Low thyroid hormone (TH) levels and myocardial damage are common in dialysis patients and are associated with mortality. However, little is known about the role of THs on myocardial damage as has been described in primary thyroid diseases. The aim of this study was to explore the potential relationship between low total triiodothyronine (total T3) and biomarkers of myocardial damage and the effect of their interaction on mortality, to ascertain if cardiovascular damage is the link between low THs and the risk of death in dialysis patients with CKD.
Material and methodsTH plasma levels, nutritional markers, inflammation and myocardial damage were studied in 296 patients undergoing peritoneal dialysis or haemodialysis, who were followed up for 16 months to ascertain the association between biochemical variables and mortality.
ResultsLow total T3 levels were found in 45% of patients, which was inversely correlated with C-reactive protein (CRP) and NT-proBNP, and directly correlated with albumin and transferrin. Diabetes, CRP and total T3 were risk factors for all-cause mortality, and CRP, NT-proBNP and total T3 for cardiovascular mortality.
ConclusionsLow total T3 levels are common in dialysis patients and are associated with inflammation, malnutrition and myocardial damage. The latter may be the link between low THs and all-cause and cardiovascular mortality.
La disminución de hormonas tiroideas (HT) y el daño miocárdico son frecuentes en pacientes en diálisis y están asociados con la mortalidad. Sin embargo, poco se conoce de la importancia de las HT como factor de daño miocárdico, como se ha descrito en las enfermedades tiroideas primarias. El objetivo de este estudio fue explorar si existe interacción entre la disminución de triyodotironina total (tT3) y los marcadores de daño miocárdico y la relación de esta interacción entre ambos con la mortalidad, para establecer si el daño cardiovascular es el vínculo entre la disminución de HT y el riesgo de muerte en pacientes con ERC en diálisis.
Material y métodosSe estudiaron los niveles plasmáticos de HT, de marcadores de nutrición, inflamación y de daño al miocardio en 296 pacientes en diálisis peritoneal o en hemodiálisis, a los que se vigiló por 16 meses para conocer la asociación de las variables bioquímicas con la mortalidad.
ResultadosEn el 45% de los pacientes se encontró tT3 disminuida, lo cual tuvo correlación inversa con la proteína C reactiva (PCR) y con el NT-proBNP y directa con la albúmina y la transferrina. La diabetes, la PCR y la tT3 fueron factores de riesgo para la mortalidad por cualquier causa y la PCR, el NT-proBNP y la tT3 para mortalidad cardiovascular.
ConclusionesLos niveles bajos de tT3 son frecuentes en pacientes en diálisis, se asocian con inflamación, desnutrición y daño miocárdico: este último puede ser el vínculo entre la disminución de HT y la mortalidad por cualquier causa y la mortalidad cardiovascular.
Functional abnormalities in the hypothalamic-pituitary-thyroid axis and in the peripheral action of thyroid hormones (THs) are common in patients with chronic kidney disease (CKD). The most consistent finding is the reduction in plasma levels of total (tT3) and free (fT3) triiodothyronine. It is common to accept that these changes are a component of the non-thyroidal illness syndrome, since the thyroid stimulating hormone (TSH) in plasma is generally within normal limits. The non-thyroidal illness syndrome and the reduced T3 levels have been interpreted as an adaptation to the process of deterioration in patients with chronic disease, with the overall objective of reducing the body's metabolism.1–4
The importance of hormonal changes in clinical outcomes of patients with CKD has only been studied in recent years. These studies have found that the decrease in T3 levels, as well as thyroxine (T4) levels, have an independent predictive value for all-cause mortality and, particularly, for cardiovascular-related death in patients with CKD in the pre-dialysis stage, in patients on haemodialysis (HD) or on peritoneal dialysis (PD).5–8
The most common cause of comorbidity and mortality in patients with CKD are cardiovascular diseases. However, little is known about the interaction between decreased TH and cardiovascular disorders in kidney patients, even though it has been shown that the primary decrease in THs has a wide range of negative effects on myocardial structure and function. Among other effects, it has been associated with decreased TH with endothelial dysfunction, as well as systolic9–11 and diastolic dysfunction, the latter as an expression of myocardial fibrosis.12
The objective of this study was to explore whether there is an association between reduced T3 levels and markers of myocardial injury—in this case with increased plasma levels of N-terminal pro-brain natriuretic peptide (NT-proBNP)—and whether this combination affects mortality and to establish whether the cardiovascular damage is a link between the decrease in THs and the risk of death in patients with CKD on dialysis.
Material and methodsDesignThis is a prospective cohort study of prevalent patients receiving dialysis in six hospitals with chronic PD and HD programmes, all attended to at the Instituto Mexicano del Seguro Social [Mexican Institute of Social Security] (IMSS) in Mexico City and in Guadalajara. The protocol was approved by the independent ethics committees of each of the participating centres. All patients signed an informed consent to participate in the study.
PatientsA sample was taken randomly of prevalent patients on chronic PD and HD at each centre. All patients were adults (≥18 years). There were no restrictions for gender, aetiology of the CKD or time receiving therapy. From one month prior to recruitment all patients were clinically stable, free from infections or acute complications. We excluded patients who were seropositive for HIV, hepatitis B or C, had cancer or were receiving immunosuppressive treatment, as well as patients previously diagnosed of thyroid diseases, cerebrovascular or peripheral vascular disease, heart failure or current or past ischaemic heart disease. The prescription of medications with a known effect on the evaluation of thyroid function was recorded. The sample size was calculated in 250 subjects, with an expected ratio of 0.40 (CI: 0.125) of patients with low TH levels.
ProceduresThe clinical files were reviewed and the demographic data and relevant information on kidney disease were extracted. An analysis was performed of body composition by electrical bioimpedance (Biodynamics, Seattle, WA, USA). Venous blood samples were obtained after a night of fasting; the plasma was separated into aliquots, which were frozen at −70°C until biochemical testing. All measurements were performed at the start of follow-up in a central laboratory. For patients on HD, the blood sample was taken on the day that they were not receiving dialysis between the first and second session of the week (mid-week). The subsequent visits were scheduled monthly. The average follow-up period was 16 months. The patients who survived were monitored for at least 12 months.
