Molecular mechanisms of increased cardiovascular mortality in chronic kidney disease (CKD) associated with biological age are not well understood. Recent studies support the hypothesis that common factors responsible for this phenomenon are cellular aging and telomere dysfunction.
ObjectivesThe purpose of this study was to investigate the relation between telomerase activity and CKD stages.
MethodsThe study included 120 patients who were followed-up for CKD stage 2–5D, composed of 30 patients of each stage and 30 healthy volunteers without any known disease who were admitted to our hospital for routine check-ups. Telomerase activity in peripheral blood mononuclear cells (PBMC) was measured using the TRAP assay.
ResultsA significant difference was observed for telomerase activity in PBMC between groups. The detected levels were lowest in the healthy control group (0.15±0.02), and highest in CKD stage 5D patients (0.23±0.04). In CKD patients, telomerase activity in PBMC was positively correlated with the CKD stage, serum creatinine, potassium and parathormone levels, and negatively correlated with estimated glomerular filtration rate (eGFR), body mass index (BMI), platelet count and serum calcium levels. According to the linear regression analysis, independent predictors for high telomerase activity in CKD patients were eGFR and BMI.
ConclusionTelomerase activity in PBMC increases with advancing CKD stage in CKD patients. Increased telomerase activity in PBMC is associated with eGFR and BMI.
Los mecanismos moleculares responsables del aumento de la mortalidad cardiovascular en la enfermedad renal crónica (ERC) asociada a la edad biológica no se conocen bien. Los estudios recientes apoyan la hipótesis de que los factores comunes responsables de este fenómeno son el envejecimiento celular y la disfunción telomérica.
ObjetivosEl objetivo de este estudio fue investigar la relación entre la actividad de la enzima telomerasa y los estadios de ERC.
MétodosEl estudio incluyó a 120 pacientes que fueron seguidos para la ERC en los estadios 2-5D; cada estadio incluyó a 30 pacientes y a 30 voluntarios sanos sin ninguna enfermedad conocida que fueron admitidos en nuestro hospital para los controles periódicos. La actividad de la telomerasa en células mononucleares de sangre periférica (CMSP) se midió usando el método de TRAP.
ResultadosSe observó una diferencia significativa en la actividad telomerasa en las CMSP entre los diferentes grupos. Los niveles más bajos fueron los del grupo de controles sanos (0,15±0,02) y los más altos los del grupo de pacientes con ERC en el estadio 5D (0,23±0,04). En los pacientes con ERC, la actividad telomerasa en las CMSP se correlacionó positivamente con el estadio de ERC y los niveles plasmáticos de potasio, hormona paratiroidea y creatinina, y se correlacionó negativamente con la tasa de filtración glomerular estimada (eTFG), el índice de masa corporal (IMC), el recuento de plaquetas y el calcio en suero. Los predictores independientes para la actividad telomerasa alta en pacientes con ERC fueron la eTFG y el IMC, de acuerdo con el análisis de regresión lineal.
ConclusiónLa actividad telomerasa en CMSP aumenta con el avance en el estadio de ERC. El aumento de la actividad telomerasa en CMSP se asocia con la eTFG y el IMC.
Epidemiological studies have shown that biological age is older than chronologic age in chronic kidney disease (CKD) patients.1–3 Molecular mechanisms of increased cardiovascular mortality in CKD associated with biological age are not well understood. Recent studies suggest that factors such as cellular aging and telomere dysfunction may be the responsible factors.4–6 Studies conducted in hemodialysis patients have shown that telomere length in peripheral blood mononuclear cells (PBMC) was reduced compared to healthy individuals, irrespective of age and gender.7,8 Telomerase enzyme maintains the length and structure of telomeres, thus provides longer cell survival.9,10 Telomerase activity in PBMC was found significantly lower in hemodialysis patients compared to the healthy control group.11
Telomerase activity levels in various CKD stages and its relation to CKD progression are not well understood. This study was aimed to determine the relation between telomerase activity and CKD stages.
