SARS-CoV-2, the pathogen responsible for coronavirus disease 2019 (COVID-19), has caused unprecedented morbidity and mortality worldwide.1 The epidemiological and clinical data on this disease in Spain have been widely commented on in publications by the Spanish Society of Nephrology (Sociedad Española de Nefrología).2–11 In general, most patients fully recover within three to four weeks of infection, but some experience persistent symptoms after they have “recovered” from the initial coronavirus infection.
A division of COVID-19 into three categories has been proposed:
- 1.
Acute COVID-19: up to four weeks since infection.
- 2.
Subacute COVID-19: includes symptoms and abnormalities present four to 12 weeks after acute COVID-19. These symptoms are explained by residual organ dysfunction due to secondary organ damage.
- 3.
Persistent COVID-19 or Long COVID (LC): includes symptoms and abnormalities that persist or are present beyond 12 weeks after acute COVID-19 onset and are not attributable to other alternative diagnoses.12
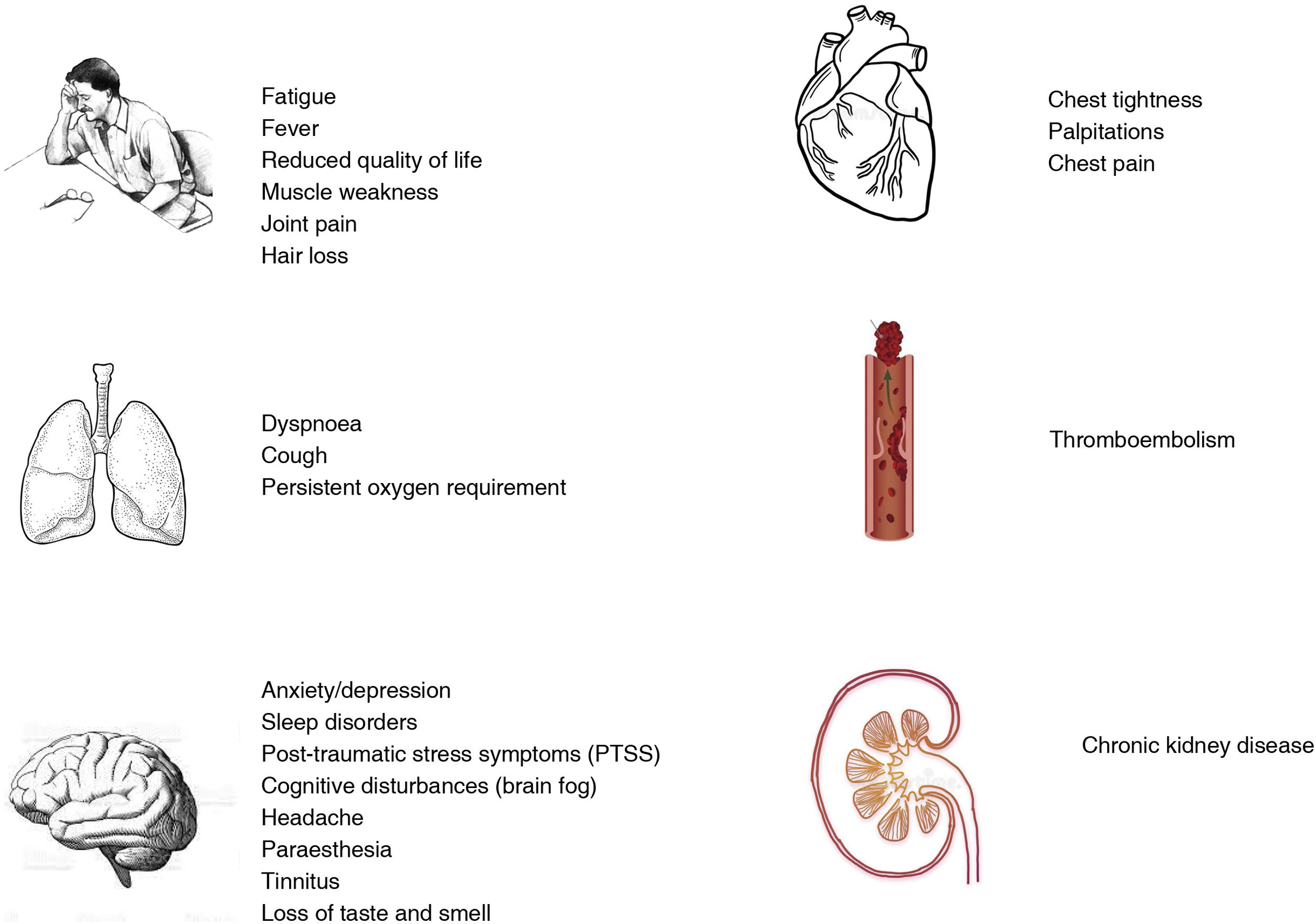
The most common symptoms of long COVID are fatigue and dyspnoea; they may be solitary, multiple, constant, transient or fluctuating, and their nature may change over time (Fig. 1).13
The prevalence of long COVID has been reported by multiple studies in different countries: USA (32%), Italy (55%), France (66%) and similar data were observed in the United Kingdom and Spain. These studies point to the association of long COVID with the severity of the disease during the acute phase (admission to the intensive care unit [ICU] and/or requirement of non-invasive care and/or invasive mechanical ventilation). It is also associated with pre-existing respiratory disease, higher body mass index, advanced age, or prior comorbidities such as cardiovascular disease, chronic kidney disease (CKD), cancer, and organ transplantation.14
We have experienced several different waves of SARS-CoV-2 infections caused by different variants: alpha, beta gamma, delta, omicron and omicron B2. In the first wave, the population was not vaccinated and clinical aggressiveness was much higher. Later, with vaccination and the immunity of those who had had the disease, the severity of the disease decreased. Still, long COVID, present even in asymptomatic patients, affects 50% of cases, as confirmed in a systematic review of October 2021.15 What we overlook are data relating the different variants with the presence of long COVID, something