both drugs the dose of MFC and corticosteroids could be reduced as shown in the RITUXVAS trial and in other retrospective studies.108 A randomized clinical trial is underway to evaluate the safety and efficacy of combined treatment; the trial will conclude in April 2025 (ENDURRANCE-1).129 Recent data support the usefulness of adding avacopan in this group of patients with more severe disease.185
Regarding the treatment of relapses of GPA and MPA without life-threatening or severe organ damage, if the relapse occurs during maintenance treatment it is important to ensure that adherence was adequate. Whether the reactivation occurred during maintenance treatment or after its suspension, it is advisable to treat the relapse with RTX and corticosteroids and perform maintenance with RTX, as this reduces recurrences and saves corticosteroids.6
In the case of maintenance treatment after reinduction in relapses with of life-threatening risk or severe organ damage, it is recommended, after reinduction, to use RTX61 and to consider the co-administration of avacopan.186 When relapse has occurred after discontinuation of maintenance therapy, it is advisable to reintroduce the same drug as it was previously administered and consider the advisability of prolonging the duration of maintenance.187 Patients who have experienced two or more relapses usually require long-term immunosuppressive treatment, and consider the addition of avacopan.186
Therapeutic strategy: special situationsRefractory vasculitisRecommendations- -
Before defining a vasculitis as refractory, it is recommended to optimize treatment and reevaluate the patient, ruling out non-adherence or other causes that may simulate persistent activity (GA: 100%).
- -
In case of refractory vasculitis, it is recommended consultation or transfering to a referral center (GA: 100%).
- -
If a patient with refractory AAV does not respond to CFM, it is recommended treatment with RTX or vice versa (GA: 100%).
- -
In the absence of response to second-line treatment, it is recommended transferring the partient to a referral center to evaluate other treatments or inclusion in clinical trials (AG: 92%).
Treatment of refractory AAV is challenging and requires a multidisciplinary approach (Fig. 10). It is important to re-evaluate the diagnosis, ensure that the treatment established is optimized and confirm that the clinical picture identified as persistent AAV activity is not explained by infection, neoplasia or other coexisting comorbidity, especially chronic AAV damage.188
Treatment of refractory anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides
RTX: rituximab; CFM: cyclophosphamide; AZA: azathioprine; LFN: leflunomide; GC: glucocorticoids; MTX: methotrexate; IVIG: intravenous immunoglobulins; MT: maintenance therapy.
a Before classifying AAV as refractory, the diagnosis should be reconsidered, ruling out other causes and assessing the activity of the damage.
The following clinical situations define refractory AAV.6,60
- -
Acute disease that does not respond to therapy or worsens after 4 weeks.
- -
Lack of adequate response, defined as a <50% reduction in BVAS score after 6 weeks of treatment.
- -
Chronic, persistently active disease, defined as the presence of at least one of the major or three minor elements in the BVAS score after >12 weeks of treatment.
- -
Those cases in which significant AEs occur that limit the use of treatment.
Before treating a case of refractory AAV, the following procedures should be performed 6,31:
- -
Disease assessment: it is important to perform a thorough evaluation of the disease and determine its severity and extent.
- -
Re-evaluation of treatment: it is important to review the patient’s current treatment and consider possible changes in dosage, type of drug or frequency of administration. Adjunctive treatments such as plasma exchange or i.v. Ig therapy may also be considered.
- -
Consider experimental treatments: in some cases, the use of specific biologic drugs may be considered, which may help reduce inflammation and improve patient outcomes. However, more research is needed to determine the efficacy and safety of these treatments in AAV before their widespread use in this disease can be recommended.
- -
Multidisciplinary and individualized management: the care of patients with refractory AAV should be multidisciplinary and include specialists in internal medicine, rheumatology, nephrology, neurology and other fields as needed. Treatment should be individualized for each patient based on their clinical presentation, response to therapy and tolerance to the treatment.189
- -
In a situation of hemodialysis, as long as there are no extrarenal manifestations, the risk-benefit of maintaining immunosuppressive therapy should be assessed. In these cases, unless there are options for recovery of renal function, tapering is recommended until immunosuppressive therapy is withdrawn (GA: 100%).
