Haemodialysis (HD) patients are a high-risk population group. For these patients, an error could have catastrophic consequences. Therefore, system that ensures the safety of these patients in an environment with high technology and great interaction of the human factor is a requirement.
ObjectivesTo show a systematic working approach, reproducible in any HD unit, which consists of recording the complications and errors that occurred during the HD session; defining which of those complications could be considered adverse event (AE), and therefore preventable; and carrying out a systematic analysis of them, as well as of underlying real or potential errors, evaluating their severity, frequency and detection; as well as establishing priorities for action (Failure Mode and Effects Analysis system [FMEA systems]).
MethodsRetrospective analysis of the graphs of all HD sessions performed during one month (October 2015) on 97 patients, analysing all recorded complications. The consideration of these complications as AEs was based on a consensus among 13 health professionals and 2 patients. The severity, frequency and detection of each AE were evaluated by the FMEA system.
ResultsWe analysed 1303 HD treatments in 97 patients. A total of 383 complications (1 every 3.4 HD treatments) were recorded. Approximately 87.9% of them were deemed AEs and 23.7% complications related with patients’ underlying pathology. There was one AE every 3.8 HD treatments. Hypertension and hypotension were the most frequent AEs (42.7 and 27.5% of all AEs recorded, respectively). Vascular-access related AEs were one every 68.5 HD treatments. A total of 21 errors (1 every 62 HD treatments), mainly related to the HD technique and to the administration of prescribed medication, were registered. The highest risk priority number, according to the FMEA, corresponded to errors related to patient body weight; dysfunction/rupture of the catheter; and needle extravasation.
ConclusionsHD complications are frequent. Consideration of some of them as AEs could improve safety by facilitating the implementation of preventive measures. The application of the FMEA system allows stratifying real and potential errors in dialysis units and acting with the appropriate degree of urgency, developing and implementing the necessary preventive and improvement measures.
La población en hemodiálisis (HD) es de alto riesgo. En estos pacientes un fallo puede tener consecuencias catastróficas, por lo que son necesarios sistemas que garanticen su seguridad en un entorno con alta tecnología y gran interacción del factor humano.
ObjetivosMostrar una sistemática de trabajo, reproducible en cualquier unidad de HD, que consiste en registrar las complicaciones y fallos ocurridos durante la sesión, definir cuáles de estas complicaciones podrían ser consideradas eventos adversos (EA) y, por tanto, prevenibles y realizar un análisis sistemático tanto de ellos como de los fallos reales o potenciales subyacentes, evaluando su gravedad, frecuencia y detección, y estableciendo prioridades de actuación (sistema de análisis modal de fallos y efectos [AMFE]).
MétodosExamen retrospectivo de las gráficas de diálisis de todas las sesiones practicadas durante un mes (octubre de 2015) en 97 pacientes, y análisis de las complicaciones registradas. La consideración de estas complicaciones como EA se basó en el consenso entre 13 profesionales y 2 pacientes. Se valoró la severidad, frecuencia y detección de cada fallo real o potencial mediante el sistema AMFE.
ResultadosSe practicaron 1.303 sesiones de HD en 97 pacientes en las que se registraron un total de 383 complicaciones (1 cada 3,4 tratamientos). De ellas, el 87,9% fueron consideradas EA y el 23,7% complicaciones relacionadas con la enfermedad de base. Se detectó un EA cada 3,8 tratamientos. Los EA más frecuentes fueron la hipertensión y la hipotensión (42,7 y 27,5% del total de EA registrados, respectivamente). Los EA relacionados con el acceso vascular fueron uno de cada 68,5 tratamientos. Se registraron un total de 21 fallos en la asistencia (1 cada 62 tratamientos), los cuales estaban relacionados con fallos en la aplicación de la técnica y en la administración de la medicación. El mayor número de prioridad de riesgo lo obtuvieron los fallos relacionados con errores en el peso, disfunción o rotura del catéter y salida de agujas.
ConclusionesLas complicaciones en HD son frecuentes y la consideración de algunas de ellas como EA podría mejorar la seguridad en la asistencia, al poner en marcha medidas preventivas. La implementación del sistema AMFE permite estratificar y priorizar los posibles fallos de las unidades de diálisis, y actuar con mayor o menor premura, desarrollando las acciones de mejora necesarias.
Patient safety is a matter of growing interest and a key component of healthcare quality. Patients, family members, managers and health professionals demand a safe, effective and efficient healthcare.1,2 The undesirable effects derived from healthcare represent a considerable cause of morbidity and mortality and result in high healthcare costs. A mean incidence of adverse events (AEs) associated with hospitalisation of 9.2% (95% CI: 4.6–12.4%) has been reported, of which 43.5% (95% CI: 39.4–49.6%) could have been avoided.3
To increase in patient safety in haemodialysis (HD) it is required, first of all, knowing what complications meet the requirements to be considered AEs and, therefore, should be registered so the causes and prevention can be analysed and, secondly, act on the implementation of safe practices in the provision of care using the Failure Mode and Effects Analysis (FMEA) method4 which allows to stratify and prioritise — according to their severity, incidence and detection — the possible actual or potential failures of HD units by calculating the risk priority numbers (RPNs)4 and acting with greater or lesser rapidity in the development and implementation of the actions required.
The HD population is a high-risk population, with many elderly patients with multi-pathology, in whom one error may have catastrophic consequences.5 The increasing complexity of the care of this type of patient, due to administrative, professional and disease-related factors, makes it necessary to implement systems ensuring patient safety in an environment with high technology and significant interaction of the human factor. Many of the aspects that are dealt globally under the different safety plans, for example, the prevention of falls, safe use of medications, hand hygiene, etc., also apply to HD; but the HD technique has a broad range of possibilities for failures that should be identified. Some key areas in the safety risk in HD units, such as the quality of water, reuse of the membrane and infection control6–10 are well recognised. However, some less grave issues that are very common in daily clinical practice, need to be defined.
The increase in patient safety in HD requires, first of all, knowing what complications meet the requirements to be considered AEs. And, therefore, should be recorded for the analysis of their causes and their prevention. In our study, the criterion to consider a complication as an AE was based on the international classification of patient safety11: an AE is an unintended damage caused during the medical care, or as a consequence of the medical care due to problems in clinical practice, products, procedures or systems, rather than the caused by patient's underlying disease, and in which a change in the pratice may prevent or minimise the AE.
The objective of this study is to show a working system, reproducible in any HD unit, that consist in recording complications and failures that have occurred during the session, defining which of these complications can be considered AEs, and, therefore, preventable. Then perform systematic analysis of the events as well as the actual or potential underlying failures, assessing their severity, frequency and detection and establishing action priorities (FMEA system).
