Ultrasound is an essential tool in the management of the nephrological patient allowing the diagnosis, monitoring and performance of kidney intervention. However, the usefulness of ultrasound in the hands of the nephrologist is not limited exclusively to the ultrasound study of the kidney. By ultrasound, the nephrologist can also optimize the management of arteriovenous fistula for hemodialysis, measure cardiovascular risk (mean intimate thickness), implant central catheters for ultrasound-guided HD, as well as the patient's volemia using basic cardiac ultrasound, ultrasound of the Cava Inferior vein and lungs.
From the Working Group on Interventional Nephrology of the Spanish Society of Nephrology (G.N.D.I. of the S.E.N.) we have prepared this Consensus Document that summarizes the main applications of ultrasound to Nephrology, including the necessary basic technical requirements, the framework normative and the level of training of nephrologists in this area. The objective of this work is to promote the inclusion of ultrasound, both diagnostic and interventional, in the usual clinical practice of the nephrologist and in the Nephrology Services portfolio with the final objective of offering diligent, efficient and comprehensive management to the nephrological patient.
La ecografía es una herramienta esencial en el manejo del paciente nefrológico permitiendo el diagnóstico, seguimiento y realización de intervencionismo sobre el riñón. La utilidad de los ultrasonidos en Nefrología no se circunscribe exclusivamente al estudio ecográfico del riñón. Mediante ecografía el nefrólogo puede, además, optimizar el manejo de la fístula arteriovenosa para hemodiálisis, medir el riesgo cardiovascular (grosor íntima media), implantar catéteres centrales para hemodiálisis ecoguiados y ayudar en la colocación de los peritoneales, así como calcular la volemia del paciente medianteecografía cardiaca básica, ecografía de la vena cava inferior y pulmonar.
Desde el G.N.D.I. de la S.E.N hemos elaborado este documento de consenso en el que se resumen las principales aplicaciones de la ecografía en Nefrología, incluyendo los requisitos técnicos básicos necesarios, el marco normativo y el nivel de capacitación de los nefrólogos en esta materia. El objetivo de este trabajo es promover la inclusión de la ecografía, tanto diagnóstica como intervencionista, en la práctica clínica habitual del nefrólogo y en la cartera de servicios de Nefrología con el objetivo final de ofrecer un manejo diligente, eficiente e integral al paciente nefrológico.
Diagnostic and interventional nephrology (DIN), developed since its initiation by nephrologists. This is the area of Nephrology that includes all the imaging and interventional techniques used by the nephrologist: insertion of catheters as vascular access for renal replacement therapy (temporary and permanent catheters for haemodialysis [HD], placement of peritoneal dialysis catheters, renal biopsy, renal (kidneys, ureters, bladder) ultrasound and arteriovenous fistula [AVF]). DIN arose out of the necessity to solve the needs and difficulties encountered during routine clinical practice, in both diagnosis and treatment. Since ultrasound was first introduced into medicine in the 1950s,1 its use has spread to multiple fields, including Nephrology, where it now plays a central role. By the end of the twentieth century, various authors had already written about the great utility of ultrasound in the management of renal patients.2,3
Compared to other areas of Nephrology, DIN has remained relegated to the background from the point of view of patient care and teaching. Until recently, there was no recognised specific training in the different techniques, and there is no official evaluation test or certification to assess the proficiency of physicians in performing these techniques.4,5
There are a number of publications showing how the creation of a DIN section in the Nephrology department is highly efficient, improves the quality of care, reduces waiting times, improves patient safety, optimises resources, and is economically viable, preventing the overload of other departments.6–11 It also has an effect on the survival and viability of patients' vascular access for renal replacement therapy and on diligence in doctor and patient decision-making.
The Sociedad Española de Nefrología (SEN) [Spanish Society of Nephrology] therefore should promote and guarantee access to specific training in ultrasound for all nephrologists interested in performing these techniques.4,6,7 The SEN needs to support the development of learning tools12 and establish routine practice standards. This should be accompanied by assessment and/or certification to officially validate the training undertaken by nephrologists and/or nephrology departments for performing diagnostic and interventional procedures. The SEN should also recommend the introduction and systematic use of this technique for any of its indications to all nephrology departments.
MethodsOn 10 October 2016, at the SEN Congress, a panel of experts, the Grupo de Nefrología Diagnóstica e Intervencionista (GNDI) [Diagnostic and Interventional Nephrology Group], met in Oviedo with the aim of encouraging and creating a document setting out the foundations of DIN for further development, teaching and quality control here in Spain. This panel of experts included nephrologists with a long track record and consolidated experience in DIN in their routine clinical practice.
To prepare this position paper, the experts drew up a discussion outline and questions based on the available data (systematic review) and the most important clinical evidence, debating each fundamental point until arriving at a consensus.
Each expert was responsible for writing an assigned section. The sections were put together and edited by MRG and RHSB in a draft, which was then approved by all panel members and presented as a consensus document. The conclusions are set out below.
