Paricalcitol, a selective vitamin D receptor activator, is used to treat secondary hyperparathyroidism in kidney transplant patients. Experimental and clinical studies in non-transplant kidney disease patients have found this molecule to have anti-inflammatory properties. In this exploratory study, we evaluated the anti-inflammatory profile of paricalcitol in kidney-transplant recipients.
MethodsThirty one kidney transplant recipients with secondary hyperparathyroidism completed 3 months of treatment with oral paricalcitol (1μg/day). Serum concentrations and gene expression levels of inflammatory cytokines in peripheral blood mononuclear cells were analysed at the beginning and end of the study.
ResultsParicalcitol significantly decreased parathyroid hormone levels with no changes in calcium and phosphorous. It also reduced serum concentrations of interleukin (IL)-6 and tumour necrosis factor-alpha (TNF-α) by 29% (p<0.05) and 9.5% (p<0.05) compared to baseline, respectively. Furthermore, gene expression levels of IL-6 and TNF-α in peripheral blood mononuclear cells decreased by 14.1% (p<0.001) and 34.1% (p<0.001), respectively. The ratios between pro-inflammatory cytokines (TNF-α and IL-6) and anti-inflammatory cytokines (IL-10), both regarding serum concentrations and gene expression, also experienced a significant reduction.
ConclusionsParicalcitol administration to kidney transplant recipients has been found to have beneficial effects on inflammation, which may be associated with potential clinical benefits.
El paricalcitol, un activador selectivo del receptor de la vitamina D, se utiliza en el tratamiento del hiperparatiroidismo secundario en el receptor de trasplante renal. Estudios tanto clínicos como experimentales realizados en pacientes renales no trasplantados muestran propiedades antiinflamatorias para esta molécula. En este estudio exploratorio, hemos evaluado el perfil antiinflamatorio del paricalcitol en receptores de trasplante renal.
MétodosTreinta y un pacientes trasplantados con hiperparatiroidismo secundario completaron 3 meses de terapia con paricalcitol oral (1μg/día). Se determinaron las concentraciones séricas y los niveles de expresión génica de citocinas inflamatorias en células mononucleares de sangre periférica al inicio y al final del estudio.
ResultadosEl paricalcitol provocó una disminución significativa en los niveles de hormona paratiroidea, sin cambios en los de calcio y fósforo. Además, indujo una reducción en las concentraciones séricas de la interleucina (IL)-6 y del factor de necrosis tumoral alfa (TNF-α), con reducciones porcentuales respecto al estado basal de un 29% (p<0,05) y de un 9,5% (p<0,05), respectivamente. Los niveles de expresión génica de la IL-6 y del TNF-α en células mononucleares de sangre periférica experimentaron un descenso de un 14,1% (p<0,001) y de un 34,1% (p<0,001), respectivamente. La proporción entre las citocinas proinflamatorias (TNF-α e IL-6) y la antiinflamatoria IL-10, tanto para los niveles séricos como para los de expresión génica, también disminuyó significativamente.
ConclusionesLa administración del paricalcitol a receptores de trasplante renal se asocia con efectos beneficiosos sobre su estado inflamatorio, lo que podría asociarse a un potencial beneficio clínico.
Although kidney transplants manage to correct many of the metabolic imbalances derived from uraemia, secondary hyperparathyroidism (SHPT) continues to be present in a high percentage of these patients.1
Paricalcitol is a selective vitamin D receptor (VDR) activator used for the prevention and treatment of SHPT. Two recently conducted prospective, randomised clinical trials show a sharp decline in concentrations of intact parathyroid hormone (iPTH) in kidney transplant recipients, in those patients who received paricalcitol,2,3 together with a reduction in frequency of post-transplant SHPT in more than half after one year of follow-up2 and an attenuation of bone remodelling processes and mineral loss.3 In addition, it causes a reduction in the urinary excretion of proteins which, together with all the above, signals the existence of pleiotropic effects of paricalcitol in this population.