Materials and methodStudy design and patient assignmentThis study was approved by the University Clinical Research Ethics Committee with approval number 2014/35 and was conducted with the support of Scientific Research Projects Coordination Unit. Written consent was obtained from all participants. This cross-sectional study was conducted at the Süleyman Demirel University Medical School Nephrology Department between May 2014 and November 2014. The study included 120 patients who were followed-up for CKD stage 2–5D, composed of 30 patients of each stage, and 30 healthy volunteers.
Inclusion criteria were age over 18 years and having CKD stage 2–5D diagnosis. Exclusion criteria included presence of an acute infection and a malignancy and using immunosuppressive therapy.
Blood samplingComplete blood count, blood levels of fasting blood glucose, serum creatinine (Cr), sodium (Na), potassium (K), calcium (Ca), phosphorus (P), albumin, alanine aminotransferase (ALT), aspartat aminotransferase (AST), uric acid, triglyceride, total cholesterol, LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C), 25-hydroxy-vitamin D (25(OH)D), parathormone (iPTH), C-reactive protein (CRP) and spot urine protein/creatinine (Upr/Ucr) values were measured in each patient. Blood samples of hemodialysis patients were collected just before mid-week dialysis session. Body mass index (BMI) was calculated. Estimated GFR (eGFR) levels were calculated using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.12
Measurement of telomerase activity in PBMCTelomerase activity in PBMC was measured using TeloTAGGG Telomerase PCR ELISA kit (Roche Applied Science, Indianapolis, IN, USA) with telomeric repeat amplification protocol (TRAP) method.13 All samples with an optical density 450nm≥0.2 were considered telomerase positive.14
Statistical analysisData analysis was performed using SPSS for Windows 15.0 software. Results were expressed as mean±standard deviation or percent values. Chi-square test, variance analysis (ANOVA) and Kruskal–Wallis variance analysis tests were used for group comparisons. Tukey's HSD test was used for post hoc analysis. Associations between qualitative variables were assessed by Spearman's or Pearson's correlation analysis. A multivariate linear regression model was used to estimate the independent effects of various predictors (analyzed variables were age, BMI, eGFR, platelet count, serum Ca, K, LDL-C, iPTH) on telomerase activity. The level of statistical significance was p<0.05.
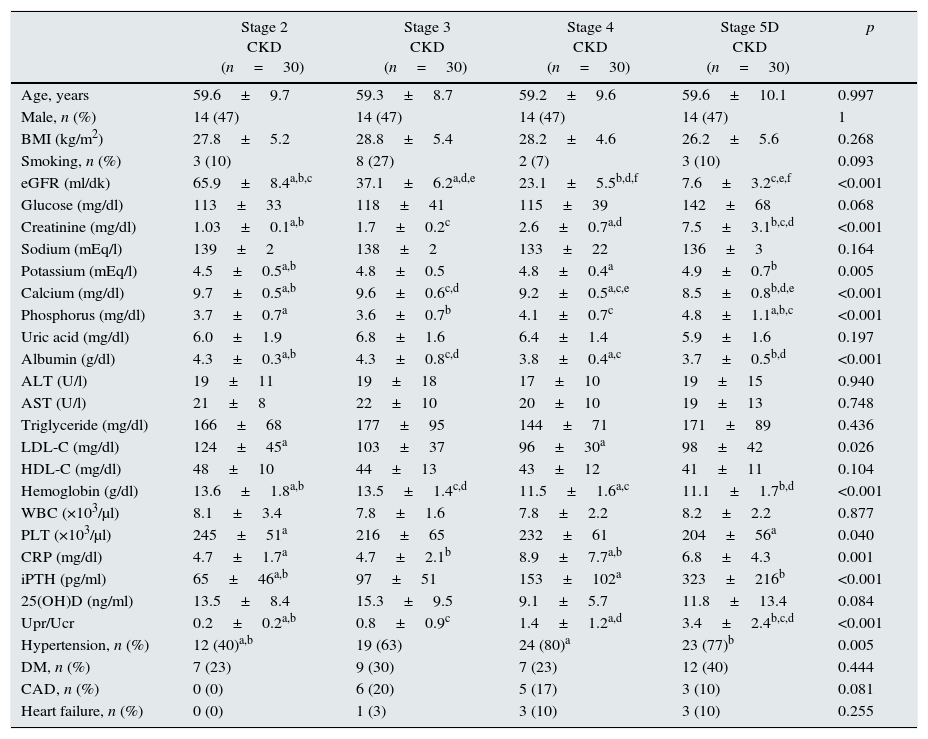
ResultsMean age was 58.6±10.4 years in the control group and 59.4±9.4 years in the patient group (p>0.05). Baseline characteristics of the patient groups are shown in Table 1. Patient groups were similar regarding BMI and smoking (p>0.05). Among the CKD stage 5D patients, 20 were on hemodialysis while 10 were on peritoneal dialysis. Mean dialysis duration for dialysis patients was 31.1±29.7 (range, 4–120) months.