Lab testsGlucose, blood urea nitrogen, creatinine, albumin, total cholesterol and triglycerides were measured by conventional methods in the piece of equipment Hitachi 902 Automatic Analyzer (Roche Diagnostics GmbH, Mannheim, Germany). CRP was measured by high-sensitivity immunoturbidimetry (Tina-quant CRP-HS, Roche Diagnostics GmbH, Mannheim, Germany). NT-proBNP was measured using immunoassays (ECLIA Roche Diagnostics GmbH, Mannheim, Germany) (the coefficients of variation were 5.4 and 6.5% for intra- and inter-assay, respectively). Cardiac troponin i (cTnI) was measured by RIA (Diagnostic Automation Inc., Calabasas, CA, USA) (the coefficients of variability were 6.1 and 6.7% for intra- and inter-assay). The THs (fT3, tT3, fT4, tT4 and TSH) were measured by RIA with standard commercial kits (CIS BIO International, Gif-sur Yvette, France). The coefficients of variation of the THs were fT3: 5.01; 5.42; tT3: 7.77; 8.17; fT4: 5.52; 8.85; tT4: 4.70; 8.00 and TSH: 2.80; 4.20%, for intra- and inter-assay, respectively.
Statistical analysisThe values are shown as frequencies and percentages or as mean±standard deviation, depending on the characteristics of the variables. For the differences between groups, the Student's t-test or Chi-square test were applied, according to the type of variable. Multiple correlation (Pearson) was used to analyse correlation between serum albumin, CRP, NT-proBNP, cTnI, and TH, controlling them by gender, diabetic status, body composition and dialysis modality. Risk factors for mortality were analysed using the Cox proportional hazards model. All statistical calculations were made using SPSS, version 17.0 (Chicago, IL, USA).
ResultsThe population under study consisted of 749 patients; of these, 400 were selected at random and invited to participate. Of these, 67 declined to participate due to problems with the sampling schedule, 21 patients were excluded due to problems coming to the hospital during the follow-up period and a further 16 patients did not attend the sampling session for the initial biochemical tests.
A total of 296 patients were included in the study. The cause of CKD was diabetic nephropathy in 50.7% of patients; unknown in 26.4%; hypertension in 6.4%; polycystic kidney in 6.1%; obstructive uropathy in 3.7%; chronic pyelonephritis in 2%; lupus in another 2% and various diseases in 2.8%. Regular medication included treatment with erythropoietin in 26%. 42% received iron supplements, mainly orally; 87% received calcium carbonate as a phosphate binder; 5% received calcitriol; 67% received at least one antihypertensive drug and 44% received two or more antihypertensive drugs; most frequently used were angiotensin-converting enzyme inhibitors. Among the 296 patients, those treated for HD (n=127) received three sessions per week, 4h per session; all were on dialysis using high-flux polysulfone filters. PD patients (continuous ambulatory PD and automated PD) (n=169) used glucose solutions.
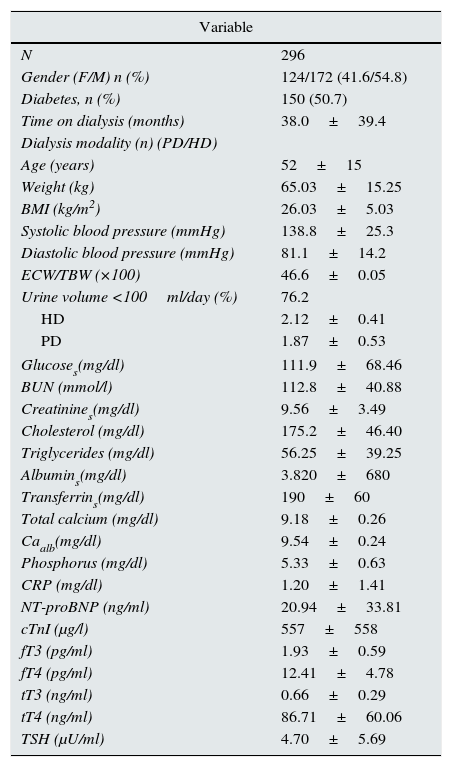
Demographic, clinical and biochemical data are shown in Table 1. None of the subjects had a history of primary thyroid disease. Table 1 also shows values of the THs. tT3 levels were below the normal limit (tT3<0.60ng/ml) in 44.3% of patients. Low values of fT3 (fT3<2.3pg/ml) were more common (52.8%). TSH was greater than 5.0μU/ml in 72 cases (25.7%). In 21 of these patients (27.0%), it was greater than 10.0μU/ml. Patients with diabetes had lower levels of fT3 (1.87±0.66 vs. 2.01±0.52pg/ml; p<0.04) and tT3 (0.59±0.29 vs. 0.74±0.28ng/ml; p<0.001) compared to non-diabetic patients and TSH was greater than 5.0μU/ml in 32.7% of diabetic patients and only in 18.5% of non-diabetic patients (p<0.01). There was no difference between TH levels according to the dialysis modalities.
Variables at the start of the study.
| Variable | |
|---|---|
| N | 296 |
| Gender (F/M) n (%) | 124/172 (41.6/54.8) |
| Diabetes, n (%) | 150 (50.7) |
| Time on dialysis (months) | 38.0±39.4 |
| Dialysis modality (n) (PD/HD) | |
| Age (years) | 52±15 |
| Weight (kg) | 65.03±15.25 |
| BMI (kg/m2) | 26.03±5.03 |
| Systolic blood pressure (mmHg) | 138.8±25.3 |
| Diastolic blood pressure (mmHg) | 81.1±14.2 |
| ECW/TBW (×100) | 46.6±0.05 |
| Urine volume <100ml/day (%) | 76.2 |
| HD | 2.12±0.41 |
| PD | 1.87±0.53 |
| Glucoses(mg/dl) | 111.9±68.46 |
| BUN (mmol/l) | 112.8±40.88 |
| Creatinines(mg/dl) | 9.56±3.49 |
| Cholesterol (mg/dl) | 175.2±46.40 |
| Triglycerides (mg/dl) | 56.25±39.25 |
| Albumins(mg/dl) | 3.820±680 |
| Transferrins(mg/dl) | 190±60 |
| Total calcium (mg/dl) | 9.18±0.26 |
| Caalb(mg/dl) | 9.54±0.24 |
| Phosphorus (mg/dl) | 5.33±0.63 |
| CRP (mg/dl) | 1.20±1.41 |
| NT-proBNP (ng/ml) | 20.94±33.81 |
| cTnI (μg/l) | 557±558 |
| fT3 (pg/ml) | 1.93±0.59 |
| fT4 (pg/ml) | 12.41±4.78 |
| tT3 (ng/ml) | 0.66±0.29 |
| tT4 (ng/ml) | 86.71±60.06 |
| TSH (μU/ml) | 4.70±5.69 |
Data presented as mean±SD or median and range unless stated otherwise.