that would have been very interesting to analyse. It has been observed that there has been less loss of smell, taste and hearing with the later variants. But there are no data on the prevalence of long COVID with different variants. Although vaccines significantly reduce the rates of severe illness and death from this condition, they are not as effective in preventing disease altogether. Long COVID can emerge even after mild or asymptomatic coronavirus infection. Countries with high infection rates could still have many cases of long COVID, even if these countries have had high rates of vaccination.16
In general, kidney disease is not included as part of long COVID because it is essentially a report of symptoms, with criteria that are not always unified, although clinical data are indicating that it is a relevant complication that requires follow-up by Nephrology. In fact, according to the National Institute for Health and Care Excellence (NICE) affects approximately to 20%–40% of patients in Europe and the USA.19–22 This complication is a sign of poor prognostic as it is associated with a 45% in-hospital mortality compared with a 7% mortality among those without AKI.20 In the study by Hirsh et al.21 on more than 5000 hospitalised patients with COVID-19, 89.7% of patients on mechanical ventilation developed AKI compared with 21.7% of patients without mechanical ventilation. And 96.8% of the patients who required renal replacement therapy were on mechanical ventilation. Of the patients who required ventilation and developed AKI, 52.2% developed AKI within 24h of intubation.
In data from the Registry of the Spanish Society of Nephrology23 on 300 patients, the majority of those registered with AKI were male (69.9%), with a mean age of 69 YO. A total of 182 patients required admission to the ICU, compared to 118 who developed mild AKI on the hospital ward. All ICU patients had developed pneumonia, requiring ventilatory support, and 84.9% required renal replacement therapy vs 12% in the group that remained hospitalised on the ward. Whereas in the group admitted to the ward, all of the patients recovered kidney function, and only 3.5% remained on chronic dialysis, in the group admitted to the ICU, basically half of the survivors remained on dialysis.
Therefore, we can conclude that AKI in COVID-19 occurs in critically ill hospitalised patients, especially those requiring assisted ICU ventilation. Overall, given the severity of the patient’s illness, mortality is high.