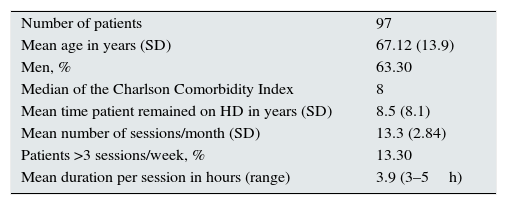
MethodsPopulation97 patients belonging to an HD unit equipped with 35 stations were included. The characteristics of the sample are described in Table 1. 47% of patients had some type of dependency (measured by the delta test12); in 13.2% the dependency was moderate and in 9% it was severe.
Characteristics of the sample.
| Number of patients | 97 |
| Mean age in years (SD) | 67.12 (13.9) |
| Men, % | 63.30 |
| Median of the Charlson Comorbidity Index | 8 |
| Mean time patient remained on HD in years (SD) | 8.5 (8.1) |
| Mean number of sessions/month (SD) | 13.3 (2.84) |
| Patients >3 sessions/week, % | 13.30 |
| Mean duration per session in hours (range) | 3.9 (3–5h) |
SD: standard deviation.
The unit has five dialysis rooms: two with isolation of seropositive patients, — one with five HCV-positive stations and another with five stations for HBsAg-positive patients — and three rooms used by patients with negative serology: two rooms with 10 stations and one with five stations. The nurse–patient ratio was 1:5 and the assistant–patient ratio was 1:10, according to the tender specifications established by the HD tender of the Ministry of Health of the Autonomous Region of Valencia. The unit has four nephrologists who work during the different shifts so all sessions are monitored by a nephrologist.
Characteristics of the dialysis techniqueAll patients are dialysed with high or medium permeability filters with a surface area measuring 1.8 and 2.1m2. The dialysis fluid is centralised with two main formulas with a calcium content of 2.5 and 3mEq/l, and a potassium content of 1.5mEq/l. Dialysis fluids with other compositions, either of calcium or potassium, are administered in glass ampoules.
Working groupThe working group was composed of 13 professionals (one intensive care specialist, four nephrologists, eight nurses from the HD unit) and the contributions of two patients.
Instrument: FMEA systemThe FMEA system4 is a technique for detecting failures present and for preventing future failures which helps to link each failure detected to its consequences. The essential components of the FMEA system are as follows:
- •
Failure mode: Potential error that may happen to the patient in any phase of the process. The potential failure modes must be described in “physical” or technical terms, not as a symptom detectable by the patient. A failure may not be detectable immediately. This is an important aspect and, therefore, should never be overlooked.
- •
Effect: Impact that the specific failure may have on the patient. It is normally the symptom detected by the patient from the failure mode, but also as an impact on the system. The unintended consequences of the failure that may be observed or detected are described.
- •
Causes: What is the aetiology that may cause it. It is necessary to link, to the greatest possible degree, all conceivable causes of failure that may be assigned to each failure mode. The causes should be related in the most concise and complete manner so the correction efforts may be addressed appropriately. A failure mode may normally be caused by two or more linked causes. It reflects the existing control and verification measures to ensure quality. The control measures should correspond with each of the causes of the failure modes.
- •
Method of detection: How the failure can be detected.
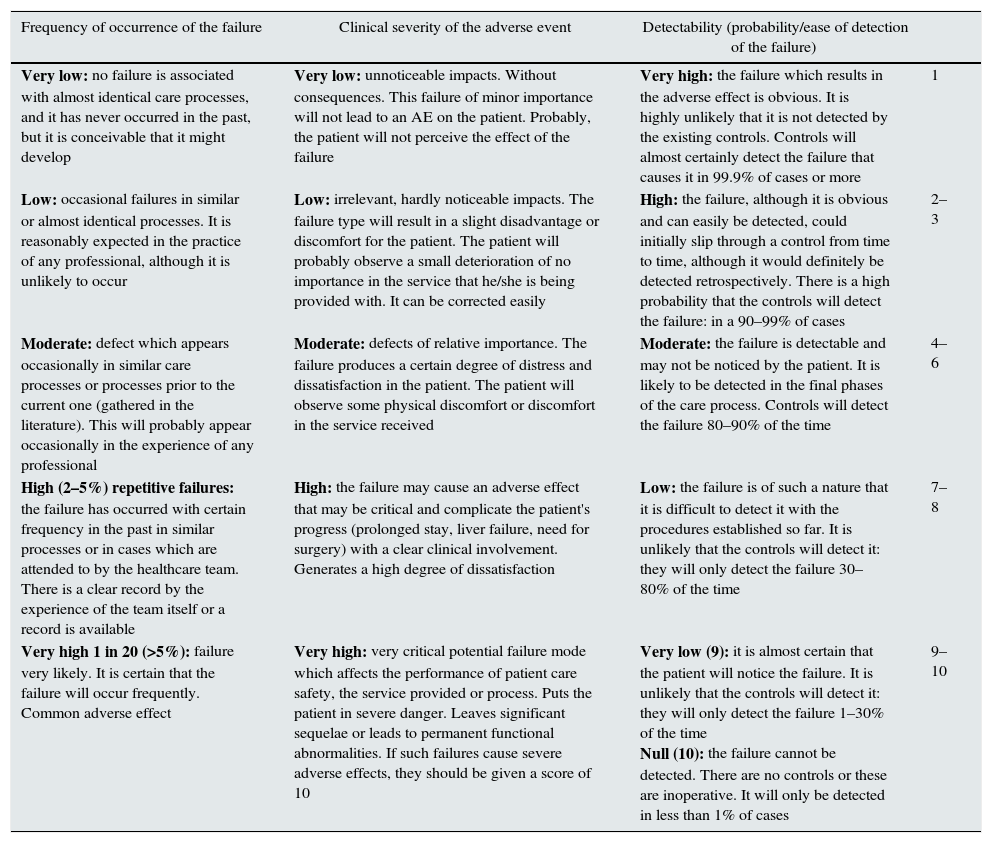
An assessment of the severity, frequency, occurrence and detection of each failure is carried out from these components, as established in Table 2.