In chronic kidney disease (CKD), the inflammatory status is associated with several complications, including anaemia, left ventricular hypertrophy, vascular calcification and atherosclerosis, and is an independent predictor of mortality.1–3 In this scenario, alterations in the regulatory cytokines of the inflammatory process are a key factor in the development of complications and in the increase of morbidity and mortality.4–8 In the case of kidney transplant recipients, inflammation has been associated with harmful consequences, such as acute rejection, long-term complications, development of coronary calcification, hypertension, as well as an increase in cardiovascular events and mortality.9–13
Both in vitro and in vivo experimental studies have shown anti-inflammatory effects of paricalcitol, including the attenuation of the expression of inflammatory cytokines, inhibition of kidney inflammation by the sequestration of NF-kB, inhibition of the Wnt pathway, and the blocking of β-catenin mediated gene transcription.14–18 However, studies that indicate the existence of anti-inflammatory effects of paricalcitol in clinical settings are limited, and none of them were conducted on kidney transplant recipients.
This preliminary study was conducted on kidney transplant recipients to determine the effect of administration of oral paricalcitol on serum concentrations and the gene expression profile in peripheral blood mononuclear cells (PBMCs) of inflammatory cytokines.
MethodsStudy designProspective, open-label, non-randomised study, which included patients who have had a kidney transplant, conducted under routine clinical practice conditions. The inclusion criteria were: patients over 18 years; stable renal function during the three months prior to the start of the study (stability defined as a variability of the serum creatinine values less than 20% with respect to the last check-up); serum calcium and phosphorus levels within the normal range; estimated glomerular filtration rate of more than 30ml/min/1.73m2; with no changes to immunosuppressive therapy in the three months beforehand and ability to provide informed consent. The exclusion criteria were: concentration of iPTH less than 100pg/ml; existence of previous episodes of acute rejection; known immunological disease; acute inflammatory episodes in the previous three months; positive for hepatitis B, C or HIV; previous treatment with calcimimetics, with vitamin D or with active vitamin D compounds and smoking, alcohol or drug abuse habits.
The patients finally included in the study and who had a serum concentration of iPTH greater than 100pg/ml were treated with paricalcitol orally at a dose of 1μg/day for three months. Once the three months of treatment were completed, the variation in serum levels of inflammatory cytokines interleukin (IL)-6, IL-10 and tumour necrosis factor (TNF)-α was determined, as well as the variation in the expression of their encoding genes in PBMCs. The study was approved by the centre's Independent Ethics Committee and conducted in accordance with the Declaration of Helsinki, with the Directive for Clinical Trials in the European Union (2001/20/EC) and following the good clinical practice guidelines. All participants signed an informed consent form prior to inclusion.
Biochemical determinationsAll determinations were performed by personnel blinded to the characteristics of the patients and the group to which they were assigned. Blood draws were performed after fasting for 8h. Serum aliquots were frozen at −80°C until they were analysed. Routine biochemical parameters were determined using standard methods. Creatinine was measured using a standardised enzymatic method (CREA Plus, Roche Diagnostic, Mannheim, Germany). The coefficients of variation (intra- and inter-assay) were less than 1.5%. The concentration of serum iPTH was determined by the Roche modular intact PTH assay (Roche Diagnostics Corp., Indianapolis, IN, USA) (normal reference range, 15–65pg/ml).
Serum levels of high-sensitivity C-reactive protein (hs-CRP) were measured using immunoturbidimetric assay (Roche Diagnostics GmbH, Mannheim, Germany) in an automated manner; the sensitivity was 0.3mg/l and the coefficients of intra- and inter-assay variability were 1.6 and 8.4%, respectively. TNF-α, IL-6 and IL-10 concentrations were determined using high-sensitivity ELISA (Quantikine Human, R&D Systems, Minneapolis, USA) using a DSXTM-4 plate ELISA processor (Vitro SA, Spain). Minimum detectable concentrations were 0.10; 0.70 and 0.50pg/ml, respectively. The intra- and inter-assay coefficients of variation were less than 10.8%. All participants were required to provide one 24-hour urine sample for the determination of the creatinine clearance value and the urinary excretion of albumin value. Patients were instructed beforehand on how to collect these urine samples and were given written instructions. The container with the urine was taken to the laboratory the same day as the collection period was completed. Urinary excretion of albumin was measured by immunoturbidimetry (Tina-quant Albumin Assay, Roche Diagnostic, Mannheim, Germany). Regarding the analytical sensitivity and specificity, the limit of blank was 2mg/dl and the limit of detection was 3mg/dl. The intra- and inter-assay coefficients of variation were less than 4%.