Baseline clinical and laboratory data of CKD patients.
| Stage 2 CKD (n=30) | Stage 3 CKD (n=30) | Stage 4 CKD (n=30) | Stage 5D CKD (n=30) | p | |
|---|---|---|---|---|---|
| Age, years | 59.6±9.7 | 59.3±8.7 | 59.2±9.6 | 59.6±10.1 | 0.997 |
| Male, n (%) | 14 (47) | 14 (47) | 14 (47) | 14 (47) | 1 |
| BMI (kg/m2) | 27.8±5.2 | 28.8±5.4 | 28.2±4.6 | 26.2±5.6 | 0.268 |
| Smoking, n (%) | 3 (10) | 8 (27) | 2 (7) | 3 (10) | 0.093 |
| eGFR (ml/dk) | 65.9±8.4a,b,c | 37.1±6.2a,d,e | 23.1±5.5b,d,f | 7.6±3.2c,e,f | <0.001 |
| Glucose (mg/dl) | 113±33 | 118±41 | 115±39 | 142±68 | 0.068 |
| Creatinine (mg/dl) | 1.03±0.1a,b | 1.7±0.2c | 2.6±0.7a,d | 7.5±3.1b,c,d | <0.001 |
| Sodium (mEq/l) | 139±2 | 138±2 | 133±22 | 136±3 | 0.164 |
| Potassium (mEq/l) | 4.5±0.5a,b | 4.8±0.5 | 4.8±0.4a | 4.9±0.7b | 0.005 |
| Calcium (mg/dl) | 9.7±0.5a,b | 9.6±0.6c,d | 9.2±0.5a,c,e | 8.5±0.8b,d,e | <0.001 |
| Phosphorus (mg/dl) | 3.7±0.7a | 3.6±0.7b | 4.1±0.7c | 4.8±1.1a,b,c | <0.001 |
| Uric acid (mg/dl) | 6.0±1.9 | 6.8±1.6 | 6.4±1.4 | 5.9±1.6 | 0.197 |
| Albumin (g/dl) | 4.3±0.3a,b | 4.3±0.8c,d | 3.8±0.4a,c | 3.7±0.5b,d | <0.001 |
| ALT (U/l) | 19±11 | 19±18 | 17±10 | 19±15 | 0.940 |
| AST (U/l) | 21±8 | 22±10 | 20±10 | 19±13 | 0.748 |
| Triglyceride (mg/dl) | 166±68 | 177±95 | 144±71 | 171±89 | 0.436 |
| LDL-C (mg/dl) | 124±45a | 103±37 | 96±30a | 98±42 | 0.026 |
| HDL-C (mg/dl) | 48±10 | 44±13 | 43±12 | 41±11 | 0.104 |
| Hemoglobin (g/dl) | 13.6±1.8a,b | 13.5±1.4c,d | 11.5±1.6a,c | 11.1±1.7b,d | <0.001 |