Assessment of the FMEA system.
| Frequency of occurrence of the failure | Clinical severity of the adverse event | Detectability (probability/ease of detection of the failure) | |
|---|---|---|---|
| Very low: no failure is associated with almost identical care processes, and it has never occurred in the past, but it is conceivable that it might develop | Very low: unnoticeable impacts. Without consequences. This failure of minor importance will not lead to an AE on the patient. Probably, the patient will not perceive the effect of the failure | Very high: the failure which results in the adverse effect is obvious. It is highly unlikely that it is not detected by the existing controls. Controls will almost certainly detect the failure that causes it in 99.9% of cases or more | 1 |
| Low: occasional failures in similar or almost identical processes. It is reasonably expected in the practice of any professional, although it is unlikely to occur | Low: irrelevant, hardly noticeable impacts. The failure type will result in a slight disadvantage or discomfort for the patient. The patient will probably observe a small deterioration of no importance in the service that he/she is being provided with. It can be corrected easily | High: the failure, although it is obvious and can easily be detected, could initially slip through a control from time to time, although it would definitely be detected retrospectively. There is a high probability that the controls will detect the failure: in a 90–99% of cases | 2–3 |
| Moderate: defect which appears occasionally in similar care processes or processes prior to the current one (gathered in the literature). This will probably appear occasionally in the experience of any professional | Moderate: defects of relative importance. The failure produces a certain degree of distress and dissatisfaction in the patient. The patient will observe some physical discomfort or discomfort in the service received | Moderate: the failure is detectable and may not be noticed by the patient. It is likely to be detected in the final phases of the care process. Controls will detect the failure 80–90% of the time | 4–6 |
| High (2–5%) repetitive failures: the failure has occurred with certain frequency in the past in similar processes or in cases which are attended to by the healthcare team. There is a clear record by the experience of the team itself or a record is available | High: the failure may cause an adverse effect that may be critical and complicate the patient's progress (prolonged stay, liver failure, need for surgery) with a clear clinical involvement. Generates a high degree of dissatisfaction | Low: the failure is of such a nature that it is difficult to detect it with the procedures established so far. It is unlikely that the controls will detect it: they will only detect the failure 30–80% of the time | 7–8 |
| Very high 1 in 20 (>5%): failure very likely. It is certain that the failure will occur frequently. Common adverse effect | Very high: very critical potential failure mode which affects the performance of patient care safety, the service provided or process. Puts the patient in severe danger. Leaves significant sequelae or leads to permanent functional abnormalities. If such failures cause severe adverse effects, they should be given a score of 10 | Very low (9): it is almost certain that the patient will notice the failure. It is unlikely that the controls will detect it: they will only detect the failure 1–30% of the time Null (10): the failure cannot be detected. There are no controls or these are inoperative. It will only be detected in less than 1% of cases | 9–10 |
All the complications that may arise in an HD unit were assessed, by: (1) retrospective analysis of all the dialysis graphs for sessions performed over one month (October 2015); (2) analysis of the patients’ laboratory test results (aminotransferase, blood aluminium, viral markers, blood count, etc.) and (3) analysis of the laboratory test results of the water plant and subsequent follow-up of patients (admissions, mortality). The consideration of these complications as an AE was performed by consensus among the working group, using the brainstorming technique and based on the international classification for patient safety,11 the existing literature13–15 and clinical experience.
Failures in the HD unit were identified from: (1) retrospective analysis of all the dialysis graphs sessions performed during one month; (2) the result of the nonconformities identified in the quality system from 2000 and (3) the result of internal and external audits performed in the unit on an annual and monthly basis, respectively. Actions recorded during the HD session which were thought to have been the cause of the actual or potential AEs were considered to be failures.
In addition to failures in the care, other circumstances occurring during the HD session that, without being able to be considered failures in themselves, could have caused adverse consequences on the patient and that were particularly common, were recorded. These are: temporary interruptions during the HD session with the patient being disconnected to go to the toilet; ultrafiltration rate (UFR)>10ml/kg/h during the HD session and sessions in which the established dry weight is not obtained.
The FMEA system was used to link each failure detected with its consequence, and vice versa. From the AEs defined and recorded, the potential failures which have been able to intervene are identified, and an assessment is carried out of the severity, frequency, occurrence and detection of each actual or potential failure. With the score obtained after this assessment, each failure mode is assigned a value called RPN, which is the result of the product of the assessment of the frequency, severity and detection of the failure (RPN=F×G×D) and that puts the risks or failures into order of importance, to establish which need to be acted on first.16 From this, the preventive measures of each one of them are defined.
Statistical analysis of the resultsA descriptive analysis of the qualitative and quantitative variables was conducted. The SPSS statistics 22 statistical package and Excel 2016 (Microsoft) spreadsheet were used.
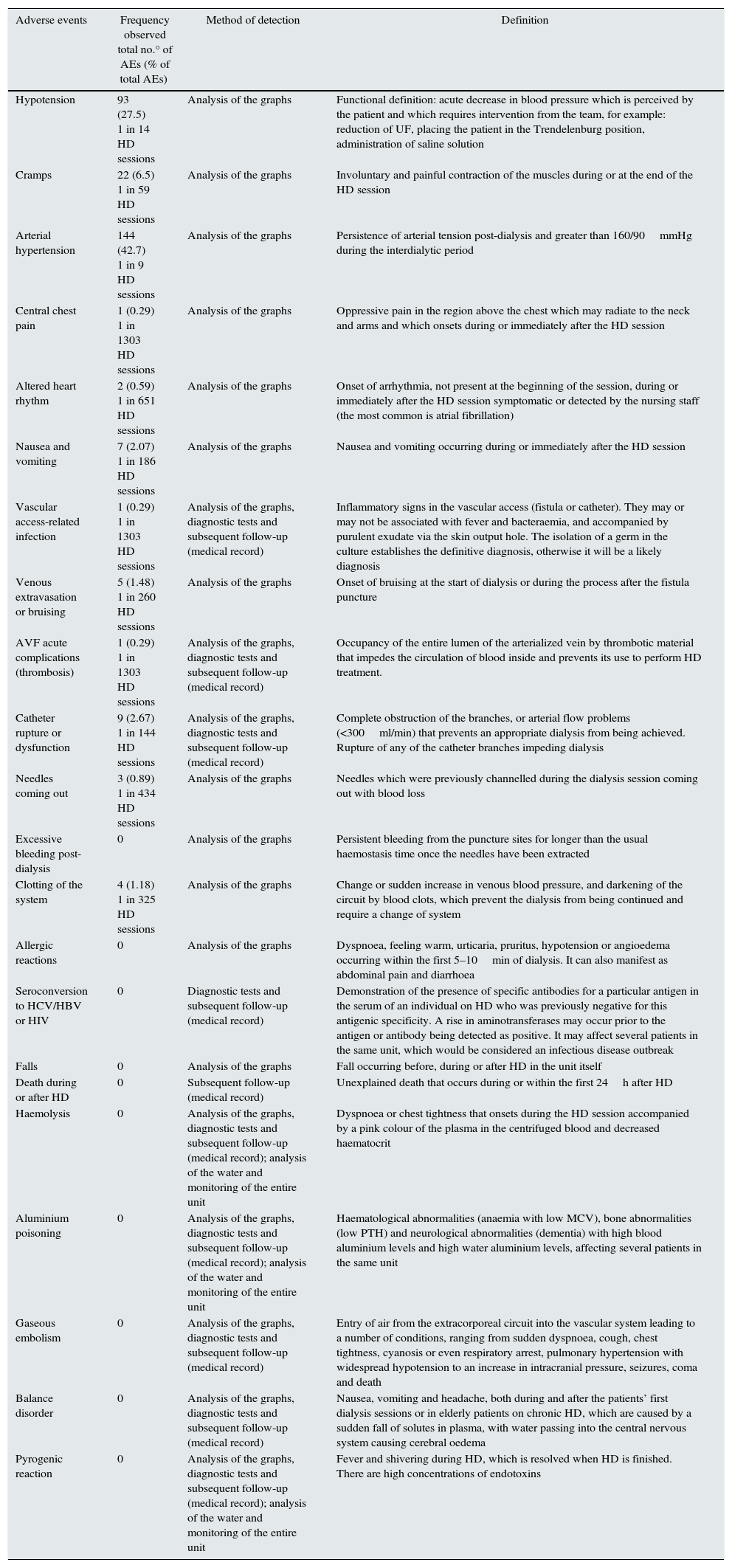