Gene expression in peripheral blood mononuclear cellsAll patients had a sample of whole blood taken (2.5ml) at baseline and at the end of the study using PAXgene Blood RNA tubes (BD, Franklin Lakes, NJ, USA). This blood draw was performed at the same time as that used to restore the serum. The PAXgene Blood RNA Kit (Qiagen, Valencia, CA, USA) was used to extract the total RNA following the manufacturer's instructions, and it was then stored at −80°C. The cDNA was synthesised using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA).
By using the TaqMan probe and quantitative real-time PCR (qRT-PCR) the expression of encoding transcripts for TNF-α, IL-6, IL-10 and for glyceraldehyde 3-phosphate dehydrogenase (GApDH) was determined. The commercial reaction mixture used was TaqMan Fast Universal PCR Master Mix (Applied Biosystems) and the TaqMan probes selected for each transcript were: Hs00174128_ml [TNFα], Hs00985639_ml [IL-6], Hs0961622_m1 [IL-10] y Hs99999905_m1 [GApDH]). Amplification was conducted in a thermocycler 7500 Fast Real-Time PCR System (Applied Biosystems). mRNA levels for each of the genes studied were calculated using relative quantification, using the comparative 2−ΔΔCt method by normalisation with respect to the expression of GApDH. The determinations of each transcript were made in triplicate and samples without reverse transcription were used as negative controls versus possible DNA contamination.
Statistical analysisGiven the exploratory nature of the study, no calculation of sample size was performed. Categorical data are presented as absolute values and percentages. Continuous variables are highlighted with mean values±standard deviation. The Shapiro–Wilk W-test was used to determine normality. The baseline comparisons were performed using the Student's t-test for independent samples if data followed a normal distribution, or using a Mann–Whitney U test when data appeared to be highly biased. To compare baseline and final values, the Mann–Whitney and Wilcoxon tests were used for paired data, as appropriate. Both the serum and gene expression values that did not have a normal distribution were converted to log-values for the calculations. Expression levels of the genes under study were calculated using the Data AssistTM v3.0 Software (Applied Biosystems). The rest of the calculations were performed in GraphPad InStat (GraphPad Software, San Diego, CA, USA). All tests were two-tailed, and a p value<0.05 was considered statistically significant.
ResultsDemographic and biochemical parametersAt the start of the study, a total of 74 patients were considered, of whom 43 were not included for the following reasons: eight had an iPTH concentration of less than 100pg/ml, 12 had an acute infectious episode, five had a history of acute rejection, five patients received treatment with nutritional vitamin D and four with active analogues, four patients had a variation in serum creatinine concentration >20% compared to the previous control and the immunosuppressive therapy was modified in five patients. Finally, 31 patients were included in accordance with the inclusion criteria (19 men; mean age 54.2±12.7 years) who were treated with paricalcitol (1μg/day for three months).
Treatment with paricalcitol was well-tolerated, meaning that all patients completed the study, and it was not necessary to withdraw, even temporarily, the treatment in any case.
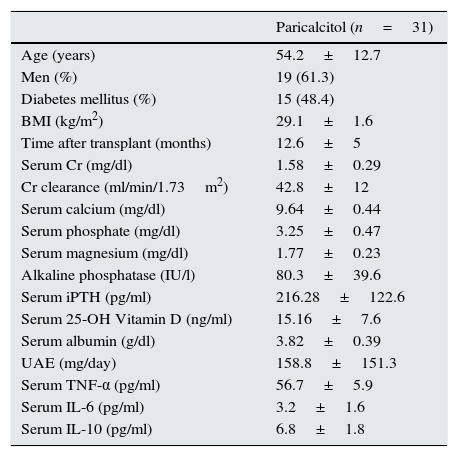
Regarding immunosuppressive therapy, all patients received therapy with steroids and with mycophenolate mofetil. Twenty-six patients received tacrolimus and five received an inhibitor of the mTOR (mammalian target of rapamycin) pathway. Finally, 15 patients (48%) were treated with renin-angiotensin system blockers. Table 1 shows the baseline clinical characteristics of the participants.