- -
We recommend deferring renal transplantation in patients with stage 5 chronic kidney disease secondary to AAV for at least six months after complete clinical response. The persistence of ANCA should not delay inclusion in the waiting list (GA: 100%).
The recurrence rate of AAV in dialysis patients is unclear, so the need to maintain immunosuppressive treatment in dialysis is questioned. An increase in patients in remission has been described as time on dialysis progresses,190 which could be explained by the exclusion of the target organ, or by the alteration of the immune response associated with renal disease. However, mortality due to infections increases significantly in patients with AAV as compared to the rest of the dialysis population, followed by mortality of CV cause.190 Therefore, it is recommended that immunosuppressant maintenance therapies be avoided in patients with AAV on dialysis.191–193
The survival of patients with AAV in a renal replacement therapy program is similar to non-diabetic patients adjusted for age and sex194; however, in renal transplantation, better patient and graft survival has been described in AAV compared to non-diabetics with CKD-5.195
The timing of inclusion in the renal transplant waiting list requires individualized analysis. The KDIGO guidelines of 2024 for the treatment of AAV82 recommend deferring renal transplantation in patients with AAV for at least six months after complete clinical response, emphasizing that persistence of ANCA should not delay inclusion on the renal transplant waiting list.
The AAV may recur after transplantation; it has been estimated at 0.02–0.03 per patient/year (5–6% of transplant recipients), with no clear relationship with the duration of remission or the level of ANCA.192 Although overall it should not be a restrictive criterion for inclusion on the waiting list, patients with ANCA should be closely monitored for the presence of symptoms suggestive of recurrence.196
An immunosuppressive regimen cannot be recommended in the patient with AAV requiring renal transplantation. The association of tacrolimus, MMF and PDN has been associated with low relapse rates,197 although there is no consensus on the most appropriate immunosuppression in AAV (Fig. 6).
The diagnosis of relapse after renal transplantation may be complex due to the heterogeneity of symptoms, and the deterioration of renal function associated with relapse requires renal biopsy. There are no specific biomarkers of relapse and the role of ANCAs in post-transplantation has not been defined.
The treatment of AAV relapses is similar to that of non-transplanted patients, without being able to provide a recommendation on the benefit of CFM or RTX associated with steroids. In case of using CFM, it is recommended to interrupt MMF temporarily, which is not necessary with the use of RTX.194 The risk of graft loss due to disease recurrence has been estimated in <1%.198
PregnancyRecommendations- -
Pregnancy in women with AAV should be managed by multidisciplinary teams and in high-risk pregnancy units (GA: 100%).
- -
Preconception counseling and pregnancy planning is recommended, informing the patient of the chances of success and associated risks (GA: 100%).
- -
It is recommended that the patient remain in stable remission for a minimum of six months prior to conception (GA: 100%).
- -
During planning of pregnancy in a woman with AAV, immunosuppressive medication should be modified, avoiding teratogenic drugs or drugs with negative effects on gestation (GA: 100%).