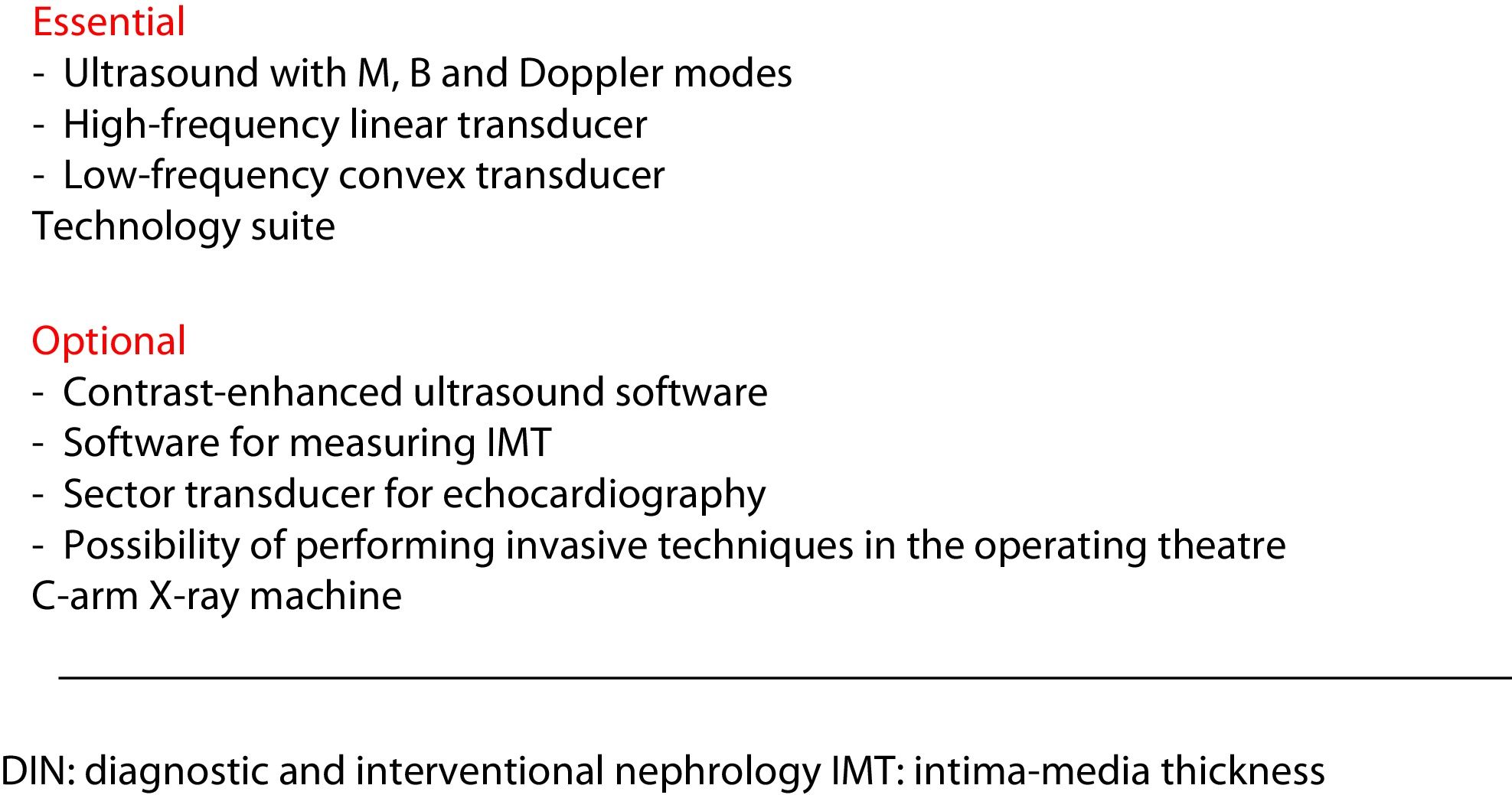
Equipment and physical space requiredNephrology departments should have an ultrasound machine capable of performing studies in two-dimensional and Doppler modes. The equipment should have at least two probes or transducers: a low-frequency convex one for the exploration of the abdominal cavity (kidneys, bladder, prostate, liver-bile duct and abdominal aorta); and a high-frequency linear one for exploration of more superficial structures (pleura-lung, parathyroid glands, carotid and femoral arteries), and exploration and cannulation of the jugular and femoral veins. The same linear transducer will also be used in puncture and exploration of the vascular access for HD.
Certain applications require specific software; for example, for the measurement of carotid intima-media thickness (IMT), plus M mode and a low-frequency sector probe for echocardiography (Fig. 1).
Ultrasound scans can be performed in any physical space, even at the bedside, as the equipment is easily transportable. Low/minimal lighting is required to optimally display the image. Ultrasound-guided interventionism, in addition to a dimly lit room, requires a height-adjustable stretcher, ideally with wheels to evacuate the patient in the event of complications, a good light source, surgical and pharmacological material according to the procedure being carried out, and nursing staff support.
The intervention suite should have computer equipment to record the activity and compile the essential clinical reports of each examination (ultrasound and interventionism).
The Nephrology department must ensure storage and custody of the images obtained in a database, so they can be reviewed and, if necessary, compared with successive examinations of the same patient, and also to allow cases to be discussed with other healthcare professionals and transmission of knowledge. If the hospital uses electronic medical records, the images should be stored with the reports in the corresponding computer storage system.