ResultsA total of 1303 HD sessions were performed, in which 383 complications were recorded (1 in 3.4 treatments). Of these, 337 were considered AEs (87.9%) and 91 were considered inevitable complications related to the underlying disease (23.7%). Table 3 shows complications considered AEs and their frequency of detection. The most frequently observed AEs are hypertension and hypotension (42.7 and 27.5% of the total number of AEs recorded, respectively). Six-month mortality when the AE were recorded was 5.1%. Two patients required hospitalisation as a result of an AE: one due to the rupture of a catheter and one due to hypotension and unresolved arrhythmia (atrial flutter) at the end of dialysis.
Frequency of the recorded adverse events: method of detection and definition.
| Adverse events | Frequency observed total no.° of AEs (% of total AEs) | Method of detection | Definition |
|---|---|---|---|
| Hypotension | 93 (27.5) 1 in 14 HD sessions | Analysis of the graphs | Functional definition: acute decrease in blood pressure which is perceived by the patient and which requires intervention from the team, for example: reduction of UF, placing the patient in the Trendelenburg position, administration of saline solution |
| Cramps | 22 (6.5) 1 in 59 HD sessions | Analysis of the graphs | Involuntary and painful contraction of the muscles during or at the end of the HD session |
| Arterial hypertension | 144 (42.7) 1 in 9 HD sessions | Analysis of the graphs | Persistence of arterial tension post-dialysis and greater than 160/90mmHg during the interdialytic period |
| Central chest pain | 1 (0.29) 1 in 1303 HD sessions | Analysis of the graphs | Oppressive pain in the region above the chest which may radiate to the neck and arms and which onsets during or immediately after the HD session |
| Altered heart rhythm | 2 (0.59) 1 in 651 HD sessions | Analysis of the graphs | Onset of arrhythmia, not present at the beginning of the session, during or immediately after the HD session symptomatic or detected by the nursing staff (the most common is atrial fibrillation) |
| Nausea and vomiting | 7 (2.07) 1 in 186 HD sessions | Analysis of the graphs | Nausea and vomiting occurring during or immediately after the HD session |
| Vascular access-related infection | 1 (0.29) 1 in 1303 HD sessions | Analysis of the graphs, diagnostic tests and subsequent follow-up (medical record) | Inflammatory signs in the vascular access (fistula or catheter). They may or may not be associated with fever and bacteraemia, and accompanied by purulent exudate via the skin output hole. The isolation of a germ in the culture establishes the definitive diagnosis, otherwise it will be a likely diagnosis |
| Venous extravasation or bruising | 5 (1.48) 1 in 260 HD sessions | Analysis of the graphs | Onset of bruising at the start of dialysis or during the process after the fistula puncture |
| AVF acute complications (thrombosis) | 1 (0.29) 1 in 1303 HD sessions | Analysis of the graphs, diagnostic tests and subsequent follow-up (medical record) | Occupancy of the entire lumen of the arterialized vein by thrombotic material that impedes the circulation of blood inside and prevents its use to perform HD treatment. |
| Catheter rupture or dysfunction | 9 (2.67) 1 in 144 HD sessions | Analysis of the graphs, diagnostic tests and subsequent follow-up (medical record) | Complete obstruction of the branches, or arterial flow problems (<300ml/min) that prevents an appropriate dialysis from being achieved. Rupture of any of the catheter branches impeding dialysis |
| Needles coming out | 3 (0.89) 1 in 434 HD sessions | Analysis of the graphs | Needles which were previously channelled during the dialysis session coming out with blood loss |
| Excessive bleeding post-dialysis | 0 | Analysis of the graphs | Persistent bleeding from the puncture sites for longer than the usual haemostasis time once the needles have been extracted |
| Clotting of the system | 4 (1.18) 1 in 325 HD sessions | Analysis of the graphs | Change or sudden increase in venous blood pressure, and darkening of the circuit by blood clots, which prevent the dialysis from being continued and require a change of system |
| Allergic reactions | 0 | Analysis of the graphs | Dyspnoea, feeling warm, urticaria, pruritus, hypotension or angioedema occurring within the first 5–10min of dialysis. It can also manifest as abdominal pain and diarrhoea |
| Seroconversion to HCV/HBV or HIV | 0 | Diagnostic tests and subsequent follow-up (medical record) | Demonstration of the presence of specific antibodies for a particular antigen in the serum of an individual on HD who was previously negative for this antigenic specificity. A rise in aminotransferases may occur prior to the antigen or antibody being detected as positive. It may affect several patients in the same unit, which would be considered an infectious disease outbreak |
| Falls | 0 | Analysis of the graphs | Fall occurring before, during or after HD in the unit itself |
| Death during or after HD | 0 | Subsequent follow-up (medical record) | Unexplained death that occurs during or within the first 24h after HD |
| Haemolysis | 0 | Analysis of the graphs, diagnostic tests and subsequent follow-up (medical record); analysis of the water and monitoring of the entire unit | Dyspnoea or chest tightness that onsets during the HD session accompanied by a pink colour of the plasma in the centrifuged blood and decreased haematocrit |
| Aluminium poisoning | 0 | Analysis of the graphs, diagnostic tests and subsequent follow-up (medical record); analysis of the water and monitoring of the entire unit | Haematological abnormalities (anaemia with low MCV), bone abnormalities (low PTH) and neurological abnormalities (dementia) with high blood aluminium levels and high water aluminium levels, affecting several patients in the same unit |
| Gaseous embolism | 0 | Analysis of the graphs, diagnostic tests and subsequent follow-up (medical record) | Entry of air from the extracorporeal circuit into the vascular system leading to a number of conditions, ranging from sudden dyspnoea, cough, chest tightness, cyanosis or even respiratory arrest, pulmonary hypertension with widespread hypotension to an increase in intracranial pressure, seizures, coma and death |
| Balance disorder | 0 | Analysis of the graphs, diagnostic tests and subsequent follow-up (medical record) | Nausea, vomiting and headache, both during and after the patients’ first dialysis sessions or in elderly patients on chronic HD, which are caused by a sudden fall of solutes in plasma, with water passing into the central nervous system causing cerebral oedema |
| Pyrogenic reaction | 0 | Analysis of the graphs, diagnostic tests and subsequent follow-up (medical record); analysis of the water and monitoring of the entire unit | Fever and shivering during HD, which is resolved when HD is finished. There are high concentrations of endotoxins |
AVF: arteriovenous fistula.