Pregnancies in patients with AAV are rare and, therefore, it is difficult to analyze.199 Most of the evidence comes from isolated cases and small series.200–203 There is a possibility of successful pregnancy in patients with vasculitis, especially when conception is planned by a multidisciplinary team and the disease is in remission. Although the prognosis has improved markedly in recent years, pregnancy in women with vasculitis entails a number of additional problems, and for this reason adequate disease control is recommended for a minimum of six months prior to conception.204
During pregnancy, women with active AAV are at increased risk of prematurity, intrauterine growth retardation, spontaneous abortion and preeclampsia.205 Preterm delivery is one of the most frequent complications, especially in GPA, with an incidence in up to 73% of cases, but this percentage drops to 7–9% in patients in remission.206,207 Miscarriages have a variable incidence, between 4–20% of pregnancies, while preeclampsia occurs in 10–30%205,207,208 (Appendix A Table S7). Cesarean deliveries were around 50% of cases.209
In addition, the effect of pregnancy on AAV may cause flares in 20–50% of patients with this diagnosis. Althoug most of these exacerbations are not usually severe, life-threatening manifestations can occur, especially in patients with renal or cardiac damage related to vasculitis.200 Active disease during pregnancy represents a great challenge due to the risk of fetal damage associated with treatments used for induction or maintenance of remission, such as CFM, MTX and MMF. In addition, there are limited data on the safety of RTX in pregnancy.208 The immunosuppressive drugs considered safest during pregnancy are: GC, AZA and calcineurin inhibitors (cyclosporine or tacrolimus), particularly in case of mild or moderate disease.210,211 Alternatives that could be considered include RTX or CFM in the second or third trimester once organogenesis is complete, although data are limited and should be assessed individually.212 Iv Ig can be used as a short-term intervention until conventional remission induction therapies can be used.212
Although there are no specific recommendations for the treatment and management of AAV in pregnancy, the advices formulated on immunosuppressive treatment in pregnancy and lactation can be applied (Appendix A Table S8).210,211 There are no general recommendations, but a follow-up visit should be done every four to six weeks including a gynecological examination and analytical control throughout the pregnancy (Fig. 6).
Blood pressure should be controlled with labetalol, nifedipine, methyldopa or hydralazine.213 There is little information on the preventive treatment of preeclampsia with aspirin in women with vasculitis, although certain experts recommend its use in patients with arterial hypertension or with previous CKD.214
The Vasculitis in Pregnancy Registry (V-PREG) is currently collecting maternal and fetal outcomes in vasculitis, in order to provide more comprehensive information for patients and physicians.215
Frail patientsRecommendations- •
Although all regimens may be recommended for frail patients, the use of RTX together with avacopan will be considered in order to minimize the use of GC (GA: 92%) (Fig. 6).
AAVs are diseases with a higher prevalence among people over 65 years of age.216 The concept of a frail patient is not well established, so it is difficult to establish recommendations. In elderly patients, diagnosis is made usually late, since symptoms of the disease, such as asthenia and constitutional syndrome, may go unnoticed or overlap with changes attributed to the age. 216–218 Additionally, it is very common for AAVs in this group of patients to occur in the context of multiple comorbidities: cardiovascular disease (CVD), diabetes or cancer. This association increases the risk of progression of CKD and mortality, especially during the first year of vasculitis treatment r.219 The main cause of mortality in this age group is complications arising from the treatment of AAV, mainly due to infectious complications, making it necessary to minimize treatment and insist on the importance of infectious prophylaxis and having a specific and updated vaccination schedule.219–222
The recruitment of these patients in controlled clinical trials conducted in AAV has been limited. As previously mentioned, in a randomized clinical trial Pagnoux et al. compared a low-dose regimen versus standard CFM doses in older patients; no differences were observed in the remission rate, and they had a lower incidence of AE.124
A recent retrospective study analyzed three types of induction immunosuppression therapies (CFM, CFM + RTX and RTX) in patients over 60 years of age. No significant differences were observed in remission rates, infectious complications or hospitalization.223 Therefore, treatment in elderly and frail patients should be individualized after stratifying their risk of relapse and AE. RTX offers advantages in frail patients to avoid the toxicity produced by CFM, but the available data are limited in this older population.216 The recommendation regarding treatment with corticosteroids is to avoid the use of PDN doses > 0.8 mg/kg/day, a risk factor for serious infection in elderly patients, and try to reach doses of 5–7.5 mg/day of PDN at five months in elderly patients with AAV and renal failure. In severe forms, it is recommended three boluses of 250 mg IV with a rapid decrease of GC dose.220,221 As an alternative to save steroids in this group of patients, the use of avacopan185 can be considered (Fig. 6).