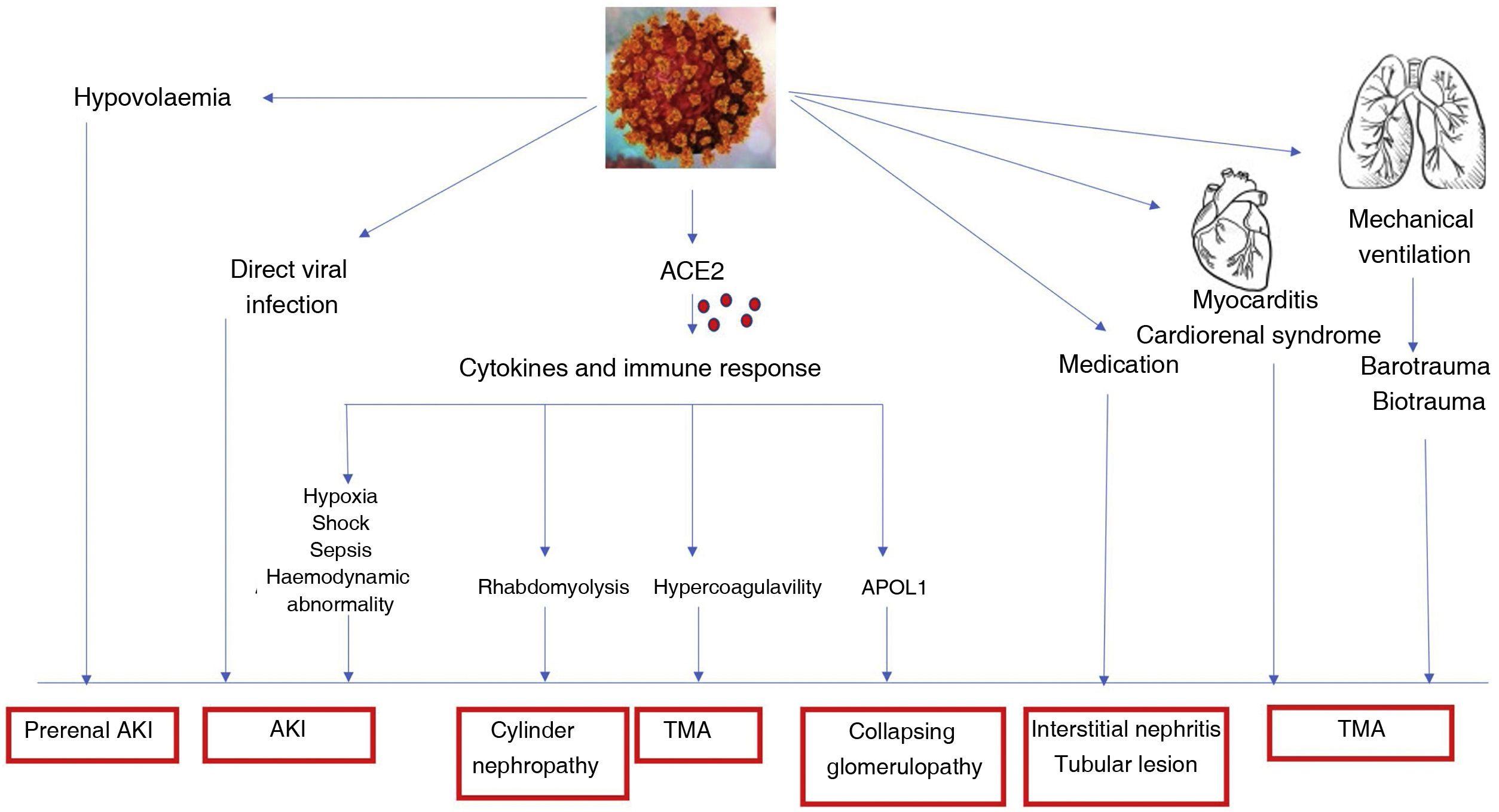
Renal involvement from COVID-19 can be caused by multiple pathogenic factors (Fig. 2).24
- •
Collapsing glomerulopathy associated with the APOL1 genotype could be related to inflammatory cytokines or podocyte injury due to the virus.25
- •
AKI may be produced by different causes:
- -
The initial negative impact could be the direct role of the virus on the tubular ACE2 receptors that are the main binding site of SARS-CoV-2.