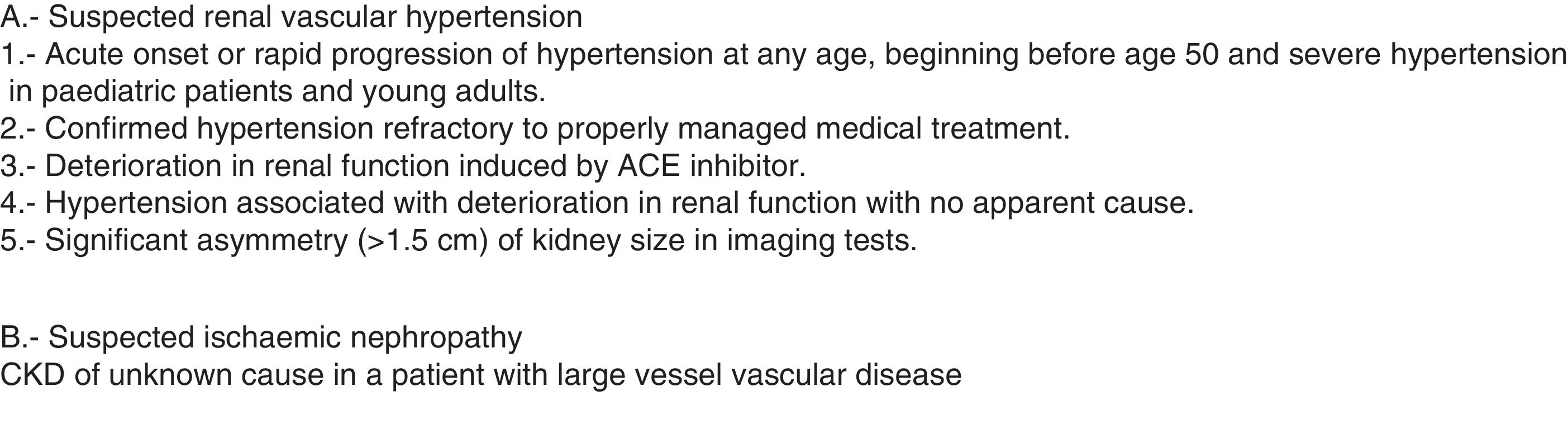
Ultrasound applied to nephrologyA. Diagnostic ultrasoundRenal, bladder and prostate ultrasoundB-mode ultrasound of the kidney, bladder and prostate is a basic tool in the assessment of both acute and chronic kidney injury. It is essential in the study of renal function deterioration in the absence of previous glomerular filtration data. It enables obstructive causes and, using colour Doppler, vascular causes to be confirmed or ruled out quickly and non-invasively for the patient.2,13 The nephrologist needs to be able to assess3 the presence and location of both kidneys, their size, symmetry and echo structure, dilation of the urinary tract, and the presence of masses, cysts or lithiasis. For the bladder, we need to be able to recognise the presence of exophytic lesions, diverticula and imprints on the bladder wall, as well as assess the wall's thickness. Inside the bladder, we have be able to recognise the presence of catheters or debris, as well as its filling volume. A full bladder and post-voiding examination should be performed to assess the presence of significant residue.
In the prostate, we have to be able to assess prostate size by measuring its three diameters in order to calculate the volume (Fig. 2).
The Doppler mode allows us to study intra- and extra-renal vessels, determine velocities for the diagnosis and follow-up of renal artery stenosis, and study vascular malformations, arterial or venous thrombosis and post-renal biopsy vascular complications.
Using colour Doppler (qualitative) we can assess the arterial and venous perfusion of the entire parenchyma, studying the renal artery and vein in all its sections. With spectral Doppler (quantitative) we can record the spectral wave of the vessel studied. From this record, we can make measurements of various parameters of vascular flow. In the indirect study, recordings are made of the intra-renal vessels, their morphology is analysed (e.g. to identify a "parvus et tardus" waveform, suggestive of stenosis), and the resistive, pulsatility and acceleration indices are calculated. The direct study (on the renal artery) is used to detect the presence of stenosis, measuring the velocities directly in the renal artery and in the aorta. The direct study is more complicated than the indirect one, as it is very difficult to visualise the renal artery in its entirety. In both the direct and indirect methods, a correct angle of insonation (angle between the ultrasound beam and the direction of blood flow) of a maximum of 60° is required for velocity measurements to be reliable (Fig. 3). Lastly, although in kidney transplantation the use of Doppler is mandatory, as there is a vascular anastomosis, in the native kidney there are only very specific indications (Fig. 4).
Ultrasound of vascular access. From pre-surgical mapping to diagnosis of complicationsGood vascular access (VA) is essential for the success of HD treatment, as it determines the quality of the dialysis, and complications can lead to high morbidity and mortality rates and increased healthcare costs.14
Preoperative mapping of the arterial and venous territory in the arms of patients who are candidates for VA, especially in patients with comorbidity, increases the rate of native VA and improves survival. Although arm mapping should be used in all patients, it is particularly necessary in cases where the habitual physical examination may be insufficient due to obesity, absent pulses, previous surgery, possible arterial disease (advanced age, diabetes, cardiovascular disease) or possible venous disease (multiple previous venous cannulation).
