Bone disease related to chronic kidney disease and, particularly, to kidney transplant patients is a common cause or morbidity and mortality, especially due to a higher risk of osteoporotic fractures. Despite the fact that this has been known for decades, to date, an appropriate diagnostic strategy has yet to be established. Apart from bone biopsy, which is invasive and scarcely used, no other technique is available to accurately establish the risk of fracture in kidney patients. Techniques applied to the general population, such as bone densitometry, have not been subjected to sufficient external validation and their use is not systematic. This means that the identification of patients at risk of fracture and therefore those who are candidates for preventive strategies is an unmet need.
Bone strength, defined as the ability of the bone to resist fracture, is determined by bone mineral density (measured by bone densitometry), trabecular architecture and bone tissue quality. The trabecular bone score estimates bone microarchitecture, and low values have been described as an independent predictor of increased fracture risk. Bone microindentation is a minimally invasive technique that measures resistance of the bone to micro-cracks (microscopic separation of mineralized collagen fibers), and therefore bone tissue biomechanical properties. The superiority over bone densitometry of the correlation between the parameters measured by trabecular bone score and microindentation with the risk of fracture in diverse populations led us to test its feasibility in chronic kidney disease and kidney transplant patients.
La enfermedad ósea asociada a la enfermedad renal crónica, y en particular en el paciente trasplantado renal, representa una causa de frecuente morbimortalidad, sobre todo porque predispone a un mayor riesgo de fractura osteoporótica. Este hecho, bien conocido desde hace décadas, no ha estimulado lo suficiente hasta la fecha el desarrollo de una adecuada estrategia diagnóstica. Si dejamos aparte la biopsia ósea, técnica invasiva y escasamente utilizada, no disponemos de herramientas capaces de estimar de manera precisa el riesgo de fractura en el paciente renal. La escasa validación externa de técnicas aplicadas en la población general como la densitometría ósea hace que su uso tampoco sea sistemático. Por tanto, la identificación de qué pacientes tienen mayor riesgo de fractura y son susceptibles de intervención preventiva es una necesidad no cubierta.
La resistencia ósea, definida como la capacidad del hueso para resistir la fractura, viene determinada por la cantidad de material mineral (medida como densidad mineral ósea por densitometría ósea), la arquitectura trabecular y la calidad del tejido óseo. El score trabecular óseo estima la microarquitectura ósea y valores bajos se han demostrado como predictores independientes de mayor riesgo de fractura. La microindentación ósea es una técnica mínimamente invasiva capaz de medir la resistencia ósea que el hueso opone a la apertura de micro-cracks (separación microscópica de fibras de colágena mineralizada), y con ello, las propiedades biomecánicas del tejido óseo. La buena correlación con el riesgo de fractura de los parámetros medidos con el score trabecular óseo o la microindentación en diversas poblaciones, superior a la propia densitometría ósea, nos ha estimulado a desarrollar su potencial aplicación en los pacientes con enfermedad renal crónica y trasplantados renales.
Achieving bone health in the population with chronic kidney disease (CKD) is a unresolved issue. The guidelines suggest that bone health should be evaluated using spine Z-ray for screening of asymptomatic vertebral fractures or bone densitometry (dual X-ray absorptiometry, DXA) to determine of bone mineral density (BMD).1–3 However, BMD is not the only feature that makes the bone able to absorb the energy of an impact and prevent a bone fracture. Other properties such as bone microarchitecture or the quality of bone tissue are determinants of bone resistance to fracture. Furthermore, although there is a good correlation between BMD and the risk of fracture in the general population, the validation of DXA as a reference diagnostic technique in the renal population has not been fully established, so it is not performed as a routine in clinical practice.4
We have reviewed the various diagnostic strategies to evaluate bone health in renal patients, with special interest in the kidney transplant (KT) recipient.