- -
Immunological alterations and the inflammatory cytokine response may explain many of the mechanisms of AKI in COVID-19 that can directly or indirectly induce sepsis, shock, hypoxia, microthrombi, and rhabdomyolysis.
- -
Hypovolaemia: induced by multiple causes.
- -
Organ interactions between heart, lung and kidney would be other possible causes of AKI induced by COVID-19.
- -
Sepsis.
- -
Kidney and COVID 19 pathophysiology of kidney damage.12
The histological data found in patients with COVID-19 are listed in Table 1.26–28
Renal histological data observed COVID-19.
| - Tubular necrosis |
| - Endothelial damage |
| - Capillary erythroid aggregates |
| - Glomerular intracapillary fibrin thrombi |
| - Coronavirus particles in tubular epithelium and podocytes |
| - Haemosiderin deposition |
| - Pigmented casts (rhabdomyolysis) |
| - Inflammation |
| - Kidney infarcts |
| - Collapsing glomerulonephritis |
| - Proliferative glomerulonephritis with IgG deposits |
| - Chronic vascular and interstitial lesions related to age and patient history |
IgG: Immunoglobulin G.
- •
Secondary to organ damage due to serious illness (AKI)
Preclinical studies show that after ischaemic renal injury and despite the return of serum creatinine concentrations to normal values, there is persistence of inflammation, renal fibrosis, abnormal gene expression profiles of the kidney, and functional impairment. Therefore, it is not clear if all cases of kidney involvement post-COVID-19 can be included as part of long COVID, because in most cases they can be explained by organ damage secondary to ischaemia and/or infection. A clear example is in a series of patients hospitalised for COVID-19 who had AKI and of whom 47% still had kidney damage at the time of hospital discharge.29 This kidney damage and subclinical inflammation and injury may persist for many months, resulting in a progressive decline in kidney function leading to CKD. A study from the USA using Veterans Health Administration (VHA) electronic health records to perform a comprehensive assessment of prolonged COVID-19 found that COVID-19 increased the risk of CKD and that this risk was higher among those with severe illness.30
- •
Secondary to the presence of the virus (long COVID)
However, it seems that it is not only a previous AKI the explanation of post-COVID-19 kidney disease. In one study published in Cell Stem Cell, researchers studied kidney tissue from COVID-19 patients admitted to the ICU. They found scar tissue compared to intensive care patients with a non-COVID-19-related lung infection and a control group. Furthermore, in autopsy samples, they observed that SARS-COV-2 directly infects renal cells and is associated with increased renal tubulointerstitial fibrosis. So, based on that, they investigated the direct effects of the virus on the kidney, independent of the systemic effects. To do this, they infected with SARS-COV-2 kidney organoids (microkidneys) derived from human pluripotent stem cells and containing many different renal cells, except immune cells. They studied the virus’s direct effect on renal cells, regardless of possible side effects caused by immune cells or other systemic effects. Single-cell RNA sequencing indicated injury and dedifferentiation of the infected cells with activation of profibrotic signalling pathways. SARS-COV-2 infection also led to increased collagen 1 protein expression in organoids. The SARS-COV-2 protease inhibitor was able to improve the infection by SARS-COV-2 of renal cells. These results suggest that SARS-CoV-2 can directly infect renal cells and induce injury with subsequent fibrosis. That is, the virus causes direct cell damage, independent of the immune system. These data could explain both acute kidney injury in patients with COVID-19 and the development of chronic kidney disease in long COVID.31
We do not yet understand completely the complexity of the kidney damage, and there may be other mechanisms that may explain kidney injury in long COVID in addition to the direct effects of the virus, such as an abnormal immune response or autoimmunity, persistent inflammation, alterations in endothelial function and in the coagulation system, or alterations in the autonomic nervous system. All this requires from us to consolidate cases and research projects to make further progress. Also, changes in social (such as reduced social contact and loneliness), financial (such as job loss), and behavioural (such as changes in diet and exercise) conditions that people with COVID-19 may experience could explain some of the general complications of long COVID, including renal complications due to non-compliance of the treatment, lack of blood pressure (BP) control, etc.