Albumins: serum albumin; BMI: body mass index; BUN: blood urea nitrogen; Caalb: total serum calcium corrected for albumin; CRP: C-reactive protein; cTnI: cardiac troponin I; ECW/TBW (×100): extracellular water/total body water (×100); fT3: free triiodothyronine; fT4: free thyroxine; Glucoses: serum glucose; HD: haemodialysis; NT-proBNP: N-terminal pro-brain natriuretic peptide; PD: peritoneal dialysis; TSH: thyroid-stimulating hormone; tT3: total triiodothyronine; tT4: total thyroxine.
The normal limits of thyroid hormones are total T3: 0.6–1.9ng/ml; free T3: 2.00–4.25pg/ml; total T4: 45–110ng/ml; free T4: 7–18pg/ml and TSH: 0.25–4.00μU/ml.
The variables relating to body composition and nutrition were out of the normal ranges in a significant number of patients. The mean BMI was in the overweight range. When BMI was assessed by gender, it was found to have low values in only 2% of patients, within normal range in 46%, and overweight or obese in 52%. Being overweight or obese was more common in women (58%) than in men (48%). The BMI and fat content measured by bioimpedance and expressed as percentage of body weight were closely linked (r=0.47; p<0.01). Mean extracellular water content expressed as a percentage of total body water was in the range of overhydration and, when it was evaluated taking into account the BMI and gender,13 overhydration was found more often in women (99%) than in men (31%). The plasma concentration of albumin was reduced (<4g/dl) in 30% of patients and serum transferrin (<200mg/dl) in 63%. In addition, CRP was high (>3.0mg/l) in 86% of cases. Concentrations of NT-proBNP and cTnI were above normal ranges in more than 50% of patients.
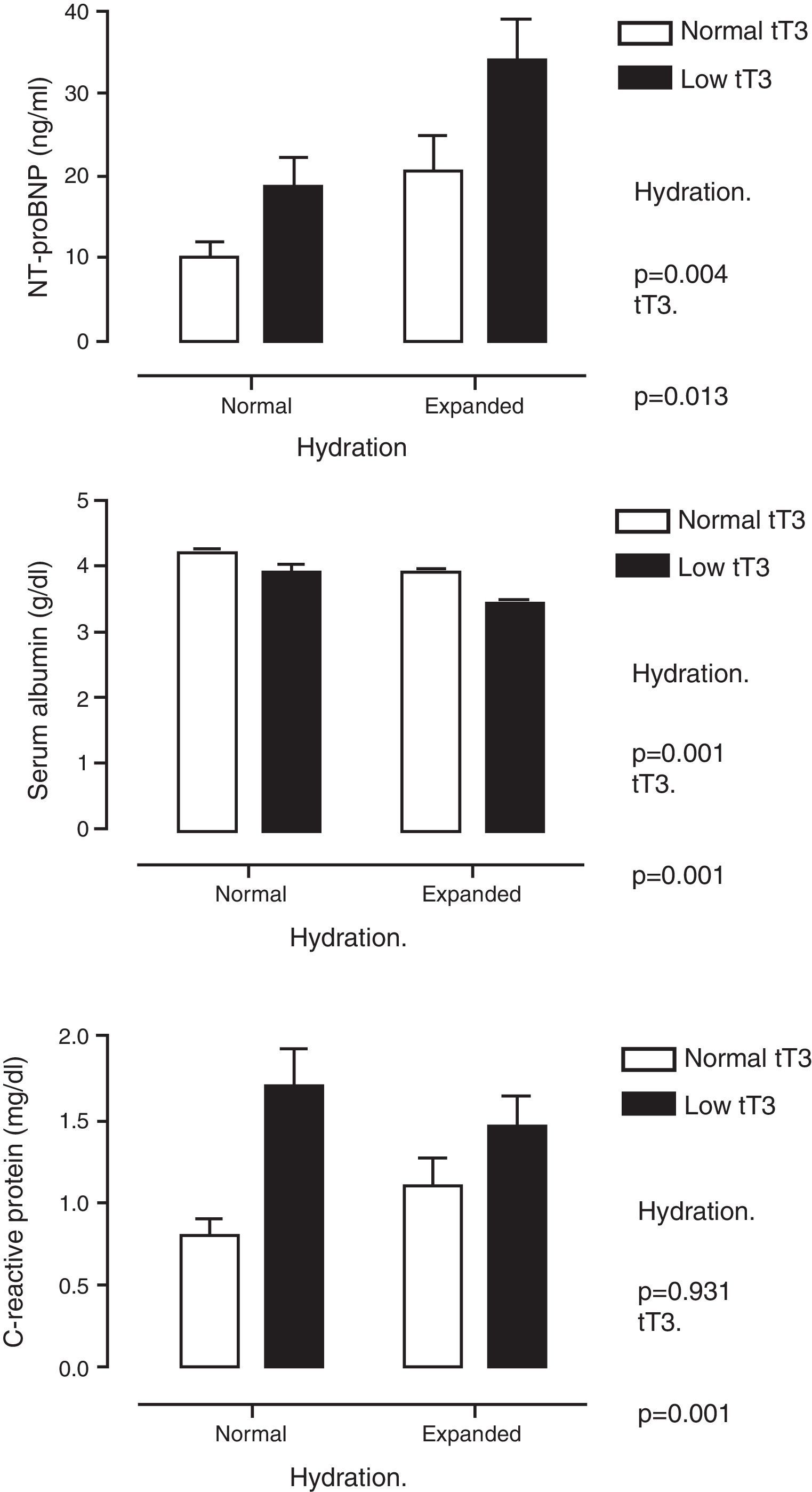
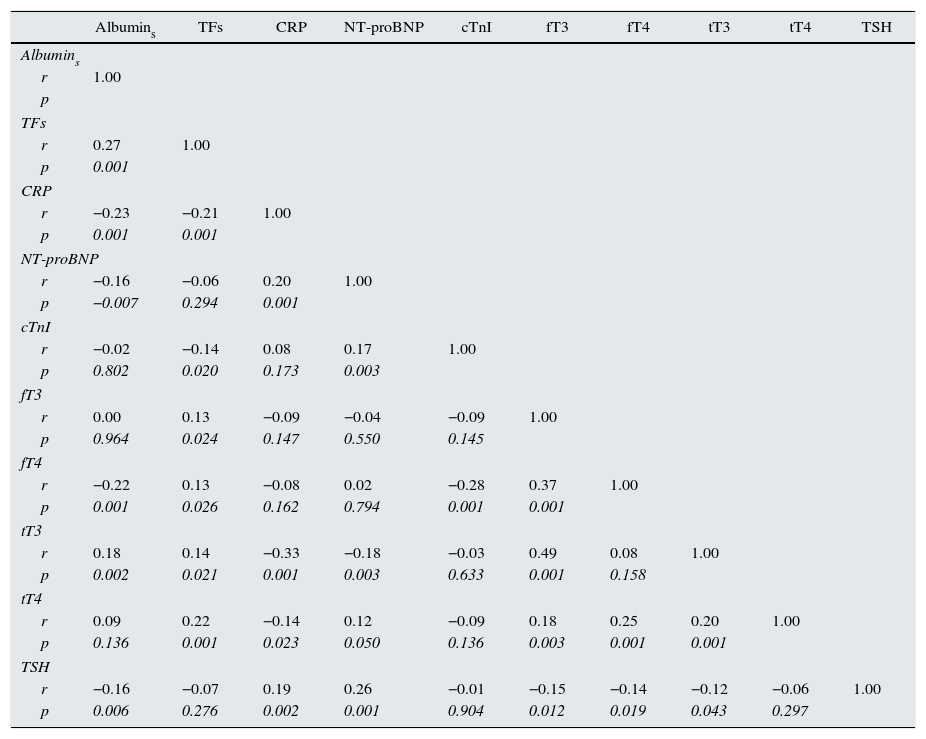
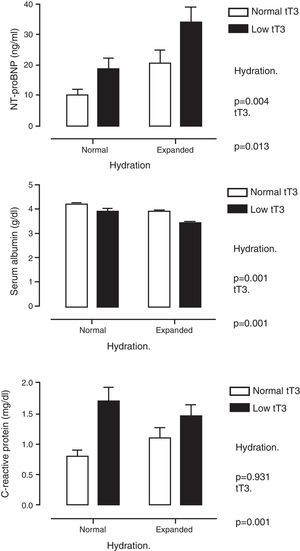
Table 2 shows multiple correlation analysis. Significant associations were found between the THs themselves, as well as with biochemical parameters of nutrition (albumin, transferrin), inflammation (CRP) and markers of myocardial injury (cTnI and NT-proBNP). Since several of these parameters are under the influence of THs, but are also affected by the state of hydration, the differences were analysed by classifying the patients according to whether or not they had low tT3 values and whether they were overhydrated. Fig. 1 shows these differences. It can be seen that NT-proBNP was higher in patients who were overhydrated than in those who had normal hydration, but, within each of these categories, those