The examination includes grey-scale B-mode ultrasound followed by a Doppler scan. Both arterial and venous exploration is from the distal area, starting at the wrist, to the proximal area, ending at the subclavian and axillary veins.14
The clinical management of VA can be optimised by the nephrologist's use of Doppler, as it enables early diagnosis of VA problems that may go unnoticed, helping to determine the degree of therapeutic urgency15 and avoiding unnecessary aggressive tests. It is therefore essential that the nephrologist knows the theoretical principles and the practical application of VA ultrasound.
The nephrologist should perform serial Doppler scans of the VA (morphological surveillance and haemodynamic study)16,17 in order to detect significant stenosis,18 monitor non-significant stenosis,19 assess AVF maturation18 and diagnose various disorders, such as thrombosis,20 aneurysms and pseudoaneurysms,18,20 and personalise the treatment of steal syndrome21 or its cardiac impact.22–24
Arterial ultrasound (carotid, femoral and abdominal aorta): assessment of vascular risk in kidney diseaseCardiovascular (CV) disease is the leading cause of death in patients with chronic kidney disease (CKD), and the main culprit is atheromatosis. It is a chronic, progressive inflammatory process of the arteries, causing a thickening of the arterial intima-media layer until the formation of atheroma plaques (thickness >1.5 mm),25 and is accelerated by traditional CV risk factors (age, sex, hypertension, diabetes, dyslipidaemia, smoking, etc.) and non-traditional factors (in patients with CKD: uraemic toxins, oxidative stress, chronic inflammation, anaemia, etc.).26 Arterial ultrasound allows the direct and precise assessment of the damage caused by risk factors on the vascular wall, it being more accurate as it is not based solely on traditional CV risk factors and scores which are not validated in the population with CKD.27 For that reason it is recommended in several different clinical practice guidelines.28,29 The diagnosis of subclinical atheromatous disease will help to detect the population most at risk and allow us to act early to prevent progression and CV events.
Ultrasound also makes it possible to assess the burden of atheromatous disease (number of territories and plaque area), analyse the composition of the plaques (lipids, fibrous tissue, calcium) and assess progression. As it is a systemic disease, it is important to explore all the accessible arterial territories: carotid (common, bifurcation or bulb, internal and external), femoral (common and superficial) and aorta (at the level of the abdomen). Various studies have shown that the prevalence of atheromatosis in femoral arteries, in the absence of carotid involvement, is in the range of 17%–20%.30
Pleuropulmonary ultrasoundLung ultrasound is a non-invasive way to detect lung congestion. It is of great help in the management of renal patients with dyspnoea, even in early subclinical stages. It is very useful in the assessment and monitoring of the response to treatment, in the prevention of volume overload in hypovolaemic heart patients treated with fluid therapy, and in the diagnosis and follow-up of pneumonia.31,32 The diagnosis of volume overload is made by evaluating ultrasound artefacts with diagnostic value such as "lung rockets" (multiple B lines) or comet tail artefacts.33,34
Ultrasound is the best technique for the detection of pleural effusion; it is far superior to conventional radiology, as it is capable of detecting effusions of as little as 5 ml, compared to 150 ml in the posterior/anterior chest X-ray. The ultrasound pattern allows us to distinguish transudative from exudative. Lastly, ultrasound enables rapid detection of pneumothorax without the need to radiate the patient and with high reproducibility.
Transthoracic echocardiogram. Measurement of hydration status: ultrasound of the inferior vena cavaTransthoracic echocardiography is recognised for its utility in renal patients35 and nephrologists should know how to perform it at a basic level.
Essentially, they need to be able to recognise pericardial effusion and left ventricular hypertrophy, and to know how to calculate the ejection fraction. A low-frequency sector probe is required. The degree of inspiratory collapse of the inferior vena cava measured by ultrasound can help estimate patients' effective volume status and, in conjunction with the symptoms, be a support in decision-making for the management of fluid therapy.36
Parathyroid ultrasoundUltrasound was the first imaging technique used to study the parathyroid glands. This is an easy technique for the nephrologist, with a quick and useful learning curve in the routine study of patients with secondary hyperparathyroidism, which provides great benefits and profitability.37 Using ultrasound, we can determine the number and location of the parathyroid hypertrophy in order to plan a parathyroidectomy. The assessment of the parathyroid mass (volume) is of important prognostic value, as any increase is associated with a poorer response to drug treatment.38 In some situations, ultrasound has been used to perform a chemical parathyroidectomy by intraglandular injection of alcohol/calcitriol.39
Ultrasound in peritoneal dialysis. Exit site and tunnel, epigastric artery detection and catheter implantationUltrasound is highly useful in the implantation of the peritoneal catheter by the nephrologist, as it allows the systematic examination of the subcutaneous tissue, musculature and fascia of the rectus muscles, and the location of the epigastric artery to avoid the risk of laceration and of the parietal peritoneum and the peritoneal cavity. It also makes it possible to locate the intestinal loops to ensure they are not in the catheter's path and to rule out adhesions between them.40–42 Lastly, an ultrasound scan to assess the bladder before implantation of the peritoneal catheter can help to prevent perforation in patients with post-void residual volume, with these patients being catheterised, if necessary, to ensure complete bladder emptying. Abdominal ultrasound also helps us guide the insertion of the catheter between the layers of the peritoneum,43 observe how the infusion of peritoneal fluid separates the loops, and facilitate direction of the guide towards the pelvis,42 enabling correct placement of the catheter. It is important to use the ultrasound to identify the tip of the catheter in the pouch of Douglas before surgical closure at the end of the procedure.