| WBC (×103/μl) | 8.1±3.4 | 7.8±1.6 | 7.8±2.2 | 8.2±2.2 | 0.877 |
| PLT (×103/μl) | 245±51a | 216±65 | 232±61 | 204±56a | 0.040 |
| CRP (mg/dl) | 4.7±1.7a | 4.7±2.1b | 8.9±7.7a,b | 6.8±4.3 | 0.001 |
| iPTH (pg/ml) | 65±46a,b | 97±51 | 153±102a | 323±216b | <0.001 |
| 25(OH)D (ng/ml) | 13.5±8.4 | 15.3±9.5 | 9.1±5.7 | 11.8±13.4 | 0.084 |
| Upr/Ucr | 0.2±0.2a,b | 0.8±0.9c | 1.4±1.2a,d | 3.4±2.4b,c,d | <0.001 |
| Hypertension, n (%) | 12 (40)a,b | 19 (63) | 24 (80)a | 23 (77)b | 0.005 |
| DM, n (%) | 7 (23) | 9 (30) | 7 (23) | 12 (40) | 0.444 |
| CAD, n (%) | 0 (0) | 6 (20) | 5 (17) | 3 (10) | 0.081 |
| Heart failure, n (%) | 0 (0) | 1 (3) | 3 (10) | 3 (10) | 0.255 |
CKD: chronic kidney disease; BMI: body mass index; eGFR: estimated glomerular filtration rate; ALT: alanine aminotransferase; AST: aspartate aminotransferase; LDL-C: low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; WBC: white blood cell; PLT: platelet; CRP: C-reactive protein; iPTH: intact parathyroid hormone; 25(OH)D: 25-hydroxy vitamin D; Upr/Ucr: protein/creatinine ratio in spot urine; DM: diabetes mellitus; CAD: coronary artery disease. Data are indicated as mean±standard deviation or percent. a,b,c,d,eThere is a significant difference between the parameters marked with the same letter (p<0.05). For the comparison between groups, analysis of variance (ANOVA) or Kruskal–Wallis test was applied, and for the post hoc study Tukey HSD test was used.