who had tT3 below normal limits had even higher NT-proBNP. In the case of serum albumin, the pattern was found to be reversed: overhydrated patients had lower albumin than those that showed normal hydration, but, within these categories, those that had low tT3 had even lower serum albumin. CRP was only found to be influenced by tT3: patients with low tT3 had higher CRP levels.
Coefficients of multiple correlations controlled by age, gender, BMI and ECW/TBW.
| Albumins | TFs | CRP | NT-proBNP | cTnI | fT3 | fT4 | tT3 | tT4 | TSH | |
|---|---|---|---|---|---|---|---|---|---|---|
| Albumins | ||||||||||
| r | 1.00 | |||||||||
| p | ||||||||||
| TFs | ||||||||||
| r | 0.27 | 1.00 | ||||||||
| p | 0.001 | |||||||||
| CRP | ||||||||||
| r | −0.23 | −0.21 | 1.00 | |||||||
| p | 0.001 | 0.001 | ||||||||
| NT-proBNP | ||||||||||
| r | −0.16 | −0.06 | 0.20 | 1.00 | ||||||
| p | −0.007 | 0.294 | 0.001 | |||||||
| cTnI | ||||||||||
| r | −0.02 | −0.14 | 0.08 | 0.17 | 1.00 | |||||
| p | 0.802 | 0.020 | 0.173 | 0.003 | ||||||
| fT3 | ||||||||||
| r | 0.00 | 0.13 | −0.09 | −0.04 | −0.09 | 1.00 | ||||
| p | 0.964 | 0.024 | 0.147 | 0.550 | 0.145 | |||||
| fT4 | ||||||||||
| r | −0.22 | 0.13 | −0.08 | 0.02 | −0.28 | 0.37 | 1.00 | |||
| p | 0.001 | 0.026 | 0.162 | 0.794 | 0.001 | 0.001 | ||||
| tT3 | ||||||||||
| r | 0.18 | 0.14 | −0.33 | −0.18 | −0.03 | 0.49 | 0.08 | 1.00 | ||
| p | 0.002 | 0.021 | 0.001 | 0.003 | 0.633 | 0.001 | 0.158 | |||
| tT4 | ||||||||||
| r | 0.09 | 0.22 | −0.14 | 0.12 | −0.09 | 0.18 | 0.25 | 0.20 | 1.00 | |
| p | 0.136 | 0.001 | 0.023 | 0.050 | 0.136 | 0.003 | 0.001 | 0.001 | ||
| TSH | ||||||||||
| r | −0.16 | −0.07 | 0.19 | 0.26 | −0.01 | −0.15 | −0.14 | −0.12 | −0.06 | 1.00 |
| p | 0.006 | 0.276 | 0.002 | 0.001 | 0.904 | 0.012 | 0.019 | 0.043 | 0.297 | |
Albumins: serum albumin; BMI: body mass index; CRP: C-reactive protein; cTnI: cardiac troponin I; ECW/TBW: extracellular water/total body water; fT3: free triiodothyronine; fT4: free thyroxine; NT-proBNP: N-terminal pro-brain natriuretic peptide; p: significance; r: Pearson's correlation coefficient; TFs: serum transferrin; TSH: thyroid-stimulating hormone; tT3: total triiodothyronine; tT4: total thyroxine.
Serum levels of N-terminal pro-brain natriuretic peptide (top panel), albumin (middle panel) and C-reactive protein (bottom panel) when patients were classified by degree of hydration and concentration of total triiodothyronine (tT3). It is shown how the effect of tT3 is independent of the degree of hydration.