In the post-implantation period, ultrasound in the hands of experienced personnel is very useful for assessing tunnel problems and complements other imaging techniques, such as plain X-ray or tomography, and can even sometimes replace them.44 Ultrasound is useful for checking for infection or peri-catheter abscesses,44,45 bleeding or haematoma post-mechanical traction or post-implantation of the catheter, extrusion of the peritoneal catheter, peri-catheter and peritoneal cavity leaks46 or catheter entrapment by omentum.47
Finally, ultrasound can also be used to determine the thickness of the peritoneal membrane and how it relates to the dialysis technique's effectiveness, possibly avoiding the need for future aggressive tests, such as peritoneal biopsy.48
Ultrasound of gastric motilityGastroparesis or slowed gastric emptying is a known complication of diseases such as diabetes or amyloidosis, and also postoperatively, such as after kidney transplantation. Gastric emptying time is reportedly lengthened in patients undergoing any of the three types of renal replacement therapy compared to healthy subjects.49 Gastroparesis can cause anorexia and malnutrition. It can be diagnosed by two-dimensional real-time ultrasound, taking dynamic images of gastric peristaltic activity and its effect on stomach emptying, and then calculating the total postprandial gastric volume.50
Hepatobiliary ultrasoundUltrasound allows early detection of fatty liver, a highly prevalent disorder in the general population and also, therefore, in renal patients. The echogenicity of the healthy renal parenchyma is very similar to that of the healthy liver. In fatty liver, the liver is whiter (hyperechogenic) than the renal parenchyma.51 Although previously considered a benign condition, progression to liver fibrosis and even cirrhosis has been reported,52 owing to which these patients should be referred to Gastroenterology for assessment.
With ultrasound, we can easily detect gallbladder sludge and gallstones, whether asymptomatic or complicated with cholecystitis, if we know how to recognise their ultrasound signs.
B. Ultrasound-guided nephrological interventionRenal biopsyPercutaneous renal biopsy (PRB) is one of the main procedures in the diagnosis of many different disorders, both of native and transplanted kidneys, and is currently the most widely used interventional technique in Nephrology departments.53 With PRB not being free of complications,54 the use of ultrasound when performing the procedure led to a clear reduction in post-biopsy complications, ranging from 10% to 15% of all patients biopsied using other procedures, to 4% to 6% after the introduction of ultrasound-guided PRB.55
We can differentiate between three uses of ultrasound in renal biopsy:
- •
Dummy run to assess structure, characteristics of the kidney, access difficulties and possible contraindications to performing the biopsy (e.g. single kidney).
- •
Performing the PRB per se, with ultrasound enabling the exact puncture point to be chosen, the visualisation of the needle inside the kidney throughout the procedure, and the structures to avoid such as colon, large vessels or renal cysts.
- •
And afterwards, to detect possible complications, such as bleeding, haematoma, arteriovenous or arteriocalyceal fistulas and pseudoaneurysms,56,57 even at the patient's bedside.
When the nephrologist is able to perform the PRB, this affords them autonomy and reduces the hospital stay by not having to depend on other departments. Acquiring skills in ultrasound-guided PRB requires specific training, experience and mastery in the morphological analysis of the healthy kidney and its pathological variables, and in the use of the Doppler mode for the analysis of post-biopsy vascular complications.
Implantation of tunnelled and non-tunnelled catheters for haemodialysisImplantation of a catheter in a central vein with subcutaneous tunnelling is a valid alternative in various clinical situations requiring VA for HD, such as contraindication for an AVF, patients awaiting an AVF, or patients with an AVF, but not sufficiently developed to be used.
For the implantation of a catheter in a central vein, whether tunnelled or not, the AV Guidelines recommend ultrasound-guided puncture rather than implantation by anatomical reference 18, as ultrasound identifies the position of each vessel and its anatomical relationship, and reduces the risk of unwanted puncture of other adjacent vascular structures or the pneumothorax. It also enables detection of thrombosis, which would rule out the vessel for catheter implantation. In short, the use of ultrasound makes catheterisation easier, quicker and safer, and reduces both the likelihood of complications58,59 and the costs.60 Ultrasound-guided puncture is ideal for cannulation of the jugular vein. However, for cannulation of the subclavian vein, the ultrasound view is limited by the interposition of the clavicle. For the implantation of a tunnelled catheter into a central, internal jugular or subclavian vein, it is recommended that after the puncture and the introduction of the guidewire the position of the guidewire be checked by radioscopy to confirm that the intravascular end is lodged in the right place, and to avoid catheter dysfunction owing to malpositioning. 18 Radioscopy also facilitates the identification of thrombosis or stenosis in central veins with the use of contrast, helping to rule out the affected vessel. Radioscopic confirmation is not considered necessary if the catheter is implanted in the femoral vein.
Implantation of peritoneal catheterAs already discussed in the "Ultrasound in peritoneal dialysis" section, ultrasound will be very useful in peritoneal catheter implantation in order to optimise placement, avoid complications such as puncture of the epigastric artery or intestinal loops, and to confirm the exact position.