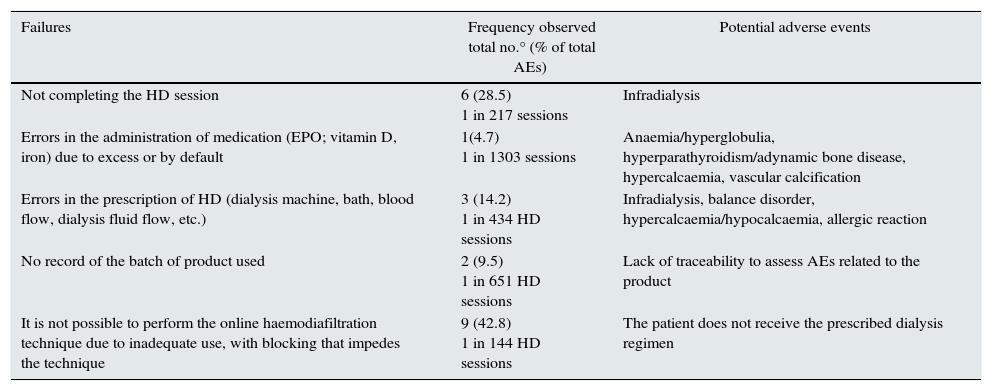
Table 4 shows the failures recorded in the study period, being considered any actions or situations that could cause an actual or potential AE. The results show that a total of 21 failures during care were recorded (1 in 62 treatments), which were related to failures in the application of the technique and the administration of the medication.
Failures recorded in the study period.
| Failures | Frequency observed total no.° (% of total AEs) | Potential adverse events |
|---|---|---|
| Not completing the HD session | 6 (28.5) 1 in 217 sessions | Infradialysis |
| Errors in the administration of medication (EPO; vitamin D, iron) due to excess or by default | 1(4.7) 1 in 1303 sessions | Anaemia/hyperglobulia, hyperparathyroidism/adynamic bone disease, hypercalcaemia, vascular calcification |
| Errors in the prescription of HD (dialysis machine, bath, blood flow, dialysis fluid flow, etc.) | 3 (14.2) 1 in 434 HD sessions | Infradialysis, balance disorder, hypercalcaemia/hypocalcaemia, allergic reaction |
| No record of the batch of product used | 2 (9.5) 1 in 651 HD sessions | Lack of traceability to assess AEs related to the product |
| It is not possible to perform the online haemodiafiltration technique due to inadequate use, with blocking that impedes the technique | 9 (42.8) 1 in 144 HD sessions | The patient does not receive the prescribed dialysis regimen |
Other recorded actions that stand out due to their frequency were: temporary interruptions during the HD session with the patient being disconnected to go to the toilet in 15 cases (1 in 86 treatments); UFR>10ml/kg/h during the HD session in 597 sessions (1 in 2 treatments) and the established dry weight not being achieved in 443 sessions (1 in 2.9 treatments). These actions were not considered failures in themselves, but they could have adverse consequences on the patient. They are therefore discussed later on.
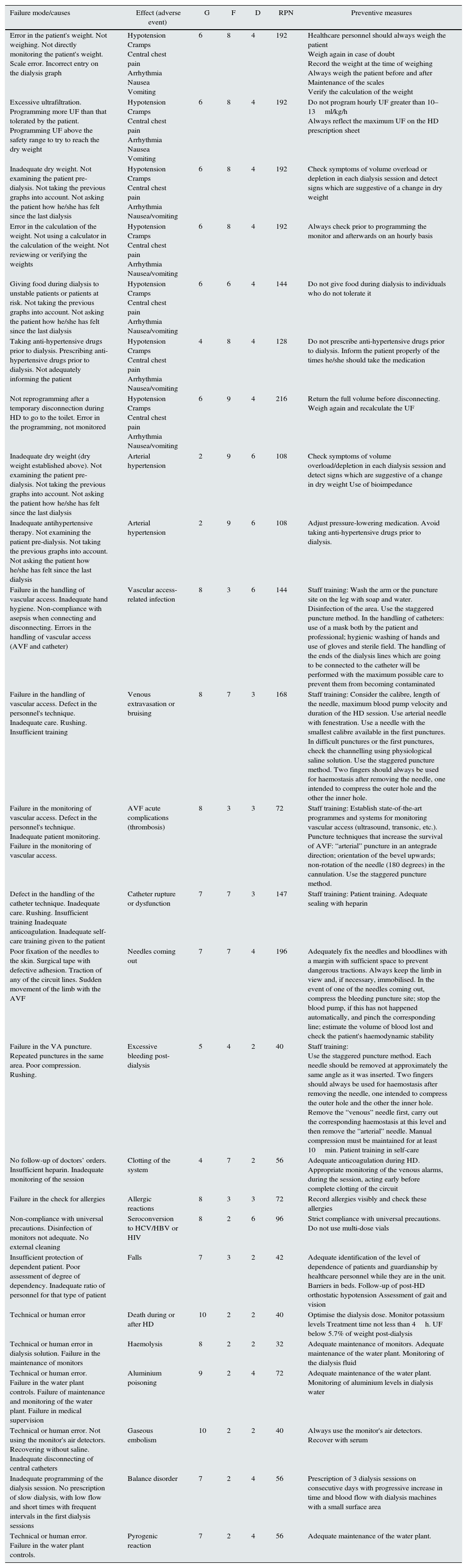
Table 5 shows the results of the implementation of the FMEA system for potential or actual failures: causes, correlation with adverse events and preventive measures in each one of them. The greatest RPN was obtained by the failures related to errors in weight, dysfunction or rupture of catheter and needles out flow.