Autoimmunity: double positivesRecommendations- -
GC, CFM and plasmapheresis are recommended for the treatment of patients with anti-GBM antibody disease and AAV (GA: 100%).
Anti-GBM disease is a small-vessel vasculitis characterized by linear IgG deposition along the glomerular basement membrane.
Circulating autoantibodies specific for the non-collagenous domain of the alpha 3 chain of collagen IV are observed in most patients with active disease. A 30–50% of patients with anti-GBM antibodies also have ANCA positivity, mainly ANCA MPO (60%), and 5–10% of ANCA-positive patients have detectable anti-GBM.224 The mechanism of this association is not known, and there are no strong pathophysiological arguments in favor of a common pathogenic mechanism.225,226 Double positivity is usually detected simultaneously in a patient presenting with an abrupt clinical presentation of alveolar hemorrhage and RPGN. Occasionally, anti-GBM antibody positivity is detected in patients previously diagnosed with AAV, suggesting that ANCA-induced glomerular inflammation may trigger an anti-GBM response, possibly by modification or exposure of CL1 domain 3 (IV) epitopes.227,228
El tratamiento de los pacientes se basa en el empleo de corticoides en dosis altas, CFM y plasmaféresis. El recambio plasmático se debe mantener hasta que negativicen los anti-MBG, excepto en pacientes que presentan 100% de glomérulos con proliferación extracapilar en la biopsia renal y no tengan HAD, en los que la plasmaféresis se considera fútil230 (Fig. 6).
Patients with double positivity tend to be older than those with isolated anti-GBM, and are predominantly male.229 The clinical presentation resembles anti-GBM disease more closely than AAV. It is manifested with hematuria and proteinuria, and nephrotic syndrome is rare. Renal failure is more severe than in isolated ANCA (mor than > 50% have Cr > 7.9 mg/dL and > 60% require renal replacement therapy at diagnosis, vs. 60% in anti-GBM and 28% of isolated ANCA). More chronic damage is observed in these patients at diagnosis than in those with isolated anti-GBM. The frequency of hemoptysis is higher, but with fewer systemic symptoms than in isolated ANCA-positive patients.230–234
The likelihood of relapse in these double-positive patients is higher than in patients with anti-GBM disease, so they should receive maintenance treatment and long-term follow-up.224,235 Mortality is also higher than in patients with isolated anti-GBM and ANCA, and in these patients, a high anti-GBM titer is associated with higher mortality.
Treatment is based on the use of high-dose corticosteroids, CFM and plasmapheresis. Plasma exchange should be maintained until anti-GBM is negative, except in patients with 100% glomeruli with extracapillary proliferation in the renal biopsy and without DAH, in whom plasmapheresis is considered useless230 (Fig. 6).
Currently there is not a solid evidence to support the use of RTX in these patients, since this treatment is derived from that used in patients with anti-GBM disease. However, there are already a number of cases treated with RTX with positive results, so it may be an alternative in patients in whom CFM cannot be used.235 Although there is some isolated experience with other therapies (imlifidase), presently there is no evidence to justify their recommendation.236
Patients with double anti-GBM and ANCA positivity should receive maintenance treatment due to the high risk of relapses.224,230,237 It is recommended the use of AZA and MMF.
ANCA negativeRecommendationsIf the diagnosis of ANCA-negative vasculitis is confirmed, it is recommended to follow the general therapeutic guideline depending on the severity of the affected organs (GA: 100%).
JustificationANCA-negative MPASmall vessel vasculitides are the most frequent cause of RPGN in adults and the elderly.238 Although most of these entities correspond to AAV, a subset of them are persistently negative. Neutrophil activation in ANCA-negative RPGs may be due to other autoantibodies that have trophism for endothelial cells (AECA) and facilitate interaction with neutrophil surface receptors (Fc and C3b)239 or anti-LAMP-2 that can activate neutrophils and produce apoptosis at the endothelial level, or to the activation of cell-mediated immunity.