The necessary nephrological presence in long COVIDThere are still not many follow-up studies on kidney involvement in long COVID, but there may be loss of kidney function months after the illness. This could be a silent disease, which also occurs in patients who did not require hospital admission. In the already mentioned work of Bowe et al.29 a cohort of 1,726,683 US veterans diagnosed with COVID-19 of varying severity and 1,637,467 uninfected controls were studied, adjusting for prior comorbidities and other covariates to ensure the separation of the pure effect of COVID-19 on outcomes. They demonstrated that after 30 days of COVID-19 the hazard ratio (HR) of AKI is 1.94;decreased glomerular filtration (GFR) ≥30% HR:1.25≥40%, HR: 1.44, ≥50% HR:1.62 and major adverse renal events (MARE) (decrease in GFR >50%, end-stage kidney disease, or all-cause mortality) HR:1.66. These data correlate with the severity of the infection. However those who were not hospitalised also had loss of kidney function. Compared with non-infected controls, 30-day COVID-19 survivors exhibited a decreased GFR of −3.26, −5.20, and −7.69ml/min/1.73m2 per year, respectively in non-hospitalised and hospitalised patients and patients admitted to intensive care during the acute phase of infection by COVID-19. What it is important is that although having AKI in the acute phase influenced the results of the loss of renal function post-COVID, the results of this study suggest that the deterioration of renal function was also observed in those who did not suffer AKI during the acute phase.
Other studies also insist on the need for post-COVID renal surveillance, finding that in patients who had AKI in the hospital, AKI associated with COVID-19 was associated with a greater rate of decline in estimated GFR (eGFR) after discharge compared to AKI in patients without COVID-19, regardless of underlying comorbidities or AKI severity.32
Therefore, considering that there have been close to 500 million recorded cases of COVID-19 globally, a figure that is undoubtedly three or four times higher, and that non-hospitalised patients who did not have AKI in the acute phase also observed deterioration of renal function, it is clear the necessity for the presence of Nephrology in monitoring and prevention of kidney function deterioration.
Renal COVID symptomsThe data we have discussed justify the critical importance of paying attention to kidney function and disease in the care of patients who have had COVID-19. However, neither in the clinical references of post-COVID syndrome nor in the consensus between societies is recognition granted to the importance of renal impairment post-COVID. One example is the Clinical Guide for LC patient care, in which 47 Spanish Societies and scientific entities participate and the Spanish Society of Nephrology is absent.33 It does not even include elemental urinalysis with sediment and detection of albuminuria.
Therefore, it is time to develop an institutional, scientific and social rationale that enables progress to be made with the following objectives:
- 1.
Scientific knowledge of renal abnormalities in patients who suffered from COVID-19.
- 2.
Registry of kidney disease in patients who suffered from COVID-19 with follow-up over time. We recommend semi-annual or annual follow-up, based on initial data, renal function and elemental urine with urinary sediment.
- 3.
Prevention of kidney damage associated with treatments derived from the numerous pathologies linked to post-COVID.
- 4.
Social information and visibility of the importance of monitoring kidney disease, even in patients who, not having had symptoms, are unaware that their kidney function may have decreased due to the infection.
- 5.
Development of lines of research with studies of risk factors, clinical and laboratory expression, renal functional reserve and biomarkers for kidney injury, among others, that improve knowledge of the mechanisms of kidney injury.
- 6.
Development by a working group of the Spanish Society of Nephrology (S.E.N.) of uniform healthcare strategy processes in terms of diagnosis and follow-up of affected patients with a single registry.
- 7.
Considering the aforementioned risks of kidney damage, the immediate incorporation of Nephrology in the multidisciplinary care of post-acute COVID-19 is necessary.
The authors declare that they did not receive any funding.
Conflicts of interestThe authors declare that they have no conflicts of interest