Application of the FMEA system to potential or actual failures: causes, correlation with adverse events and preventive measures.
| Failure mode/causes | Effect (adverse event) | G | F | D | RPN | Preventive measures |
|---|---|---|---|---|---|---|
| Error in the patient's weight. Not weighing. Not directly monitoring the patient's weight. Scale error. Incorrect entry on the dialysis graph | Hypotension Cramps Central chest pain Arrhythmia Nausea Vomiting | 6 | 8 | 4 | 192 | Healthcare personnel should always weigh the patient Weigh again in case of doubt Record the weight at the time of weighing Always weigh the patient before and after Maintenance of the scales Verify the calculation of the weight |
| Excessive ultrafiltration. Programming more UF than that tolerated by the patient. Programming UF above the safety range to try to reach the dry weight | Hypotension Cramps Central chest pain Arrhythmia Nausea Vomiting | 6 | 8 | 4 | 192 | Do not program hourly UF greater than 10–13ml/kg/h Always reflect the maximum UF on the HD prescription sheet |
| Inadequate dry weight. Not examining the patient pre-dialysis. Not taking the previous graphs into account. Not asking the patient how he/she has felt since the last dialysis | Hypotension Cramps Central chest pain Arrhythmia Nausea/vomiting | 6 | 8 | 4 | 192 | Check symptoms of volume overload or depletion in each dialysis session and detect signs which are suggestive of a change in dry weight |
| Error in the calculation of the weight. Not using a calculator in the calculation of the weight. Not reviewing or verifying the weights | Hypotension Cramps Central chest pain Arrhythmia Nausea/vomiting | 6 | 8 | 4 | 192 | Always check prior to programming the monitor and afterwards on an hourly basis |
| Giving food during dialysis to unstable patients or patients at risk. Not taking the previous graphs into account. Not asking the patient how he/she has felt since the last dialysis | Hypotension Cramps Central chest pain Arrhythmia Nausea/vomiting | 6 | 6 | 4 | 144 | Do not give food during dialysis to individuals who do not tolerate it |
| Taking anti-hypertensive drugs prior to dialysis. Prescribing anti-hypertensive drugs prior to dialysis. Not adequately informing the patient | Hypotension Cramps Central chest pain Arrhythmia Nausea/vomiting | 4 | 8 | 4 | 128 | Do not prescribe anti-hypertensive drugs prior to dialysis. Inform the patient properly of the times he/she should take the medication |
| Not reprogramming after a temporary disconnection during HD to go to the toilet. Error in the programming, not monitored | Hypotension Cramps Central chest pain Arrhythmia Nausea/vomiting | 6 | 9 | 4 | 216 | Return the full volume before disconnecting. Weigh again and recalculate the UF |
| Inadequate dry weight (dry weight established above). Not examining the patient pre-dialysis. Not taking the previous graphs into account. Not asking the patient how he/she has felt since the last dialysis | Arterial hypertension | 2 | 9 | 6 | 108 | Check symptoms of volume overload/depletion in each dialysis session and detect signs which are suggestive of a change in dry weight Use of bioimpedance |
| Inadequate antihypertensive therapy. Not examining the patient pre-dialysis. Not taking the previous graphs into account. Not asking the patient how he/she has felt since the last dialysis | Arterial hypertension | 2 | 9 | 6 | 108 | Adjust pressure-lowering medication. Avoid taking anti-hypertensive drugs prior to dialysis. |
| Failure in the handling of vascular access. Inadequate hand hygiene. Non-compliance with asepsis when connecting and disconnecting. Errors in the handling of vascular access (AVF and catheter) | Vascular access-related infection | 8 | 3 | 6 | 144 | Staff training: Wash the arm or the puncture site on the leg with soap and water. Disinfection of the area. Use the staggered puncture method. In the handling of catheters: use of a mask both by the patient and professional; hygienic washing of hands and use of gloves and sterile field. The handling of the ends of the dialysis lines which are going to be connected to the catheter will be performed with the maximum possible care to prevent them from becoming contaminated |
| Failure in the handling of vascular access. Defect in the personnel's technique. Inadequate care. Rushing. Insufficient training | Venous extravasation or bruising | 8 | 7 | 3 | 168 | Staff training: Consider the calibre, length of the needle, maximum blood pump velocity and duration of the HD session. Use arterial needle with fenestration. Use a needle with the smallest calibre available in the first punctures. In difficult punctures or the first punctures, check the channelling using physiological saline solution. Use the staggered puncture method. Two fingers should always be used for haemostasis after removing the needle, one intended to compress the outer hole and the other the inner hole. |
| Failure in the monitoring of vascular access. Defect in the personnel's technique. Inadequate patient monitoring. Failure in the monitoring of vascular access. | AVF acute complications (thrombosis) | 8 | 3 | 3 | 72 | Staff training: Establish state-of-the-art programmes and systems for monitoring vascular access (ultrasound, transonic, etc.). Puncture techniques that increase the survival of AVF: “arterial” puncture in an antegrade direction; orientation of the bevel upwards; non-rotation of the needle (180 degrees) in the cannulation. Use the staggered puncture method. |
| Defect in the handling of the catheter technique. Inadequate care. Rushing. Insufficient training Inadequate anticoagulation. Inadequate self-care training given to the patient | Catheter rupture or dysfunction | 7 | 7 | 3 | 147 | Staff training: Patient training. Adequate sealing with heparin |
| Poor fixation of the needles to the skin. Surgical tape with defective adhesion. Traction of any of the circuit lines. Sudden movement of the limb with the AVF | Needles coming out | 7 | 7 | 4 | 196 | Adequately fix the needles and bloodlines with a margin with sufficient space to prevent dangerous tractions. Always keep the limb in view and, if necessary, immobilised. In the event of one of the needles coming out, compress the bleeding puncture site; stop the blood pump, if this has not happened automatically, and pinch the corresponding line; estimate the volume of blood lost and check the patient's haemodynamic stability |
| Failure in the VA puncture. Repeated punctures in the same area. Poor compression. Rushing. | Excessive bleeding post-dialysis | 5 | 4 | 2 | 40 | Staff training: Use the staggered puncture method. Each needle should be removed at approximately the same angle as it was inserted. Two fingers should always be used for haemostasis after removing the needle, one intended to compress the outer hole and the other the inner hole. Remove the “venous” needle first, carry out the corresponding haemostasis at this level and then remove the “arterial” needle. Manual compression must be maintained for at least 10min. Patient training in self-care |