The histology of ANCA-negative RPGNs is based on the demonstration in the renal biopsy of a percentage of crescents greater than 50% and an intensity for Ig staining by direct immunofluorescence of 0–1 on a scale of 0–4.240 The few series that have been published with a review of RPGNs estimate the percentage of negative ANCAs at 10–30%.241,242 The main characteristics of its clinical presentation use to be its presence in younger patients than AAV, and its extrarenal involvement is less frequent. Although a more severe renal involvement has been described in ANCA-negative RPGN compared to AAV, this could be attributed to the delay in diagnosis due to persistent ANCA negativity.243 In vitro and in vivo studies suggest with relevant evidence the role of ANCA in the pathogenesis of AAV as a diagnostic marker, and linking chemotactic and inflammatory activity and complement activation by the alternative pathway with neutrophil activation.71 Neutrophils are also the main effector cells of ANCA-negative RPGN, as observed in the renal biopsies analyzed.244 Anti-endothelial antibodies or antibodies against LAMP-2 may explain the humoral activation of neutrophils, together with the mechanism of interleukin (Il-8 or IL-17) mediated cellular activation in ANCA-negative RPGN.240
There are no controlled trials of treatment of ANCA-negative RPGN, and the accepted protocols are those for the treatment of AAV,245 and there are series published with worse evolution than AAV.240 There are patients described with MPA with peripheral neurological involvement and ANCA negative.
ANCA-negative GPAApproximately 10% of patients with GPA are ANCA negative and in these cases the diagnosis is based on histology. There are very few studies on the evolution and treatment of this group of patients. Negative ANCAs are more frequent in patients with localized forms of the disease, usually granulomatous, with involvement of the upper or lower respiratory tract and without renal involvement.246 BVAS is usually lower than in ANCA-positive patients. Biopsy is essential for diagnosis.
There are described cases of ocular (orbital pseudotumor), neurological (pachymeningitis, hypophysitis, multiple cranial nerve involvement), ENT (subglottic tracheal stenosis, otitis media), cutaneous (pyoderma-gangrenosum-like, skin ulcers), pulmonary (solitary pulmonary nodules) involvement with persistently negative ANCA. Holle et al.246 described that 10% of patients with localized forms progressed to generalized forms and 46% relapsed. Puéchal et al.247 found a relapse rate similar to that of ANCA-positive GPA patients, and similar overall survival.
There are no studies comparing the treatment of ANCA-positive and ANCA-negative GPA patients, and usually the same therapeutic guidelines are used as in ANCA-positive patients.
Therapeutic strategy: non-immunosuppressive treatmentControl of cardiovascular risk factorsRecommendations- •
It is recommended to treat modifiable CVD risk factors (high blood pressure, dyslipidemia, obesity, smoking, sedentary lifestyle) (GA: 100%).
- •
It is recommended, in all patients with AAV, to maintain a blood pressure ≤ 120/80 mmHg and a plasma LDL-cholesterol ≤ 70 mg/dL. In case of arterial hypertension or proteinuria, salt restriction is recommended (<2 g of sodium per day, or < 90 mmol sodium per day, or < 5 g of sodium chloride per day) (GA: 70%).
Patients with AAV have a significantly higher risk of CVD and stroke than the general population. A study including 144 patients, from an European and a Canadian center, showed that 73% of patients had insulin resistance at the time of inclusion, regardless of concurrent treatment with PDN.248 Another study carried out in Minnesota, with a cohort of 58 patients followed for 10 years, showed that patients with AAV had a more than three times greater risk of CVD and up to eight times greater risk of stroke as compared to the general population, despite have a similar prevalence of CV risk factors.249 Finally, a meta-analysis showed that patients with AAV have a 1.65 times higher risk of suffering from CVD compared to the general population.250 This risk varies geographically, it can increase up to 24% in some regions of the world and occurs within the first five years after diagnosis.