Baseline clinical characteristics of the patients included in the study.
| Paricalcitol (n=31) | |
|---|---|
| Age (years) | 54.2±12.7 |
| Men (%) | 19 (61.3) |
| Diabetes mellitus (%) | 15 (48.4) |
| BMI (kg/m2) | 29.1±1.6 |
| Time after transplant (months) | 12.6±5 |
| Serum Cr (mg/dl) | 1.58±0.29 |
| Cr clearance (ml/min/1.73m2) | 42.8±12 |
| Serum calcium (mg/dl) | 9.64±0.44 |
| Serum phosphate (mg/dl) | 3.25±0.47 |
| Serum magnesium (mg/dl) | 1.77±0.23 |
| Alkaline phosphatase (IU/l) | 80.3±39.6 |
| Serum iPTH (pg/ml) | 216.28±122.6 |
| Serum 25-OH Vitamin D (ng/ml) | 15.16±7.6 |
| Serum albumin (g/dl) | 3.82±0.39 |
| UAE (mg/day) | 158.8±151.3 |
| Serum TNF-α (pg/ml) | 56.7±5.9 |
| Serum IL-6 (pg/ml) | 3.2±1.6 |
| Serum IL-10 (pg/ml) | 6.8±1.8 |
BMI: body mass index; Cr: creatinine; IL: interleukin; iPTH: intact parathyroid hormone; IU: international units; NS: not significant; TNF-α: tumour necrosis factor alpha; UAE: urinary excretion of albumin.
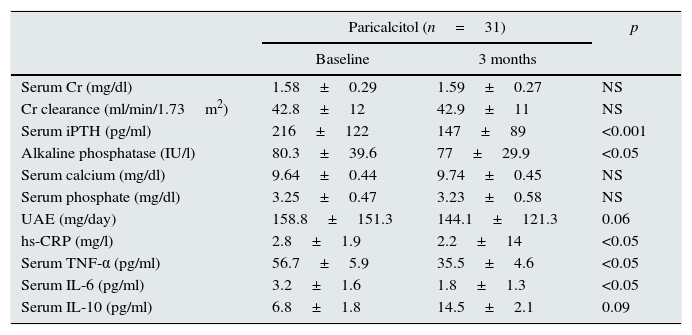
After three months of paricalcitol administration, there were no significant changes in renal function (serum creatinine values and creatinine clearance values) or in mineral metabolism parameters (serum phosphorus and calcium and calcium-phosphorus product) except for iPTH (Table 2). Thus, iPTH serum concentrations decreased significantly from 216.3±122.6pg/ml at baseline to 147±89pg/ml at the end of the study (p<0.001), representing a mean percentage decrease of 31.9% in relation to the baseline values. Similarly, the serum alkaline phosphatase levels also decreased in subjects who received paricalcitol. There were no episodes of hypercalcaemia or hyperphosphataemia during the study.
Biochemical characteristics of the patients at the start and end of the study.
| Paricalcitol (n=31) | p | ||
|---|---|---|---|
| Baseline | 3 months | ||
| Serum Cr (mg/dl) | 1.58±0.29 | 1.59±0.27 | NS |
| Cr clearance (ml/min/1.73m2) | 42.8±12 | 42.9±11 | NS |
| Serum iPTH (pg/ml) | 216±122 | 147±89 | <0.001 |
| Alkaline phosphatase (IU/l) | 80.3±39.6 | 77±29.9 | <0.05 |
| Serum calcium (mg/dl) | 9.64±0.44 | 9.74±0.45 | NS |
| Serum phosphate (mg/dl) | 3.25±0.47 | 3.23±0.58 | NS |
| UAE (mg/day) | 158.8±151.3 | 144.1±121.3 | 0.06 |
| hs-CRP (mg/l) | 2.8±1.9 | 2.2±14 | <0.05 |
| Serum TNF-α (pg/ml) | 56.7±5.9 | 35.5±4.6 | <0.05 |
| Serum IL-6 (pg/ml) | 3.2±1.6 | 1.8±1.3 | <0.05 |
| Serum IL-10 (pg/ml) | 6.8±1.8 | 14.5±2.1 | 0.09 |
Cr: creatinine; hs-CRP: high-sensitivity C-reactive protein; IL: interleukin; iPTH: intact parathyroid hormone; IU: international units; NS: not significant; TNF-α: tumour necrosis factor alpha; UAE: urinary excretion of albumin.
Regarding the urinary excretion of albumin, there were no significant changes in this parameter, although a decrease of 9.3% was observed from the baseline values (p=0.057).