Ultrasound-guided puncture of the arteriovenous fistulaPuncture of the AVF can be difficult due to poor maturation, small diameter, juxta-anastomotic stenosis or, around the puncture site, proximity to arteries or nerves, existence of collaterals, previous unsuccessful punctures with haematomas, or even not knowing the direction of cannulation in the case of deep AVF. The characteristics of the HD programme population, with a progressive increase in age and vascular comorbidity, have led to an increase in this type of puncture with extra difficulties.
To counteract these difficulties, Doppler ultrasound performed at the patient's bedside by the nephrologist and/or haemodialysis unit nurse can increase the sensitivity of early detection of abnormalities, resulting in reduced morbidity and mortality rates.61–63 Doppler provides information on the patency, flow, depth and diameter of the vessel when performing the targeted puncture,64,65 and allows the path of the needle to be tracked within the vessel.66–70 This last aspect is essential, as it allows the needle to be repositioned during the HD session if necessary.
Although there are limited studies on the utility of Doppler ultrasound for ultrasound-guided puncture of the VA, its recent incorporation into routine clinical practice has shown that it can reduce the number of punctures, facilitate cannulation in difficult-to-access AVF, and reduce patient discomfort deriving from the puncture. It is therefore necessary to include strategies aimed at training professionals in this specific area.71
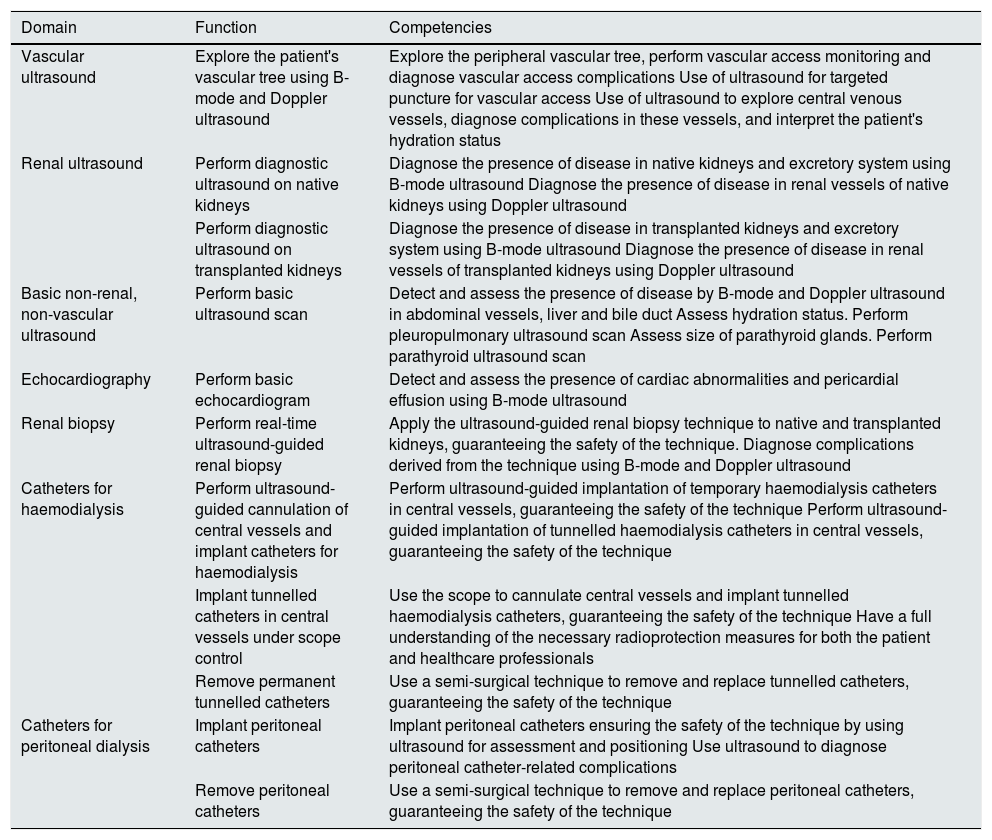
Competencies of the interventional nephrologistThe technical competencies that the interventional nephrologist needs to acquire in each of the domains that define this subspeciality of Nephrology are listed in Table 1.
Technical competencies of the interventional nephrologist in each of the domains that define this subspeciality of Nephrology.