In turn, the disease itself and its treatment determine the presence of vascular risk factors such as high blood pressure and diabetes in these patients. However, beyond traditional risk factors, there are mechanisms specific to the disease that contribute to increased CV risk, such as endothelial dysfunction that causes a procoagulant state and precedes the formation of atherosclerotic plaque. Elevated levels of circulating proinflammatory cytokines, the formation of neutrophil extracellular traps, the activation of the complement system, and the defective regulation of T lymphocytes are just some of the multiple factors that enhance this process.251
For all these reasons, it is necessary to carry out a double approach in the management of patients with AAV. Firstly it is very important early diagnosis and initiation of adequate treatment of the disease to control inflammatory activity. Concomitantly, an intensive approach to CVD risk factors, especially modifiable ones (hypertension, dyslipidemia, smoking, sedentary lifestyle), should be carried out during the first years after the diagnosis of AAV, recommending adjustments in lifestyle, treatment pharmacological and periodic and rigorous medical monitoring. In fact, one study has shown that in this group of patients the recommended targets for LDL cholesterol and blood pressure are rarely achieved250 (Fig. 11).
SuggestionsThe 2016 EULAR recommendations suggested an annual review of traditional Framingham risk factors. The latest 2022 guidelines do not establish a specific temporal recommendation, but they underline the importance of controlling vascular inflammation, as well as screening and treatment of classic CV risk factors.6
Cardio-nephroprotective drugsRecommendations- •
In case of proteinuria, blockade of the renin-angiotensin-aldosterone system should be performed with angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin II receptor antagonists (ARBs) at the maximum tolerated dose (according mainly, to blood pressure and kidney function) to achieve proteinuria < 0.5 g/day. As alternatives or complementary treatments can be used antialdosterone drugs (spironolactone, eplerenone, finerenone, currently accepted only for patients with type 2 diabetes mellitus) or sodium-glucose cotransporter type 2 (SGLT2i) inhibitors (GA: 91%).
- •
In advanced CKD stages, it is also important to address other CV risk factors, such as anemia and secondary hyperparathyroidism, following the available guidelines in this respect (GA: 100%).
In general, patients with AAV experience an increased risk of long-term death after their first year of diagnosis compared to the general population of the same age and sex, and CVD remains the most important cause of death along with malignancies, and infections.252,253
In addition to the inflammatory nature of the disease itself, including endothelial dysfunction and arterial stiffness, the long-term effects of immunosuppressive therapy, especially if not adequately controlled, contribute significantly to the increased CV risk in this group of patients.254,255 Among the independent risk factors of a high mortality, CKD remains one of the main predictors of poor prognosis.256 Therefore, patients with AAV and renal involvement have a significantly increased risk of CV morbidity and mortality as part of the inherent association with CKD.257 In addition, several unique pathophysiological features affecting the cardiorenal axis occur more frequently in patients with AAV: diastolic dysfunction, pulmonary hypertension and impaired systolic function.258,259 The iSGLT-2s exert unequivocal cardioprotective and nephroprotective effects by reducing albuminuria and delaying CKD progression because they reduce glomerular hyperfiltration and modulate tubular workload. These profound clinical effects suggest that SGLT-2 inhibition is an ideal therapeutic pathway for patients with AAV, especially when signs of cardiac or renal damage have been manifested. The DAPA-CKD trial significantly changed our view of the treatment of CKD, which (with all its diverse etiologies, ranging from diabetes and hypertension to various forms of GN) should be viewed primarily as a unique form of organ dysfunction that can be successfully treated.260 Trials are currently in preparation to test dapagliflozin in AAV, such as DAPA-vasculitis. Patients with CKD (isolated albuminuria or with reduced GFR) should receive potent nephroprotective therapies such as RAS inhibitors and iSGLT-2 that affect both hyperfiltration and tubular capacity, thereby reducing CKD progression and CV risk (Fig. 11).
In addition, it should be consider the use of GLP1 receptor agonists in selected patients (obese and/or metabolic syndrome), drugs that have demonstrated their nephroprotective and cardioprotective efficacy in different clinical trials.261
Antibiotic prophylaxis and vaccinationsRecommendations- -
Systematic screening for latent infection by Mycobacterium tuberculosis, hepatotropic viruses and human immunodeficiency virus (HIV) is recommended in all patients with AAV. If present, they will be managed according to that established for immunocompromised patients of other origin (GA: 100%).