In CKD stage 5D patients, serum Ca (8.5±0.8mg/dl), serum albumin (3.7±0.5g/dl) and hemoglobin levels (11.1±1.7g/dl) and eGFR (7.6±3.2ml/min.) were significantly lower (p<0.001) while serum Cr (7.5±3.1mg/dl), serum P (4.8±1.1mg/dl), iPTH (323±216pg/ml) levels and Upr/Ucr ratio (3.4±2.4) were significantly higher (p<0.001). Serum LDL-C levels were found highest in CKD stage 2 patients (124±45mg/dl) (p=0.026) (Table 1).
Comorbidity rates for CKD patients were, hypertension 64.2%, diabetes mellitus 29.2%, coronary artery disease 11.7% and heart failure 5.8%. Hypertension was most prevalent in CKD stage 4 patients (Table 1).
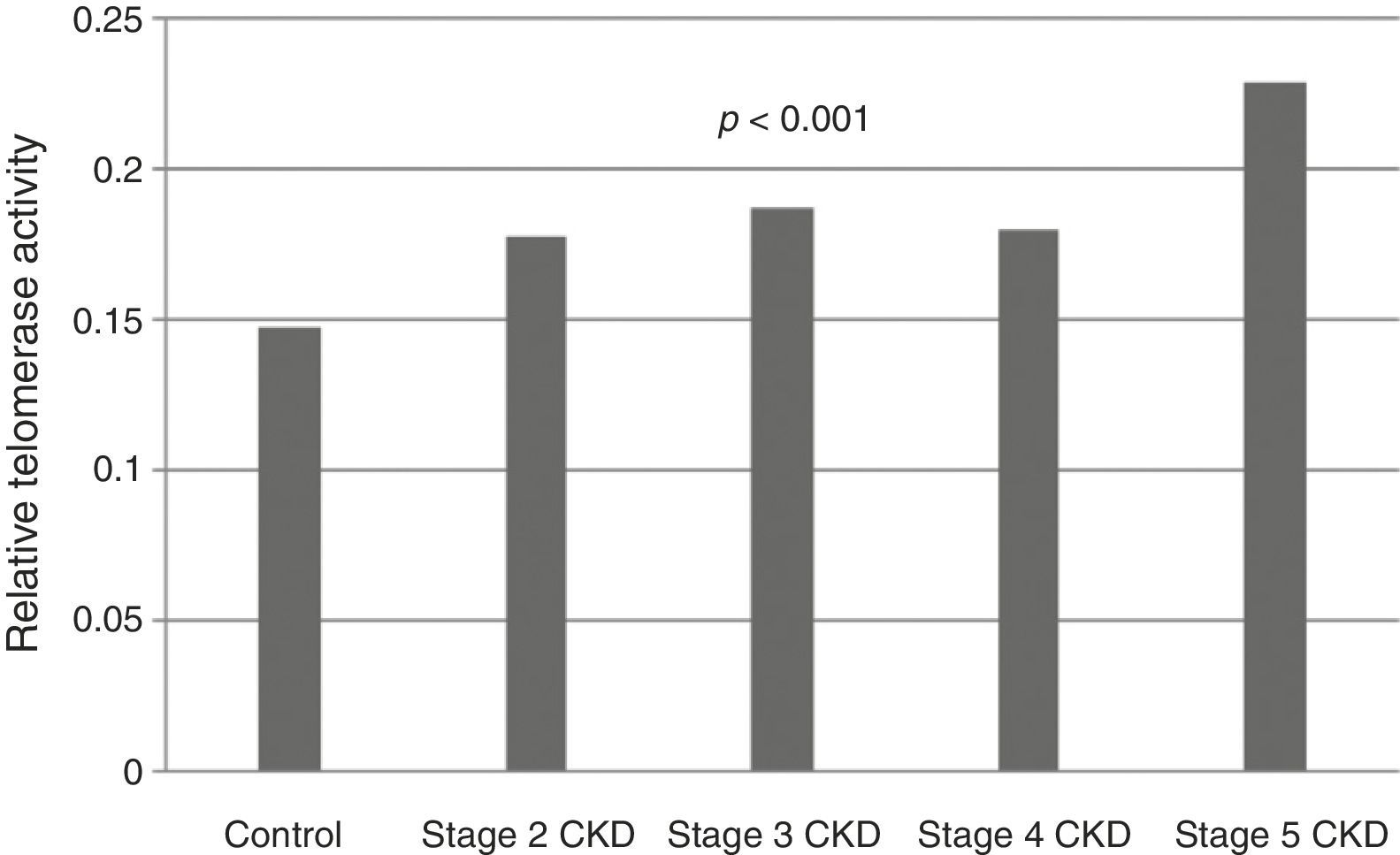
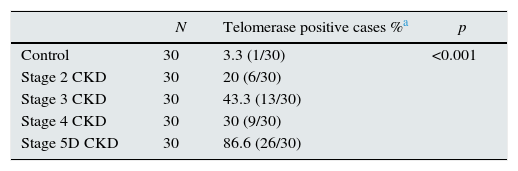
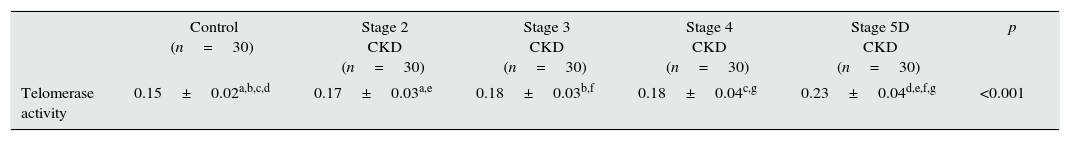
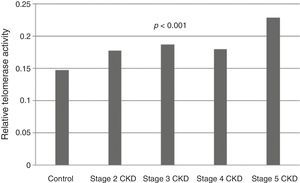
Telomerase activity in PBMCTelomerase activity in PBMC was positive in 86% (26/30) of CKD stage 5D patients while healthy control group was only 3% positive (1/30). Telomerase activity was increased with CKD stage (Table 2) (p<0.001). A significant difference was found for telomerase activity between patient and control groups (p<0.001) (Fig. 1). The levels were lowest in the healthy control group (0.15±0.02) and highest in CKD stage 5 group (0.23±0.04). Telomerase activities in PBMC were similar in CKD stages 2, 3 and 4 (p>0.05) while in stage 5 CKD it was significantly higher compared to the other stages (p<0.05). It was significantly lower in the healthy control group compared to all other groups (p<0.05) (Table 3).