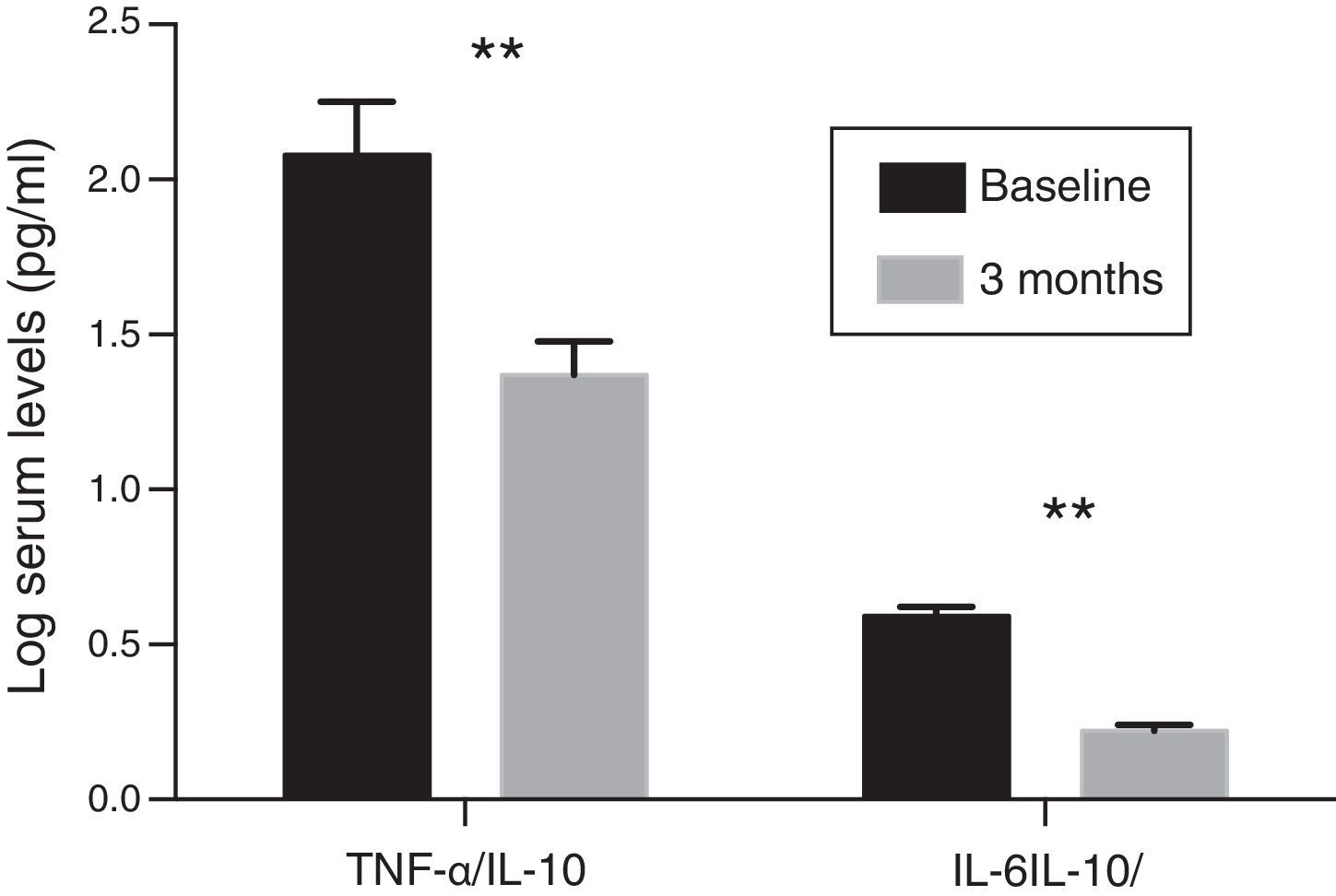
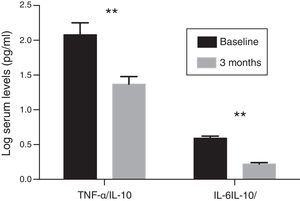
Inflammatory profileThe concentration of hs-CRP experienced a significant decrease after treatment with paricalcitol (2.8±1.9 vs. 2.2±1.4; p<0.05). Serum levels of IL-10 following treatment with paricalcitol showed an upward trend (p=0.09), while both the IL-6 and TNF-α values decreased significantly (Table 2). Thus, in relation to the baseline values, the mean percentage decreases of IL-6 and TNF-α were respectively: 29.08 and 9.58% (p<0.05, in both cases). These changes were unrelated to the variations in serum iPTH levels or to the change in the urinary excretion of albumin. The change in the balance between pro- and anti-inflammatory strengths was also evaluated by the evolution of the TNF-α and IL-6 ratios relative to the anti-inflammatory cytokine IL-10. Thus, and compared to the baseline values, significant reductions were observed for both the TNF-α/IL-10 ratio and IL-6/IL-10 ratio, with percentage decrease values of 33 and 36.9%, respectively (p=0.01) (Fig. 1).