- -
Any patient with AAV on treatment with GC at doses greater than 15 mg/day or with RTX or CFM at induction doses should receive cotrimoxazole prophylaxis (GA: 100%).
- -
All patients diagnosed with AAV should receive vaccination against Streptococcus pneumoniae, herpes zoster virus (VHZ), as well as a vaccine or booster dose against SARS-CoV2 and seasonal influenza (GA: 100%).
- -
In all patients undergoing treatment with RTX, monitoring of serum Ig levels is recommended every six months, indicating replacement therapy in case of IgG < 200 mg/dL, and also assessing its administration in patients with less marked deficiencies (IgG >200 mg/dL) and repeated infections (GA: 91%).
Severe infections are frequent in patients with AAV, with a prevalence between 20 and 60%, and constitute the first cause of death in the first six to 12 months,130,262,263 with bacterial pneumonia being the most frequent.264,265
Risk factors of infection are: age, smoking, severe renal failure or at debut, the cumulative dose of CFM, leukopenia, hypogammaglobulinemia, number of B lymphocytes CD19 + or T CD4 +, and high doses of GC at baseline.217,264–267 In the PEXIVAS trial, two GC regimens were compared, and the results showed the lower dose group being safer in terms of severe infection.110 Therefore, minimizing the use of GC is considered an effective measure to reduce the risk of infection. Recent studies suggest that avacopan, a C5aR2 receptor antagonist for the complement C5a fraction, allows to reduce doses of GCS and thus the risk of serious infection.114
The prevention of infections in patients with AAV has been reviewed in the Recommendations for the Prevention of Infection in Systemic Autoimmune Rheumatic Diseases of the Spanish Society of Rheumatology.268 General hygiene measures, use of masks in the case of close contacts or cohabitants with active infection, etc., are described in detail.
Prophylaxis against Pneumocystis jirovecii should be performed, usually with cotrimoxazole, in all patients with PDN > 15 mg/day, or T-CD4 lymphopenia < 200 cells/mcl.268 Its use has been associated with a lower rate of severe infection and lower mortality from infection in patients with AAV, particularly in those treated with RTX.263,267 The latest EULAR recommendations for the management of AAV suggest maintaining cotrimoxazole while the patient receives treatment with RTX or CFM.6
The recommendations for prevention of reactivation of latent tuberculosis do not differ from those for other immunocompromised patients. Interferon-gamma release assay (IGRA) tests are preferred for screening, particularly in the presence of immunosuppressive therapy.269 Screening for previous or latent infection by hepatitis viruses (HBV, HCV) and HIV is also mandatory, and there are specific recommendations regarding the use of antivirals in patients with serology of past HBV infection who are going to undergo treatment with GC or RTX.268
In general, vaccines can be considered safe in these patients.268,270 Vaccination against pneumococcus and influenza is recommended in all cases268,271 and it is usually immunogenic in patients with established disease under standard treatment.272 A booster dose at four weeks has been recommended, in addition to the use of the adjuvanted tetravalent vaccine, in immunocompromised patients.268
Patients with AAV are at increased risk of varicella-zoster virus reactivation273 and are therefore candidates for vaccination with the recombinant vaccine, although there is no direct evidence in this population.271 Appendix A Table S9 summarizes the vaccines commonly recommended.