Group comparisons for telomerase activity positivity.
| N | Telomerase positive cases %a | p | |
|---|---|---|---|
| Control | 30 | 3.3 (1/30) | <0.001 |
| Stage 2 CKD | 30 | 20 (6/30) | |
| Stage 3 CKD | 30 | 43.3 (13/30) | |
| Stage 4 CKD | 30 | 30 (9/30) | |
| Stage 5D CKD | 30 | 86.6 (26/30) |
CKD: chronic kidney disease. Chi-square test was applied for the comparison between groups.
Group comparisons for telomerase activity.
| Control (n=30) | Stage 2 CKD (n=30) | Stage 3 CKD (n=30) | Stage 4 CKD (n=30) | Stage 5D CKD (n=30) | p | |
|---|---|---|---|---|---|---|
| Telomerase activity | 0.15±0.02a,b,c,d | 0.17±0.03a,e | 0.18±0.03b,f | 0.18±0.04c,g | 0.23±0.04d,e,f,g | <0.001 |
CKD: chronic kidney disease. a,b,c,d,e,f,gData are indicated as mean±standard deviation. There is a significant difference between the parameters marked with the same letter (p<0.05). For the comparison between groups, analysis of variance (ANOVA) was applied, and for the post hoc study Tukey HSD test was used.
Dialysis patients were grouped according to dialysis duration (median duration 20 months); among the 30 patients dialysis duration was long (range 39–120 months) in 6 of the patients and short (range 4–38 months) in 24 of the patients. Telomerase activity in PBMC was similar in both subgroups (0.24±0.03, 0.23±0.03; p>0.05).
In CKD patients, telomerase activity in PBMC was positively correlated with CKD stage (r=0.412, p<0.001) serum Cr (r=0.404, p<0.001), serum K (r=0.189, p=0.038) and iPTH levels (r=0.245, p=0.007) and; negatively correlated with BMI (r=−0.248, p=0.006), eGFR (r=−0.407, p<0.001), platelet count (r=−0.252, p=0.006), LDL-C (r=−0.243, p<0.007) and serum Ca levels (r=−0.357, p<0.001). A positive correlation was found between telomerase activity and age in healthy controls (r=0.623, p<0.001).
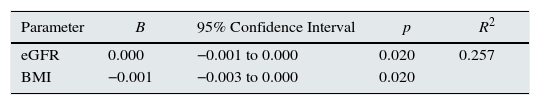
Telomerase activity in CKD patients and dependent variables age, BMI, eGFR, platelet count, serum Ca, LDL-C, iPTH and K levels were evaluated by linear regression analysis. Only eGFR and BMI were found to be independent predictors for high telomerase activity (R2=0.257, β=0.001 for eGFR, CI=−0.001 to 0.000, p=0.020; β=−0.001 for BMI, CI=−0.003 to 0.000, p=0.020) (Table 4).
Linear regression analysis of factors associated with telomerase activity in CKD patients.
| Parameter | B | 95% Confidence Interval | p | R2 |
|---|---|---|---|---|
| eGFR | 0.000 | −0.001 to 0.000 | 0.020 | 0.257 |
| BMI | −0.001 | −0.003 to 0.000 | 0.020 |
eGFR: estimated glomerular filtration rate; BMI: body mass index. Dependent variables included in the analysis were age, BMI, eGFR, platelet count, Ca, LDL-C, iPTH and K levels.
To our knowledge, unlike the previous studies, the current study is the first to report increased telomerase activity in PBMC in CKD patients. In this study, telomerase activity in PBMC was found significantly increased in CKD patients compared to healthy controls. This increase was found independent of age, gender, inflammation, hyperlipidemia and hyperparathyroidism but it was correlated with eGFR and BMI.