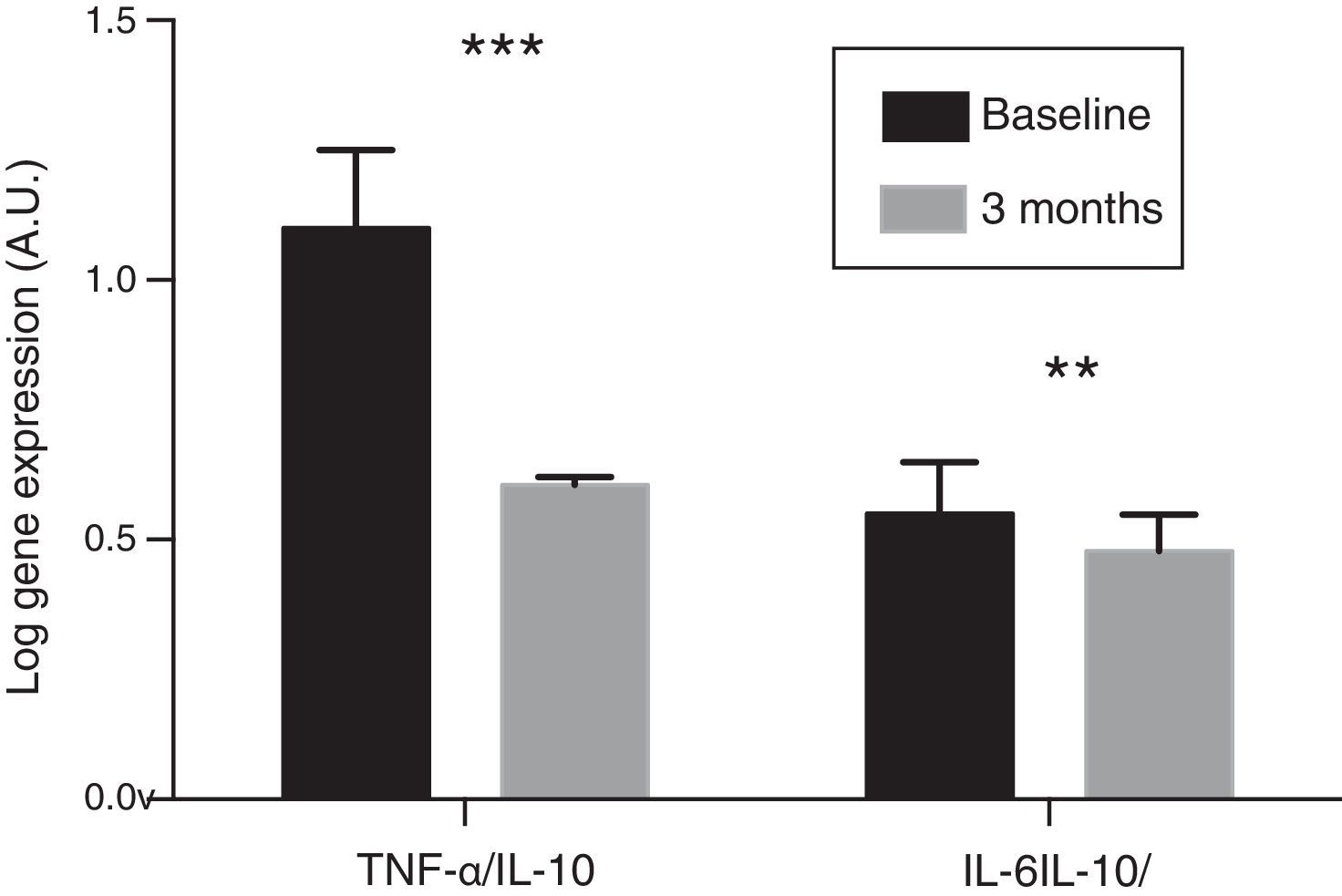
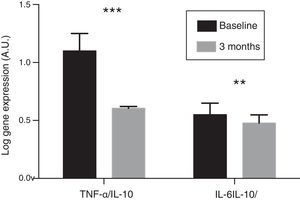
In the resulting PBMCs, the evolution of the gene expression levels of pro-inflammatory cytokines IL-6 and TNF-α were analysed. After three months of oral paricalcitol administration, mRNA levels in these cytokines showed a significant decrease, with a mean percentage value of 14.13 and 34.17%, respectively (p<0.001). Meanwhile, the expression level of IL-10 showed no significant variations. No correlation was found either between the change in expression levels of IL-6 and TNF-α and the variations observed in serum concentrations of these cytokines. Finally, the ratios of the expression levels of TNF-α and IL-6 with respect to those of IL-10 experienced a significant reduction of 45.6% and 13.4%, respectively, after treatment (p<0.01 and p<0.001, respectively) (Fig. 2).