RTX therapy substantially reduces the response to vaccinations, and it is recommended that vaccination be performed at least two weeks before the dose of RTX. However, influenza vaccination should not be delayed for this reason. It has been recommended that MTX be suspended two weeks after flu vaccination in order to increase the response.271
Hypogammaglobulinemia has been associated with severe infections.274–276 The risk of hypogammaglobulinemia with RTX has been associated with age, GC use and repeated doses.130,275 It is recommended that IgG levels be monitored before each course of RTX6 together with an overall estimate of the risk of infection. Although the available evidence is limited, it is suggested to administer Ig in RTX-treated patients with repeated infections, whatever the IgG level.276
COVID can be more critical in patients with immune-mediated systemic disease,277–280 particularly in those treated with immunosuppressants and, mainly RTX279,280 (Fig. 11). It is recommended to vaccinate as soon as possible, regardless of disease activity, and to complete three doses and two more booster doses in case of inadequate response.6,279,280 In case of SARS-CoV-2 infection, it is recommended to suspend disease-modifying antirheumatic drugs (DMARDs) for two weeks and, according to local guidelines, early use of antivirals in order to reduce the risk of progression to severe COVID.279–282 Paxlovid® and molnupiravir are recommended in case of moderate infection and remdesvir in case of severe infection.283 Passive immunization using monoclonal antibodies is under review.283
Prevention of osteoporosisRecommendations- -
Periodic evaluation for osteoporosis is recommended in patients with AAV, especially in those who have received or are on active treatment with GC (GA: 100%).
- -
In patients with AAV on GC treatment for ≥ 3 months, it is recommended calcium and vitamin D supplementation, individualizing the introduction of antiresorptive therapy (GA: 100%).
Patients with AAV are at risk for complications and comorbidities.284,285 Osteoporosis may be secondary to underlying vasculitis (due to inflammation), impaired renal function or treatment of the disease, mainly due to the use of GC. In a cohort of Swedish patients with AAV, osteoporosis was the most prevalent comorbidity, and it was 4 times more frequent than in the general population.286 Fractures have also been described to be more frequent in patients with AAV than in the general population.287,288
It is important to consider both the beneficial effects of GCS in disease control and their potential toxicity. Several studies have shown that osteoporosis is a frequent complication in AAV patients treated with GCS (14–20%)284,289 and that the cumulative dose of GCS is associated with bone loss.289 In general, it is recommended to use the lowest dose and the shortest possible duration of GC therapy and to introduce other immunosuppressants as GC-sparing agents.290 However, some patients with AAV may require long-term low-dose of GC to maintain remission.61 In this regard, some recent studies in AAV patients have shown encouraging results using a treatment regimen with low-dose GC or without GC.220,291 The use of avacopan has shown be promising as a GC-sparing treatment, although further work is still required.114
The prevention and management of CG osteoporosis is addressed in several guidelines and reviews.290,292 These guidelines recommend measuring bone mineral density (BMD) by dual-energy X-ray absorptiometry (DEXA) at the start of CG therapy and after one year. If BMD remains stable, it could be measured every two years. In addition, the ACR guidelines recommend evaluation for vertebral fractures.290 Generally speaking, in all patients receiving any oral dose of GC with an expected duration of ≥ 3 months, calcium and vitamin D supplementation is suggested. In patients with previous fracture, BMD T-score less than -2, or GC with doses ≥ 20 mg/day, pharmacological treatment should be instituted, preferably with antiresorptives (Fig. 11). This is based on the evidence that patients receiving GCs have fractures with higher BMD values, since GCs act by several mechanisms that affect both bone quality and BMD.293
CRediT authorship contribution statementAll authors participated in the periodic meetings of the recommendations; they developed at least one specific section of the main document, including the bibliographic search and review, approved and signed the final document, including the main manuscript, the main tables and figures and the supplementary material before shipment.
Enrique Morales, Roser Solans and Iñigo Rúa-Figueroa designed and coordinated the study and performed the general editing of the manuscript.
Carmen Mon Mon and Néstor Oliva Dámaso provided comments during the public review phase of the document, which were partially adopted.
FinancingThis work has not received specific support from public sector agencies, commercial sectors or non-profit entities.
The authors of this manuscript thank CSL Vifor for their collaboration in the logistics of the manuscript preparation meetings.
The authors of this manuscript would like to thank Adalia Farma S.L. for their support in the preparation, graphics and final execution of the manuscript.