Telomere length was reported to decrease independent of age and gender in hemodialysis patients.7,8 In renal cells of cats with CKD, increased cellular aging and short telomeres were detected accompanied by normal telomerase activity.15 Tsirpanlis et al. reported that telomerase activity in PBMC was lower in hemodialysis patients compared to the healthy control group.11 In contrast with this study, telomerase activity in PBMC was found increased in our study. Despite being an unexpected result, higher prevalence of diabetes in CKD stage 5D patients in our study might be the reason for high detected telomerase activity considering high telomerase activity reported in patients with metabolic syndrome in previous studies.16 Additionally, insignificantly lower BMI in CKD stage 5D patients might have contributed to the detected high telomerase activity in this subgroup. Thus, previous studies have reported that obesity associated with telomere shortening.17–19
Telomerase activity was reported to decrease with age.20 Although no correlation was found between age and telomerase activity in CKD patients, a positive correlation was detected between age and telomerase activity in the healthy controls in our study. This may be associated with other factors other than age that influence telomerase activity.
The combination of high telomerase activity and short telomere was suggested to be a sign of activated cell stress.21 Epel et al. in their study on caregivers of dementia patients found increased telomerase activity in PBMC accompanied by increased serum cortisol levels related to acute psychological stress.22 Lack of acute stress and serum cortisol levels assessments is a limitation of this study. Further studies may clarify this issue.
Telomerase activity in PBMC was reported to be potentially associated with inflammation.23 In cell cultures, increased telomerase activity in macrophages was shown in response to inflammatory stimulants.24 In the study by Rentoukas et al. telomerase activity in PBMC, IL6, TNFα and asymmetric dimethylarginine (ADMA) levels were found significantly higher in metabolic syndrome patients compared to healthy controls. Additionally, a positive correlation was observed between telomerase activity and ADMA levels while no correlation was found between telomerase activity and IL6 or TNFα.16 In this study, CRP was the only evaluated inflammatory marker. A significant relation was not found between telomerase activity in PBMC and CRP. However, this result may be due to the fact that this study excluded patients with acute infection.
Lin et al. measured telomerase activity and telomere length in various subtypes of T and B cells (CD4+, CD8+CD28+ and CD8+CD28− T and B cells). They found the longest telomere and highest telomerase activity was in B cells, while shortest telomere and lowest telomerase activity was in CD8+CD28− cells.25 In patients with unstable angina, telomerase activity in polymorphonuclear neutrophils in aterosclerotic plaques was found significantly higher compared to circulating neutrophils and this was suggested to be related to activation of inflammatory cells in early stage of instability and their locally prolonged survival.26 In our study, telomerase activity assessment in T and B subtypes was lacking since subtype analysis for PBMC was not performed. However, accelerated atherosclerosis and chronic micro-inflammation can be speculated as contributing factors to the findings in this study. Further studies will set light to this issue.
Obesity was reported to accelerate cellular aging and was found associated with telomere shortening.17–19 In our study, high BMI was associated with low telomerase activity in CKD patients. Telomerase activity levels were lower in obese patients. This result supports the previous observations suggesting obesity is related with telomere shortening in CKD patients.
Telomerase activity in PBMC was found negatively correlated with oxidized LDL-C.27 Statin treatment was associated with high telomerase activity and long telomeres, independent from age, lipid levels and inflammation.28 In our study, unlike previous studies, telomerase activity in PBMC was not associated with LDL-C. This result may be due to the fact that 1/5 of our patients were on statins.
Cross-sectional design and relatively small number of patients and controls are the other limitations of our study.
In conclusion; to our knowledge, this study is important for being the first to evaluate the relation between CKD stages and telomerase activity in PBMC. Based on the results of our study, it can be concluded that telomerase activity in PBMC is increased in CKD patients compared to healthy individuals. Telomerase activity in PBMC is increased with advancing stage in CKD patients and particularly in end-stage renal failure. Confirmation of this result in further studies may open new horizons in prevention of biological aging.
Author contributionsConcept – V.K.; design – V.K., AY.A.; supervision – M.T.S., B.A.; resource – V.K., AY.A.; materials – V.K., AY.A.; data collection and/or processing – V.K., AY.A., AT.A., S.I.; analysis and/or interpretation – V.K., S.I.; literature search – V.K.; writing – V.K.; critical reviews – M.T.S.
Conflict of interestThe authors declare that they have no conflict of interest.
This study was presented at the ASN Kidney Week 2015, 3–8 November 2015, San Diego, USA.