DiscussionIn this study, we evaluated the effects of paricalcitol on the inflammatory profile of kidney transplant patients with persistent SHPT. Recent studies in this field show that treatment with this compound has a positive impact that leads to a greater decrease in iPTH concentrations and a reduction in the frequency of post-transplant SHPT.2,3 In addition, it causes an attenuation in the processes of bone remodelling and mineral loss, as well as a reduction in the urinary excretion of protein, which indicates the existence of beneficial pleiotropic effects derived from the activation of the VDR.3 Our results provide new data in this regard, and point to a modulating effect of the inflammatory profile of kidney transplant patients by paricalcitol. Specifically, the administration of this selective VDR activator causes a significant decrease in serum levels of pro-inflammatory cytokines IL-6 and TNF-α, as well as a reduction in the expression levels of genes IL-6 and TNF-α in PBMCs.
According to previous observational studies and several clinical trials,2,3,19 serum iPTH levels showed a rapid decrease after three months of paricalcitol treatment, with a significant mean percentage decrease of 31.9% compared to the baseline values, with no changes in calcium and phosphorous values. In addition, there was a significant reduction in the serum concentration of alkaline phosphatase, which has been observed previously.20,21 This decrease has been associated with a concomitant decrease in iPTH, as well as a suppressing effect of paricalcitol on osteoblasts.21
The increase in urinary albumin excretion is common in kidney transplant recipients, with more than 45% of subjects presenting with proteinuria one year after the transplant.22 In most cases, the degree of proteinuria is below 500mg/day, but even at low levels—ranging from 150 to 500mg/day—it is a powerful predictor of reduced long-term kidney graft survival.23,24 In our study, we analysed the evolution of albuminuria and found that it tends to decrease at the end of treatment, which does not become statistically significant. A previous retrospective study also shows a slight, although significant, decrease of proteinuria in 58 kidney transplant patients, after three months of paricalcitol administration.19 Similarly, our results concur with those of a recent clinical trial conducted by Trillini et al.3 where, again, there is a tendency to reduce proteinuria after three months of therapy with paricalcitol. This decrease reached statistical significance at six months of follow-up. Some experimental studies also highlight this association between the use of paricalcitol and the antiproteinuric effects, and point to multiple mechanisms, including the downregulation of the renin–angiotensin system, the attenuation of renal fibrosis, the stimulation of nephrin synthesis and the anti-inflammatory effect.15,16,25,26
Activation of the VDR, beyond its classic effects on mineral metabolism, is associated with important pleiotropic capacities, including the regulation of immune functions and the modulation of oxidative stress and inflammation.27,28 Although experimental studies have provided solid evidence of the anti-inflammatory effects of paricalcitol as a selective VDR,15,29,30 clinical studies are limited and those which do exist have been conducted with patients with CKD or patients on dialysis.31–34 This study, approached as proof of concept, is the first one specifically designed to evaluate the potential effects of considering the selective activation of the VDR as a target when modulating the inflammatory profile of kidney transplant patients. Our results show that treatment with paricalcitol is associated with a significant reduction in serum concentrations of the pro-inflammatory cytokines TNF-α and IL-6, as well as a decrease in the TNF-α/IL-10 and IL-6/IL-10 ratios. To our knowledge, only one previous study has analysed these effects in kidney transplant patients.19 In this study, the progression of 58 patients was evaluated retrospectively, and the inflammatory status of the patients was determined only by measuring serum levels of C-reactive protein. This value was considered a tertiary outcome. The results at the end of the study showed a drop in the levels of C-reactive protein after three months of treatment with paricalcitol. This reduction continued to be significant after 24 months of treatment. However, the serum levels of inflammatory cytokines or their gene expression levels were not considered.