| Domain | Function | Competencies |
|---|---|---|
| Vascular ultrasound | Explore the patient's vascular tree using B-mode and Doppler ultrasound | Explore the peripheral vascular tree, perform vascular access monitoring and diagnose vascular access complications Use of ultrasound for targeted puncture for vascular access Use of ultrasound to explore central venous vessels, diagnose complications in these vessels, and interpret the patient's hydration status |
| Renal ultrasound | Perform diagnostic ultrasound on native kidneys | Diagnose the presence of disease in native kidneys and excretory system using B-mode ultrasound Diagnose the presence of disease in renal vessels of native kidneys using Doppler ultrasound |
| Perform diagnostic ultrasound on transplanted kidneys | Diagnose the presence of disease in transplanted kidneys and excretory system using B-mode ultrasound Diagnose the presence of disease in renal vessels of transplanted kidneys using Doppler ultrasound | |
| Basic non-renal, non-vascular ultrasound | Perform basic ultrasound scan | Detect and assess the presence of disease by B-mode and Doppler ultrasound in abdominal vessels, liver and bile duct Assess hydration status. Perform pleuropulmonary ultrasound scan Assess size of parathyroid glands. Perform parathyroid ultrasound scan |
| Echocardiography | Perform basic echocardiogram | Detect and assess the presence of cardiac abnormalities and pericardial effusion using B-mode ultrasound |
| Renal biopsy | Perform real-time ultrasound-guided renal biopsy | Apply the ultrasound-guided renal biopsy technique to native and transplanted kidneys, guaranteeing the safety of the technique. Diagnose complications derived from the technique using B-mode and Doppler ultrasound |
| Catheters for haemodialysis | Perform ultrasound-guided cannulation of central vessels and implant catheters for haemodialysis | Perform ultrasound-guided implantation of temporary haemodialysis catheters in central vessels, guaranteeing the safety of the technique Perform ultrasound-guided implantation of tunnelled haemodialysis catheters in central vessels, guaranteeing the safety of the technique |
| Implant tunnelled catheters in central vessels under scope control | Use the scope to cannulate central vessels and implant tunnelled haemodialysis catheters, guaranteeing the safety of the technique Have a full understanding of the necessary radioprotection measures for both the patient and healthcare professionals | |
| Remove permanent tunnelled catheters | Use a semi-surgical technique to remove and replace tunnelled catheters, guaranteeing the safety of the technique | |
| Catheters for peritoneal dialysis | Implant peritoneal catheters | Implant peritoneal catheters ensuring the safety of the technique by using ultrasound for assessment and positioning Use ultrasound to diagnose peritoneal catheter-related complications |
| Remove peritoneal catheters | Use a semi-surgical technique to remove and replace peritoneal catheters, guaranteeing the safety of the technique |
The following are the basicor mandatory areas that the nephrologist must have a mastery of:
- •
Renal and bladder/prostate ultrasound, both 2-D and Doppler modes.
- •
AVF ultrasound and ultrasound-guided puncture of the same.
- •
Cannulation of temporary HD catheters, both jugular and femoral.
- •
Ultrasound-guided renal biopsy, both native and transplanted kidneys.
The following would be secondary or optional areas:
- •
Cardiopulmonary ultrasound.
- •
Vascular ultrasound: carotid, femoral and abdominal aorta.
- •
Vascular mapping of the upper limb prior to AVF.
- •
Gastrointestinal ultrasound: gastric and hepatobiliary.
- •
Parathyroid ultrasound.
- •
Implantation of tunnelled catheters for HD.
- •
Implantation of catheters for peritoneal dialysis{2}