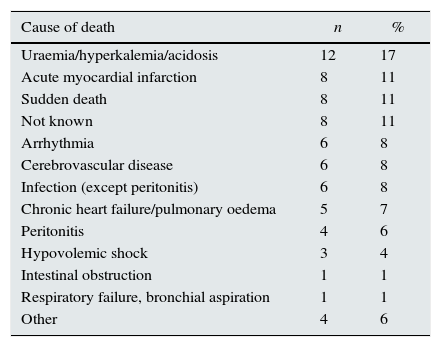
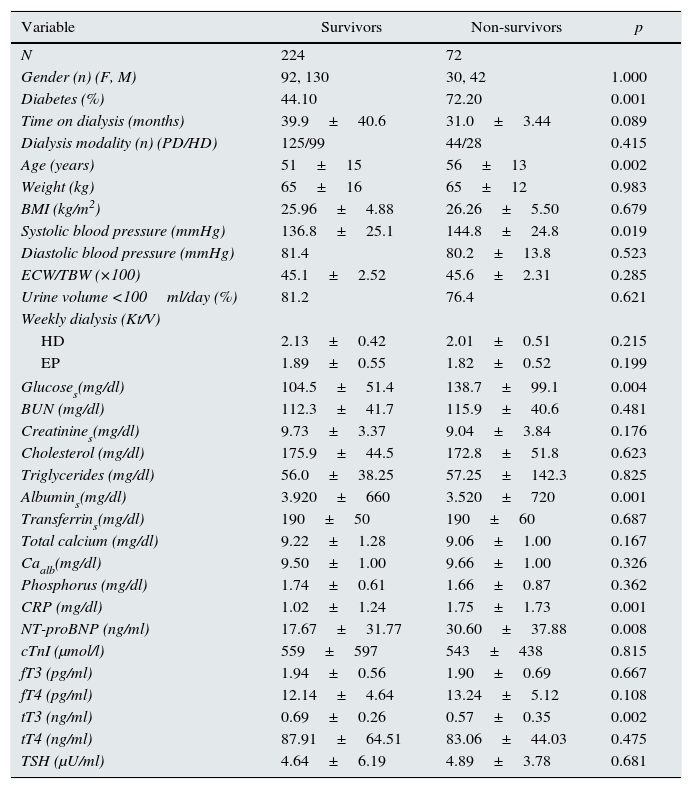
The follow-up period was 16.2±2.0 months. There were 72 deaths. The causes of death are shown in Table 3; cardiovascular deaths (sudden death, myocardial infarction, arrhythmia, heart failure, cerebrovascular disease) (n=33) accounted for more than 45% of all deaths. Table 4 shows the differences in the relevant variables among those who survived and those who did not. In addition to the expected differences in age, blood pressure and presence of diabetes, there were also significant differences in variables related to nutrition and inflammation (albumin and CRP), as well as with variables associated with myocardial injury (NT-proBNP). The difference between the two groups in values of the THs was only significant for tT3. There was no difference in the frequency or the type of medication, or the dialysis modality.
Cause, number and percentage of deaths.
| Cause of death | n | % |
|---|---|---|
| Uraemia/hyperkalemia/acidosis | 12 | 17 |
| Acute myocardial infarction | 8 | 11 |
| Sudden death | 8 | 11 |
| Not known | 8 | 11 |
| Arrhythmia | 6 | 8 |
| Cerebrovascular disease | 6 | 8 |
| Infection (except peritonitis) | 6 | 8 |
| Chronic heart failure/pulmonary oedema | 5 | 7 |
| Peritonitis | 4 | 6 |
| Hypovolemic shock | 3 | 4 |
| Intestinal obstruction | 1 | 1 |
| Respiratory failure, bronchial aspiration | 1 | 1 |
| Other | 4 | 6 |
Comparisons of different variables between survivors and non-survivors.
| Variable | Survivors | Non-survivors | p |
|---|---|---|---|
| N | 224 | 72 | |
| Gender (n) (F, M) | 92, 130 | 30, 42 | 1.000 |
| Diabetes (%) | 44.10 | 72.20 | 0.001 |
| Time on dialysis (months) | 39.9±40.6 | 31.0±3.44 | 0.089 |
| Dialysis modality (n) (PD/HD) | 125/99 | 44/28 | 0.415 |
| Age (years) | 51±15 | 56±13 | 0.002 |
| Weight (kg) | 65±16 | 65±12 | 0.983 |
| BMI (kg/m2) | 25.96±4.88 | 26.26±5.50 | 0.679 |
| Systolic blood pressure (mmHg) | 136.8±25.1 | 144.8±24.8 | 0.019 |
| Diastolic blood pressure (mmHg) | 81.4 | 80.2±13.8 | 0.523 |
| ECW/TBW (×100) | 45.1±2.52 | 45.6±2.31 | 0.285 |
| Urine volume <100ml/day (%) | 81.2 | 76.4 | 0.621 |
| Weekly dialysis (Kt/V) | |||
| HD | 2.13±0.42 | 2.01±0.51 | 0.215 |
| EP | 1.89±0.55 | 1.82±0.52 | 0.199 |
| Glucoses(mg/dl) | 104.5±51.4 | 138.7±99.1 | 0.004 |
| BUN (mg/dl) | 112.3±41.7 | 115.9±40.6 | 0.481 |
| Creatinines(mg/dl) | 9.73±3.37 | 9.04±3.84 | 0.176 |
| Cholesterol (mg/dl) | 175.9±44.5 | 172.8±51.8 | 0.623 |
| Triglycerides (mg/dl) | 56.0±38.25 | 57.25±142.3 | 0.825 |
| Albumins(mg/dl) | 3.920±660 | 3.520±720 | 0.001 |
| Transferrins(mg/dl) | 190±50 | 190±60 | 0.687 |
| Total calcium (mg/dl) | 9.22±1.28 | 9.06±1.00 | 0.167 |
| Caalb(mg/dl) | 9.50±1.00 | 9.66±1.00 | 0.326 |
| Phosphorus (mg/dl) | 1.74±0.61 | 1.66±0.87 | 0.362 |
| CRP (mg/dl) | 1.02±1.24 | 1.75±1.73 | 0.001 |
| NT-proBNP (ng/ml) | 17.67±31.77 | 30.60±37.88 | 0.008 |
| cTnI (μmol/l) | 559±597 | 543±438 | 0.815 |
| fT3 (pg/ml) | 1.94±0.56 | 1.90±0.69 | 0.667 |
| fT4 (pg/ml) | 12.14±4.64 | 13.24±5.12 | 0.108 |
| tT3 (ng/ml) | 0.69±0.26 | 0.57±0.35 | 0.002 |
| tT4 (ng/ml) | 87.91±64.51 | 83.06±44.03 | 0.475 |
| TSH (μU/ml) | 4.64±6.19 | 4.89±3.78 | 0.681 |
Data presented as mean±SD or median and range unless stated otherwise. The comparisons were made by the Student's t-test or the Chi-square test, depending on the characteristics of the variables.