| No follow-up of doctors’ orders. Insufficient heparin. Inadequate monitoring of the session | Clotting of the system | 4 | 7 | 2 | 56 | Adequate anticoagulation during HD. Appropriate monitoring of the venous alarms, during the session, acting early before complete clotting of the circuit |
| Failure in the check for allergies | Allergic reactions | 8 | 3 | 3 | 72 | Record allergies visibly and check these allergies |
| Non-compliance with universal precautions. Disinfection of monitors not adequate. No external cleaning | Seroconversion to HCV/HBV or HIV | 8 | 2 | 6 | 96 | Strict compliance with universal precautions. Do not use multi-dose vials |
| Insufficient protection of dependent patient. Poor assessment of degree of dependency. Inadequate ratio of personnel for that type of patient | Falls | 7 | 3 | 2 | 42 | Adequate identification of the level of dependence of patients and guardianship by healthcare personnel while they are in the unit. Barriers in beds. Follow-up of post-HD orthostatic hypotension Assessment of gait and vision |
| Technical or human error | Death during or after HD | 10 | 2 | 2 | 40 | Optimise the dialysis dose. Monitor potassium levels Treatment time not less than 4h. UF below 5.7% of weight post-dialysis |
| Technical or human error in dialysis solution. Failure in the maintenance of monitors | Haemolysis | 8 | 2 | 2 | 32 | Adequate maintenance of monitors. Adequate maintenance of the water plant. Monitoring of the dialysis fluid |
| Technical or human error. Failure in the water plant controls. Failure of maintenance and monitoring of the water plant. Failure in medical supervision | Aluminium poisoning | 9 | 2 | 4 | 72 | Adequate maintenance of the water plant. Monitoring of aluminium levels in dialysis water |
| Technical or human error. Not using the monitor's air detectors. Recovering without saline. Inadequate disconnecting of central catheters | Gaseous embolism | 10 | 2 | 2 | 40 | Always use the monitor's air detectors. Recover with serum |
| Inadequate programming of the dialysis session. No prescription of slow dialysis, with low flow and short times with frequent intervals in the first dialysis sessions | Balance disorder | 7 | 2 | 4 | 56 | Prescription of 3 dialysis sessions on consecutive days with progressive increase in time and blood flow with dialysis machines with a small surface area |
| Technical or human error. Failure in the water plant controls. | Pyrogenic reaction | 7 | 2 | 4 | 56 | Adequate maintenance of the water plant. |
AVF: arteriovenous fistula; VA: vascular access.
Our first objective was to show a working system, reproducible in any HD unit, consisting of recording complications and failures that have occurred during the session, and defining which of these complications could be considered AEs.
The results have shown that complications in HD are not uncommon, and they affect more than a quarter of the sessions during one month. The prevalence of AEs (1 in 3.8 treatments) and failures (1 in 62 treatments) detected in this study is higher than that reported by other studies.13,17,18 Holley shows a failure rate of only1 in 733 HD treatments.13 The reason is that, we included AEs and failures which were not contemplated in other analyses and, unlike the rest of reports, our results do not dependent on the voluntary reports by the healthcare personnel — which, in turn, depends on the degree of involvement in the safety habits in this sector — but rather on the observation of daily practice.
In dialysis, it is not easy to differentiate which complications are due to the patient's disease and which are due to the technique and the performance of the professionals, as both types of situations coexists in many cases. Therefore, instead of AEs we will continue to talk about complications19 which, to some extent, appears to relieve us of responsibility and prevents us from implementing strategies necessary to analyse and determine the failures that lead to such AEs.
The complications considered as AEs in various studies include: hypotension, air embolism, haemolysis, acute myocardial infarction, acute cerebrovascular accident, pulmonary thromboembolism or death during or after dialysis, clotting of the system, vascular access infection, transmission of viral diseases, adverse drug reactions, needles coming out during the dialysis session, excessive bleeding from the puncture sites, subcutaneous infiltration, falls and cramps.13 Other studies add balance disorder, pyrogenic reactions and systemic bleeding or bruising to the aforementioned list of complications.15
The five most common safety problems in HD described by the National ESRD Patient Safety Initiative, published in 2011, were: (1) patient falls; (2) medication errors (including deviation from dialysis prescription, allergic reactions, and medication omissions); (3) access-related events (clotting, infiltrates, poor blood flow, difficult cannulation); (4) dialyzer errors (incorrect dialyzer or dialysate and equipment-related sepsis) and (5) excess blood loss or prolonged bleeding.20
In our study, more than 80% of the complications observed met the definition of an AE. The most common were intradialytic hypertension (1 in 9 treatments) and symptomatic hypotension occurring during (or immediately after) the HD (1 in 14 treatments). Intradialytic hypertension is a complication that may be prevented by appropriate adjustment of the target weight, of the dialysate sodium content and of high blood pressure medication.21,22 However, it is not usually included as an AE in the different studies. Symptomatic hypotension, when it is considered to be an AE (in other studies it is not),17 is the most commonly reported.16,23 Although it may be caused by underlying diseases, and be enhanced by factors that are not related to the technique,21 it is common for it to be generated in the absence of these conditions. In fact, many of the factors that contribute to this intradialytic hypotension are due to actions taken by healthcare professionals who, if they had performed them in a different way, could have avoided it (rapid or excessive ultrafiltration; rapid reduction in plasma osmolality; prescription of an incorrectly low dry weight objective; prescription of antihypertensive drugs pre-dialysis; food intake during the session; composition of the dialysis fluid low in sodium and in calcium; dialysate temperature higher than body temperature; error in the weighing of the patient, etc.). In our study, cramps, which could be of a similar origin to hypotension, accounted for 6.5% of the total number of AEs recorded (1 in 59 HD sessions). Nausea and vomiting, which accounted for 2.07% (1 in 186 HD sessions), may be prevented, avoiding hypotension and intake during the HD session.
Considering the above-mentioned complications as AEs may be questionable. However, although they are very often secondary to the patient's underlying disease, they can be avoided or, at least, minimised with certain actions during the HD treatment. We therefore believe that they should be integrated into a safety plan, in which their incidence is recorded and the possible causes analysed.