The training programme for the speciality of Nephrology (ORDER SCO/2604/2008. BOE [Official State Gazette] 15/09/2008) in point 5.3.4 establishes renal ultrasound, renal biopsy, temporary vascular access and peritoneal catheters as within the "knowledge, skills and development of investigation, diagnosis and treatment techniques".72 In the new training programme, currently under development, Interventional Nephrology is included among the skills that the nephrologist must master. Lastly, in the 2016–2020 SEN strategic plan, the Governing Board included reassessment of the speciality of Nephrology as a priority, defending its own competencies and developing emerging areas such as Interventional Nephrology.73
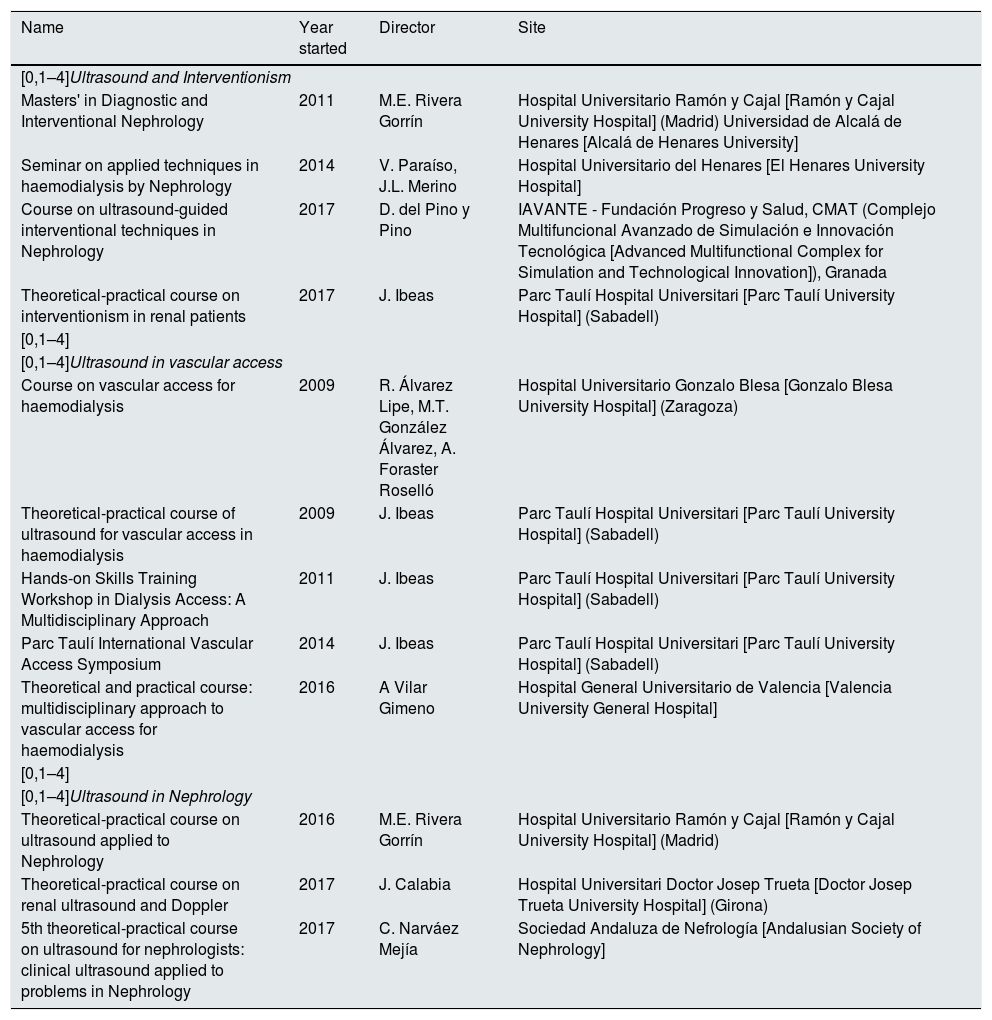
Last of all, we, as members of the GNDI, believe it is necessary for the SEN to establish an accreditation and training system in ultrasound applied to Nephrology in order to guarantee quality and homogeneous training and clinical practice in this area for Spanish nephrologists. While there are no accredited centres, Spanish nephrologists have a wide range of courses available to them, the majority accredited and with SEN endorsement, in addition to university degrees. Table 2 shows a list of the most consolidated courses in Spain.
Consolidated courses in Diagnostic and Interventional Nephrology in Spain.
| Name | Year started | Director | Site |
|---|---|---|---|
| [0,1–4]Ultrasound and Interventionism | |||
| Masters' in Diagnostic and Interventional Nephrology | 2011 | M.E. Rivera Gorrín | Hospital Universitario Ramón y Cajal [Ramón y Cajal University Hospital] (Madrid) Universidad de Alcalá de Henares [Alcalá de Henares University] |
| Seminar on applied techniques in haemodialysis by Nephrology | 2014 | V. Paraíso, J.L. Merino | Hospital Universitario del Henares [El Henares University Hospital] |
| Course on ultrasound-guided interventional techniques in Nephrology | 2017 | D. del Pino y Pino | IAVANTE - Fundación Progreso y Salud, CMAT (Complejo Multifuncional Avanzado de Simulación e Innovación Tecnológica [Advanced Multifunctional Complex for Simulation and Technological Innovation]), Granada |
| Theoretical-practical course on interventionism in renal patients | 2017 | J. Ibeas | Parc Taulí Hospital Universitari [Parc Taulí University Hospital] (Sabadell) |
| [0,1–4] | |||
| [0,1–4]Ultrasound in vascular access | |||
| Course on vascular access for haemodialysis | 2009 | R. Álvarez Lipe, M.T. González Álvarez, A. Foraster Roselló | Hospital Universitario Gonzalo Blesa [Gonzalo Blesa University Hospital] (Zaragoza) |
| Theoretical-practical course of ultrasound for vascular access in haemodialysis | 2009 | J. Ibeas | Parc Taulí Hospital Universitari [Parc Taulí University Hospital] (Sabadell) |
| Hands-on Skills Training Workshop in Dialysis Access: A Multidisciplinary Approach | 2011 | J. Ibeas | Parc Taulí Hospital Universitari [Parc Taulí University Hospital] (Sabadell) |
| Parc Taulí International Vascular Access Symposium | 2014 | J. Ibeas | Parc Taulí Hospital Universitari [Parc Taulí University Hospital] (Sabadell) |
| Theoretical and practical course: multidisciplinary approach to vascular access for haemodialysis | 2016 | A Vilar Gimeno | Hospital General Universitario de Valencia [Valencia University General Hospital] |
| [0,1–4] | |||
| [0,1–4]Ultrasound in Nephrology | |||
| Theoretical-practical course on ultrasound applied to Nephrology | 2016 | M.E. Rivera Gorrín | Hospital Universitario Ramón y Cajal [Ramón y Cajal University Hospital] (Madrid) |
| Theoretical-practical course on renal ultrasound and Doppler | 2017 | J. Calabia | Hospital Universitari Doctor Josep Trueta [Doctor Josep Trueta University Hospital] (Girona) |
| 5th theoretical-practical course on ultrasound for nephrologists: clinical ultrasound applied to problems in Nephrology | 2017 | C. Narváez Mejía | Sociedad Andaluza de Nefrología [Andalusian Society of Nephrology] |
The authors declare that they have no conflicts of interest.
Please cite this article as: Gorrín MR, Barrios RHS, López CR-Z, Fernández JM, Robayna SM, López JI et al. Documento de Consenso para la Formación en Ecografía en la especialidad de Nefrología. Nefrologia. 2020;40:623–633.