Albumins: serum albumin; BMI: body mass index; BUN: blood urea nitrogen; Caalb: total serum calcium corrected for albumin; CRP: C-reactive protein; cTnI: cardiac troponin I; ECW/TBW: extracellular water/total body water; fT3: free triiodothyronine; fT4: free thyroxine; Glucoses: serum glucose; HD: haemodialysis; NT-proBNP: N-terminal pro-brain natriuretic peptide; PD: peritoneal dialysis; TSH: thyroid-stimulating hormone; tT3: total triiodothyronine; tT4: total thyroxine.
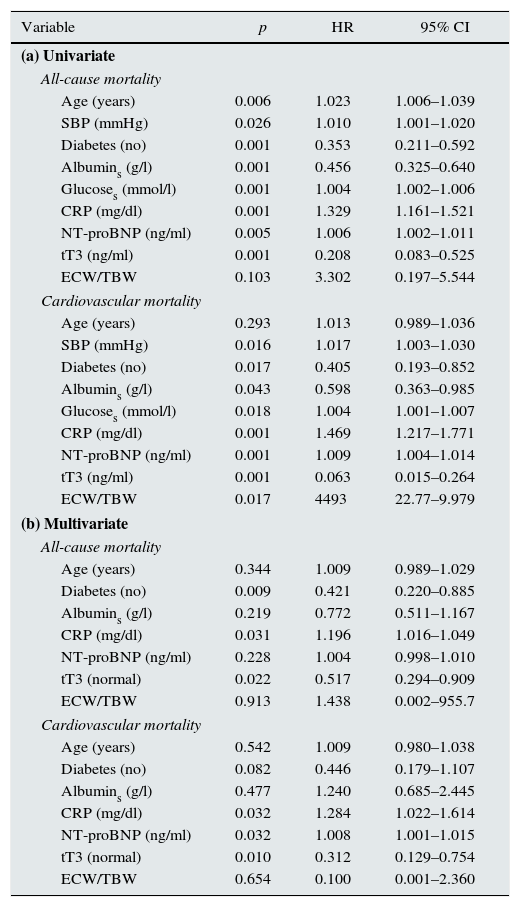
Variables which were different between those who survived and those who did not were considered in the survival analysis. Section a of Table 5 shows the univariate analysis. Risk factors for all-cause mortality were: age, systolic blood pressure, diabetes, serum albumin, glucose, CRP and NT-proBN; of the THs, only tT3 was a significant predictor. In the univariate analysis of cardiovascular mortality, all the above-mentioned variables, with the exception of age, were significant predictors.
Analysis of mortality.
| Variable | p | HR | 95% CI |
|---|---|---|---|
| (a) Univariate | |||
| All-cause mortality | |||
| Age (years) | 0.006 | 1.023 | 1.006–1.039 |
| SBP (mmHg) | 0.026 | 1.010 | 1.001–1.020 |
| Diabetes (no) | 0.001 | 0.353 | 0.211–0.592 |
| Albumins (g/l) | 0.001 | 0.456 | 0.325–0.640 |
| Glucoses (mmol/l) | 0.001 | 1.004 | 1.002–1.006 |
| CRP (mg/dl) | 0.001 | 1.329 | 1.161–1.521 |
| NT-proBNP (ng/ml) | 0.005 | 1.006 | 1.002–1.011 |
| tT3 (ng/ml) | 0.001 | 0.208 | 0.083–0.525 |
| ECW/TBW | 0.103 | 3.302 | 0.197–5.544 |
| Cardiovascular mortality | |||
| Age (years) | 0.293 | 1.013 | 0.989–1.036 |
| SBP (mmHg) | 0.016 | 1.017 | 1.003–1.030 |
| Diabetes (no) | 0.017 | 0.405 | 0.193–0.852 |
| Albumins (g/l) | 0.043 | 0.598 | 0.363–0.985 |
| Glucoses (mmol/l) | 0.018 | 1.004 | 1.001–1.007 |
| CRP (mg/dl) | 0.001 | 1.469 | 1.217–1.771 |
| NT-proBNP (ng/ml) | 0.001 | 1.009 | 1.004–1.014 |
| tT3 (ng/ml) | 0.001 | 0.063 | 0.015–0.264 |
| ECW/TBW | 0.017 | 4493 | 22.77–9.979 |
| (b) Multivariate | |||
| All-cause mortality | |||
| Age (years) | 0.344 | 1.009 | 0.989–1.029 |
| Diabetes (no) | 0.009 | 0.421 | 0.220–0.885 |
| Albumins (g/l) | 0.219 | 0.772 | 0.511–1.167 |
| CRP (mg/dl) | 0.031 | 1.196 | 1.016–1.049 |
| NT-proBNP (ng/ml) | 0.228 | 1.004 | 0.998–1.010 |
| tT3 (normal) | 0.022 | 0.517 | 0.294–0.909 |
| ECW/TBW | 0.913 | 1.438 | 0.002–955.7 |
| Cardiovascular mortality | |||
| Age (years) | 0.542 | 1.009 | 0.980–1.038 |
| Diabetes (no) | 0.082 | 0.446 | 0.179–1.107 |
| Albumins (g/l) | 0.477 | 1.240 | 0.685–2.445 |
| CRP (mg/dl) | 0.032 | 1.284 | 1.022–1.614 |
| NT-proBNP (ng/ml) | 0.032 | 1.008 | 1.001–1.015 |
| tT3 (normal) | 0.010 | 0.312 | 0.129–0.754 |
| ECW/TBW | 0.654 | 0.100 | 0.001–2.360 |
Albumins: serum albumin; 95% CI: 95% confidence interval; CRP: C-reactive protein; ECW/TBW: extracellular water/total body water; Glucoses: serum glucose; HR: hazard ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide; p: significance; SBP: systolic blood pressure; tT3: total triiodothyronine.