Similar questions have been raised regarding arrhythmias (0.59% of the AEs in our study, 1 in 651 sessions) or precordial pain (0.29% of AEs, 1 in 1303 treatments) occurring during (or immediately after) HD. As with those described above, both complications may be enhanced or avoided during the HD session, depending on prescription of higher or lower UFR, changes in concentrations of calcium or potassium in the dialysate, etc.24
The most commonly reported AEs in other studies are those related to vascular access,13 which in our case only represented 5.62% of the total number of AEs (1 in 68.5 treatments), including vascular access-related infection (1 in 1303 HD sessions); venous extravasation or bruising (1 in 260 HD sessions); arteriovenous fistula (AVF) thrombosis (1 in 1303 HD sessions); catheter dysfunction or rupture (1 in 144 HD sessions) and needles coming out (1 in 434 HD sessions). Other frequently reported complications are clotting of the circuit (1 in 325 HD sessions in our study and 1 in 3396 HD sessions in Holley's study13) and patient falls13,17,18 (between 0.01313 and 0.3%17 of the total number of recorded AEs), although in our study we did not identify any falls during the month of observation.
Some AEs are unexpected incidents which result in — or there is a risk that they result in — death or a serious physical or mental injury. These events are called “sentinel” because they warn of the need for research and an immediate response. Despite their low frequency, they involve very high risks and, therefore, must be subject to an assessment of causes and prevention. These include acute myocardial infarction, acute cerebrovascular accident or death during the dialysis session or after it, air embolism, haemolysis and aluminium poisoning. Dialysis with low potassium (<2mEq/l)25; treatment time of less than 3.5h; an ultrafiltration rate greater than 5.7% of the post-dialysis weight and a Kt/V<1.226 may contribute to the sudden death of patients during or after dialysis. Air embolism is basically due to a human error as a result of not using the monitor's air detectors and recovering without saline, and has been associated with failures in the disconnection systems from central catheters.27 Haemolysis tends to be related to problems with the dialysis solution, but also with the lack of proper maintenance of the monitors leading to malfunctioning of the roller pumps in the HD monitors. Although there are increasingly fewer cases of aluminium poisoning after improvement of the water plants and not using phosphorus captors with aluminium, accidental poisonings with a significant impact on patient safety are sporadically reported.28,29 The seroconversion of patients to HCV or HBV in the unit is a problem widely accepted as an AE, secondary to a breaking of the safety measures, such as hand hygiene30 or the inappropriate handling of multi-dose vials.31
Regarding the severity of the AEs observed, on only two occasions did the AE lead to hospital referral with admission, which was less than 48h in both cases. Other studies have not shown any AEs that resulted in hospitalisation,17 and a Spanish study reported 0.7% hospitalisations after 4797 procedures.18
In our study, the most commonly recorded failure was not being able to perform the online haemodiafiltration due to inadequate use of the monitor attributed to skipping of some steps to save time, and causing a block that impedes the technique (42.8% of the total number of failures, 1 in 144 HD sessions). The analysis of this failure and the investigation into its causes allowed to correct a human failure that could have gone unnoticed can be attributed to the monitor itself. In 1 in 217 HD sessions (28.5% of the failures) the session was not completed. Sometimes, due to hypotension that occurs almost at the end of dialysis, it is decided to disconnect the patient instead of establishing mechanisms, such as decreasing the ultrafiltration rate or leaving it at zero, and continuing with dialysis. If a patient has a tendency to hypotension and this practice is maintained, it possible that intradialysis is being performed. Therefore, it is advisable to record the times and to determine whether it is common and frequent practice in the unit, which would be regarded as a failure, or if they are only sporadic and totally justified cases. Another common failure was the lack of consistency between the prescribed HD regimen and the HD regimen performed (14.2%; 1 in 434 HD sessions). The most commonly described failures in the literature include failures in medication, in particular, their omission.17,18 In our study, the frequency observed in relation to medication was one case (1 in 1303 HD sessions) as a result of not changing the patient's dose of erythropoietin when it had been modified previously by the doctor in the dialysis regimen.
In the analysis of the circumstances related to the dialysis that could adversely affect the patient, and that we have designated as “potential failures” in our study, attention is drawn to the high incidence of dialysis sessions in which patients did not reach their dry weight (32.8%) and in whom dialysis is performed with high UFRs (>10ml/kg/h) (46.0%). Rapid ultrafiltration (UFR>10ml/kg/h) takes place routinely in many dialysis units in order to achieve the dry weight target in patients with high interdialytic weight gain. This action, whether or not it causes hypotension, has been shown to be an independent risk factor for mortality32 associated with an elevation of troponin I.33 That is why it should be regarded as an action to be recorded and analysed, and it should be subject to assessment as a possible “failure”, due to its potential ability to trigger an AE in the medium or long term. In any case, to avoid these “potential failures” important organisational changes — such as increasing the frequency of the sessions34 or lengthening their duration,35 or even reducing the patient–nurse ratio, especially in units with a high risk of infection36 or with dependent patients37 — are required. They are often difficult to implement as they are not authorised by the managers because of the higher cost they can entail.
Our second objective was to perform a systematic analysis of adverse events, as well as the actual or potential underlying failures, assessing their severity, frequency and detection, and establishing action priorities (FMEA system). In our study, the failures that required priority actions according to the RPN were those related to the patient's weight, catheter dysfunction or rupture and needles coming out. Therefore, in an attempt to resolve these failures, a set of preventive measures was established in each case.
An extensive recent review38 described how HD units are complex organisations involving professionals from multiple disciplines using advanced technologies to treat patients who are often elderly and have multiple conditions. As the organisations become more complex, the possibility of failure increases. Therefore, the potential risks should be identified and prioritised in order to increase the safety of care. The person responsible for the dialysis unit should establish a routine on safety as part of the quality assessment and performance improvement process. Several lines of research, including conducting surveys on patients and dialysis professionals, have helped to identify important areas of safety risk in the dialysis centres. The evaluation of these responses helps to improve the understanding of attitudes and predominant concerns. In any case, personnel and patients should be encouraged to express and openly share their concerns and the difficulties which exist in a blame-free environment.
The main limitations of the study were that the observation period was short and that, as the detection of failures was based on the records of the dialysis graphs, it is possible that some have not been recorded. The study was conducted in a single centre. This means that the decisions about which complications should be considered AEs, and which actions should be considered failures, should be reviewed and analysed by a wider group of professionals and patients.
In conclusion, HD complications are common and the consideration of some of them as AEs could improve safety in the care through the implementation of preventive measures. In order to assess the need and priority of new measures, after a period of time established in advance, each failure mode should be re-assessed and assigned a new RPN in accordance with the FMEA system.
Conflict of interestThe authors declare that they have no conflicts of interest and that they have not received financial support for this study.
We would like to thank all individuals, professionals and patients who helped in the consideration of complications as adverse events and in the assessment of the FMEA system.
Please cite this article as: Arenas Jiménez MD, Ferre G, Álvarez-Ude F. Estrategias para aumentar la seguridad del paciente en hemodiálisis: Aplicación del sistema de análisis modal de fallos y efectos (sistema AMFE). Nefrologia. 2017;37:608–621.