Our study demonstrates that, in addition to its effect on serum concentrations, paricalcitol is able to act on PBMCs by modulating the transcription of genes encoding inflammatory cytokines. In a previous in vitro study, it was observed that paricalcitol was able to reduce the production of baseline TNF-α and IL-8 and lipopolysaccharide-induced production in PBMCs of healthy subjects.35 More recently, it has been confirmed that, by culturing PBMCs isolated from patients with CKD and stimulating them with phytohaemagglutinin A, there is a resulting increase in the production of inflammatory cytokines (IL-17, IL-6, IL-1β and TNF-α). However, if the cells are obtained from patients with CKD who received paricalcitol, there is a significant decrease in the production of these cytokines compared to patients who did not receive the treatment and who recovered baseline values similar to those of the cells of healthy subjects.35 In our study, we determined that mRNA levels of IL-6 and TNF-α in PBMCs show significant reductions of 14 and 34% compared to baseline values (p<0.001) after treatment with paricalcitol. A similar effect of paricalcitol on gene expression of inflammatory cytokines in PBMCs has been observed in patients with CKD and on haemodialysis.33,34
Nowadays, atherosclerosis is considered to be an inflammatory disease. In turn, inflammation is characterised by activation in peripheral blood of immunocompetent cells, leading to the production of inflammatory cytokines. In addition, the activation and ingress of PBMCs from the blood circulation into the vascular wall is a critical step in the pathogenesis of atherosclerosis36,37 and, from a clinical perspective, the increase in the expression of inflammatory cytokines in PBMCs is related to cardiovascular disease.38–40 It is therefore possible to speculate that the anti-inflammatory capacity of paricalcitol, a pleiotropic and extraskeletal effect, may be associated with a positive clinical impact, similar to that seen in patients undergoing haemodialysis.41
Although this study presents new information, we have to recognise certain limitations. As a result of the exploratory nature of this study, it was not conducted following a randomised design. The sample size was small, and the follow-up period of the patients was short, which may have determined that some of the results have not reached statistical significance and, therefore, we have not been able to draw definitive conclusions. In addition, the effects of paricalcitol therapy were not compared with those produced by vitamin D or with another of its active metabolites. However, based on the homogeneity of the study in terms of the general care, follow-up and the therapeutic approach of the patients, that did not vary during the study, it can be assumed that the results obtained are due to the treatment with paricalcitol.
In conclusion, the results of this study show that the selective activation of the VDR by paricalcitol in kidney transplant patients is associated with a modulating and beneficial effect on the serum inflammatory profile and on the cytokine gene expression in PBMCs, resulting in a favourable impact on the balance between pro-inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokine IL-10. These findings indicate that the benefits of paricalcitol therapy observed previously in patients with CKD and dialysis may be extended to kidney transplant recipients. Long-term prospective trials, which are properly conducted, are needed in order to achieve a better assessment of the potential impact of this treatment and of the translation of these pleiotropic beneficial effects on the clinical results in kidney transplant recipients, including graft survival and the morbidity and mortality of the patient.
FundingJDC, MMF, CMF and JFNG are members of RETIC (Thematic Networks of Corporate Research)/REDinREN (Kidney Disease Research Network)/RD16/0009/0022, (ISCIII – Carlos III Health Institute) and GEENDIAB (Diabetic Nephropathy Working Group of the Spanish Society of Nephrology). The research activity of JDC, EMN and JFNG is funded by the ISCIII (CD16/00165, FI14/00033 and INT15/00004, respectively). This study has been partly funded by research grants from the Spanish Society of Nephrology (SEN) and the Scientific Association for Nephrology Research (ACINEF).
Authorship/collaborationsThe authors JDN, EHP and EMN have contributed equally to the preparation of this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Donate-Correa J, Henríquez-Palop F, Martín-Núñez E, Hernández-Carballo C, Ferri C, Pérez-Delgado N, et al. Perfil antiinflamatorio del paricalcitol en el receptor de trasplante renal. Nefrologia. 2017;37:622–629.