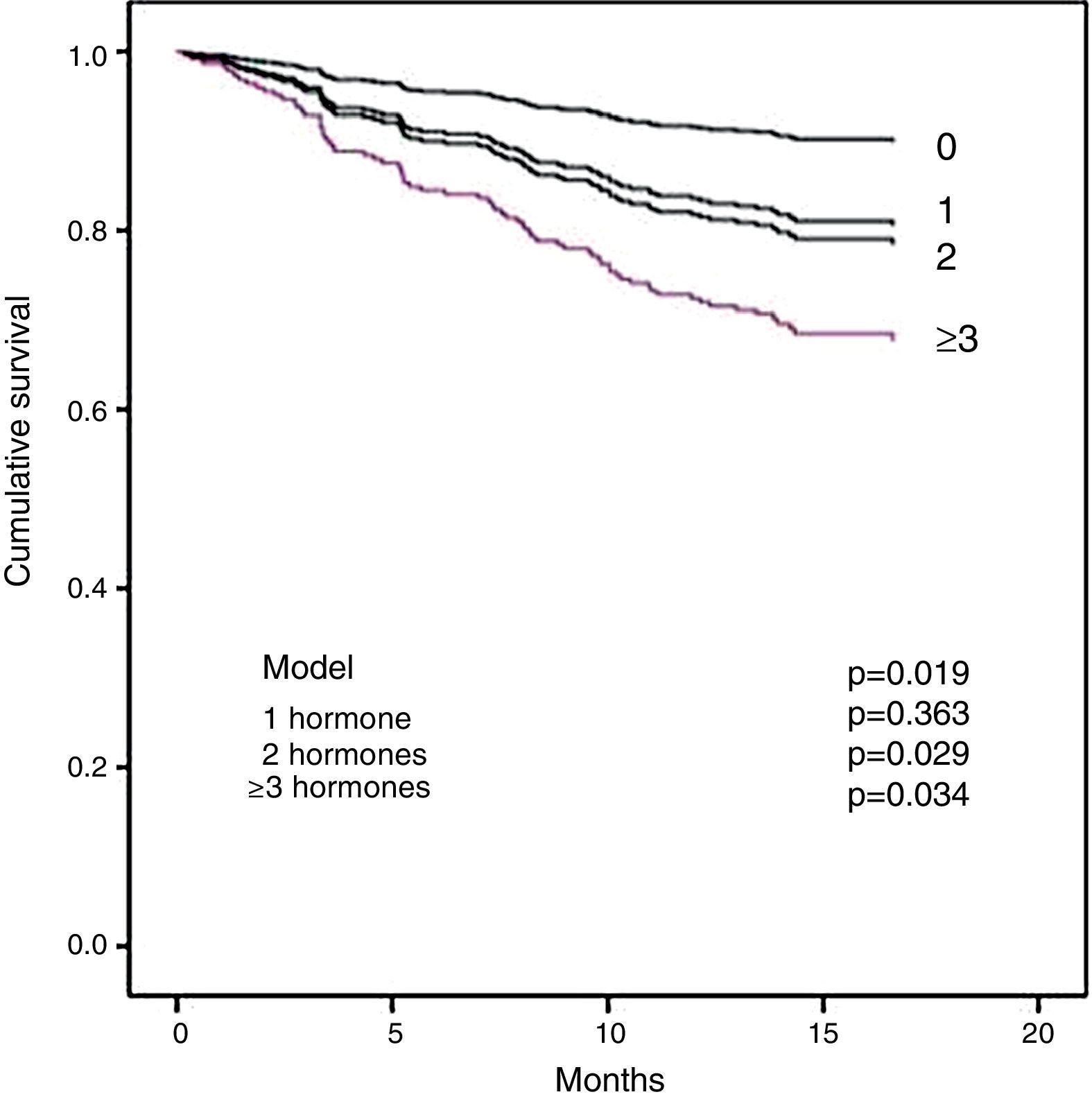
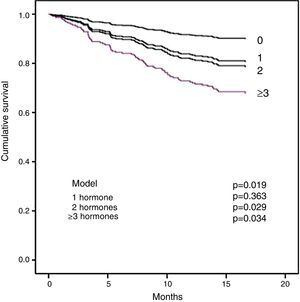
Section b of Table 5 shows the multivariate analysis. Diabetes, CRP and tT3 were independent significant predictors of all-cause mortality. For cardiovascular mortality, the significant factors were CRP, NT-proBNP and tT3. It is important to note that tT3 maintained its significance as an independent risk factor for all-cause mortality and for cardiovascular mortality. In order to examine whether the extent of the changes in the THs affected survival, the effect of the number of hormones outside normal limits on mortality was analysed. Fig. 2 shows that the patients with two or more hormones considered to be in the range of hypothyroidism had a shorter survival.
DiscussionThe main findings of this study can be summarised as follows: (1) Reduced concentration of T3 in the blood is common in patients on dialysis. (2) The concentration of tT3 had, as expected, a significant relationship with biochemical markers of nutrition and inflammation. (3) A new finding was the inverse relationship between tT3 and NT-proBNP, a marker of myocardium injury, which may be the nexus between low T3 levels and cardiovascular mortality and, probably, also with all-cause mortality.
Reduced serum levels of tT3 and fT3 have previously been reported both in patients with CKD in pre-dialysis and in patients on HD and PD. The frequency of these changes has been found in between 10 and 30% of cases.1,14 Changes in T4 and TSH are less common. The direct mechanism for reducing T3 is the reduction in the enzymatic activity of 5′-monodeiodinase, responsible for converting T4 into T3, and an increase in 5′-monodeiodinase, which converts T4 to reverse T3, which is inactive in peripheral tissue.15 The conversion of T4 to T3 is much better preserved in the pituitary gland: this is because T3 and TSH remain within normal levels. However, as the state of patients’ health declines, the changes in T3 are more pronounced and, in more severe stages, there may be a progressive reduction in T4 levels and further changes in TSH.15 The proportion of patients with low T3 found in this study (44.3%) was higher than that reported in others. A small number of patients also showed a decrease in T4 levels and increased levels of TSH. It is important to mention that the patients included in this study did not have a history of thyroid disease. Elevated TSH may be linked to higher intensity of non-thyroidal illness syndrome or to changes secondary to patient improvement, as has been described in other studies,16–18 and not necessarily to primary hypothyroidism. This is why they were analysed together. The extent and the number of hormones affected were associated with worse clinical outcomes, as discussed below.
The highest proportion of TH abnormalities in this study can be explained by the larger proportion of patients with biochemical data of malnutrition and inflammation, as indicated in the correlation of T3 with serum albumin and CRP. The inverse relationship between low T3 levels and inflammation has been consistent with previous studies.19 Although the association between these factors is poorly understood, the effect of pro-inflammatory cytokines is a possible explanation. The administration of exogenous interleukin-6 to mice and humans reduces the conversion of T4 to T3 in tissue and speeds up their metabolism.20,21 Moreover, in cases of primary malnutrition, T3 levels falls significantly, probably as a mechanism to reduce energy expenditure in the body.22,23 In patients from the present study, it is difficult to separate the effect of inflammation from that of the combination of malnutrition-metabolic burnout, because both are closely associated.24 Loss of THs is another factor that has been considered to explain the low T3 levels. In patients treated with PD, up to 10% of the THs produced daily and variable quantities of thyroxine-binding globulin may be lost in the dialysate. In patients with proteinuria, there is also loss of THs in the urine.25–27 In this study, there were no differences in TH levels between HD and PD, nor with the urine volume, indicating that it had little effect.
The inverse correlation found between T3 and NT-proBNP indicates that low T3 levels may be involved in the remodelling of the myocardium, but this concept requires a more detailed analysis., Hypothyroid patients with a healthy heart have normal or low levels of NT-proBNP or BNP, and patients with hyperthyroidism and a healthy heart have elevated levels of NT-proBNP or BNP.28,29 In patients with CKD, the interaction between THs and cardiac function and remodelling occurs under different conditions: the thyroid gland is essentially normal and the myocardium has major functional and anatomical abnormalities. Two articles have reported a lower ejection fraction and a larger ventricular mass in PD and HD patients with reduced T3 levels,30,31 and another study found an association between decreased T3 and diastolic dysfunction.12 It should be noted that in these clinical conditions elevated, rather than reduced Nt-proBNP has been found, as would be expected if T3 is reduced. Despite this relationship, the reports concerning the relationship between THs and natriuretic factors—a widely recognised marker of myocardial injury—are limited in patients with CKD. additionally, abnormal remodelling also has an influence on this relationship: in a non-uraemic patient with dilated cardiomyopathy and a severe reduction in THs, contrary to what was expected, there has been found an increased myocardial atrial natriuretic peptide gene expression; this is corrected by levothyroxine replacement therapy.32 These data together suggest that the interaction between T3 and heart depends on which organ fails first. In CKD patients, volume overload, pressure overload and myocardial remodelling appear to precede thyroid disorders. In the long term, diastolic and systolic dysfunctions linked to inflammation and malnutrition lead to more significant thyroid disorders, which, in turn, lead to more severe myocardial injury, initiating a vicious cycle leading to progressive patient deterioration. Myocardial fibrosis and diastolic dysfunction are presented early on in CKD and are associated with a decrease in THs,33–35 this is similar to the observed in subclinical and experimental hypothyroidism.9,36 The role of other markers of myocardial injury, such as cTnI, may be masked by the co-linearity with NT-proBNP, although it must be mentioned that its importance in patients with CKD is not consistent.37
While diabetes, low serum albumin, high CRP and high NT-proBNP values are known as important factors associated with all-cause mortality and cardiovascular mortality,38,39 the role of the changes in THs as risk factors has only recently been recognised in kidney patients.5–7 However, the pathophysiological link between THs and mortality remains a debatable issue. In some studies, the fT3 level was involved as a risk factor for mortality,5 but, in other studies, and in accordance with the present study, tT3 was found to have greater predictive value in a panel of TH tests.7 We do not know why would tT3 be a better predictor of mortality than fT3? It has been argued that tT3, and fT3, decrease as a result of a reduction in the conversion of T4 to T3, but tT3 is also affected by a reduced number of proteins binding to THs. This reduction is induced by inflammation. This means that low tT3 levels represent a low level of hormones, while they also serve as an inflammatory marker.7 In this study, the decrease in tT3 had the greatest influence on mortality, but the number of THs in the range compatible with hypothyroidism was also associated with the progressive reduction in survival.
A causal relationship between THs and all-cause mortality and cardiovascular mortality cannot be established with the information currently available. The strong association of low T3 levels with markers of inflammation and malnutrition makes it difficult to weigh up the individual influence of each factor on mortality. Despite this, low T3 levels and high CRP levels are still independent risk factors for all-cause mortality and cardiovascular mortality, indicating that they may have an additive effect. It is also plausible that low T3 levels and high levels of inflammatory markers act in parallel and remain another chance factor. Inflammatory markers in patients with CKD have various possible origins, some of which can also be the triggering factor for non-thyroidal illness syndrome.
The data reported in this article show that low T3 levels may be an additional element, acting together with other traditional and non-traditional risk factors, in mortality. Intervention studies are necessary for a better understanding of this relationship between THs and myocardial remodelling in patients with CKD. It is important to discuss some progress that has been made in this regard in patients with dilated cardiomyopathy: acute T3 supplementation significantly reduced circulating levels of norepinephrine, aldosterone and NT-proBNP, and increased left ventricular end-diastolic volume, indicating that supplementation with THs has a significant role in the function of the myocardium in patients with chronic diseases or secondary thyroid disorders.34,40
Our study is descriptive so it has limitations, as causality cannot be established in the observed associations. In addition, the population studied has a significant number of confounding variables (such as the treatment modality, the prevalence of diabetes and the fact that it involves prevalent patients) and even the influence of other non-identified factors, as well as co-linearity between the variables that were measured, means that the lack of reproducibility of findings reported in other publications is expected. An appropriate response to this observation can only be provided with intervention studies.
With the results obtained, we can conclude that low tT3 levels are common in dialysis patients, which are associated with inflammation, malnutrition and myocardial injury. The latter may be the link between decreased THs and cardiovascular mortality. However, as some authors have indicated, interventional studies are required to stablish a cause–effect relationship between changes in THs with myocardial remodelling and mortality.
FundingThe authors express their thanks to the Coordination of Health Research of the Instituto Mexicano del Seguro Social for their sponsorship of this study (2008-785-041).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Prado-Uribe MdC, Ventura M-d-J, Ávila-Díaz M, Mora CJ, Méndez-Durán A, Villanueva-Noches D, et al. La disminución de triyodotironina se asocia con la elevación del péptido natriurético cerebral N-terminal y con la mortalidad en pacientes en diálisis. Nefrologia. 2017;37:598–607.