Justification: bone disease and fractures after kidney transplantationThe bone mineral disease in CKD patients is characterized by abnormalities in bone turnover, mineralization and bone density, which cause bone fragility and an increased risk of fractures.2 In the transplant patient, bone disease has its own peculiarities that are associated to the recovery of renal function, persistent hyperparathyroidism5 and immunosuppressive treatment which is inherent in the transplant process.6
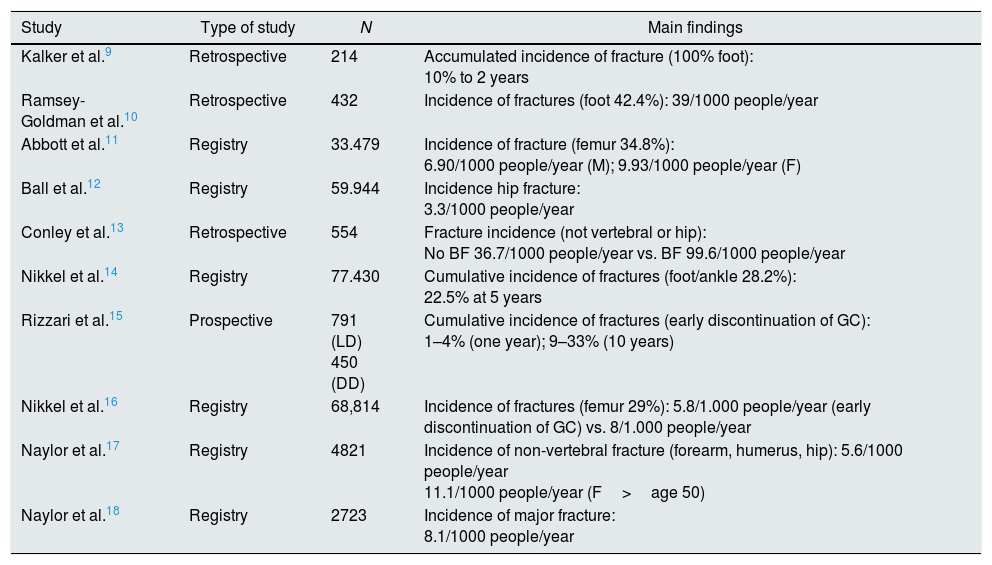
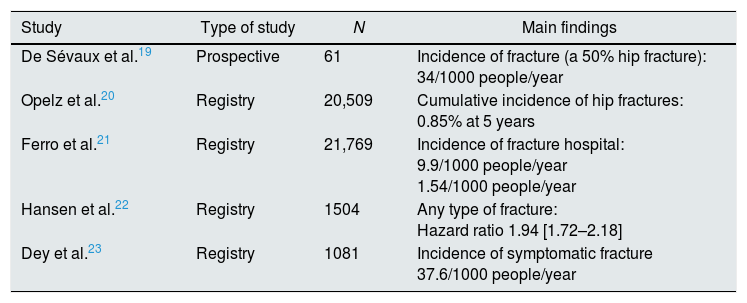
The main alteration in bone remodeling after renal transplantation is the reduction in bone formation and mineralization, with a persistent predominance of resorption. In fact, during the first 6 months after transplantation, there is a rapid decrease in BMD,7 which is subsequently attenuated,8 most likely related to the decrease in the dose of steroids. As a result, a high risk of fracture has been described in patients after KT (Tables 1 and 2),9–23 which, initially, is even higher than that in dialysis patients.12 In addition, it has been proven that this increase in risk persists in the late post-transplant period.17
Main studies describing the risk of fracture in kidney transplant patients in the EE UU and Canada.
| Study | Type of study | N | Main findings |
|---|---|---|---|
| Kalker et al.9 | Retrospective | 214 | Accumulated incidence of fracture (100% foot): 10% to 2 years |
| Ramsey-Goldman et al.10 | Retrospective | 432 | Incidence of fractures (foot 42.4%): 39/1000 people/year |
| Abbott et al.11 | Registry | 33.479 | Incidence of fracture (femur 34.8%): 6.90/1000 people/year (M); 9.93/1000 people/year (F) |
| Ball et al.12 | Registry | 59.944 | Incidence hip fracture: 3.3/1000 people/year |
| Conley et al.13 | Retrospective | 554 | Fracture incidence (not vertebral or hip): No BF 36.7/1000 people/year vs. BF 99.6/1000 people/year |
| Nikkel et al.14 | Registry | 77.430 | Cumulative incidence of fractures (foot/ankle 28.2%): 22.5% at 5 years |
| Rizzari et al.15 | Prospective | 791 (LD) 450 (DD) | Cumulative incidence of fractures (early discontinuation of GC): 1–4% (one year); 9–33% (10 years) |
| Nikkel et al.16 | Registry | 68,814 | Incidence of fractures (femur 29%): 5.8/1.000 people/year (early discontinuation of GC) vs. 8/1.000 people/year |
| Naylor et al.17 | Registry | 4821 | Incidence of non-vertebral fracture (forearm, humerus, hip): 5.6/1000 people/year 11.1/1000 people/year (F>age 50) |
| Naylor et al.18 | Registry | 2723 | Incidence of major fracture: 8.1/1000 people/year |
BF: bisphosphonates; DD: deceased donor; LD: living donor; EE UU: United States; GC: glucocorticoids; M: male; F: female.
Main studies describing the risk of fracture in kidney transplant patients in European population.
| Study | Type of study | N | Main findings |
|---|---|---|---|
| De Sévaux et al.19 | Prospective | 61 | Incidence of fracture (a 50% hip fracture): 34/1000 people/year |
| Opelz et al.20 | Registry | 20,509 | Cumulative incidence of hip fractures: 0.85% at 5 years |
| Ferro et al.21 | Registry | 21,769 | Incidence of fracture hospital: 9.9/1000 people/year 1.54/1000 people/year |
| Hansen et al.22 | Registry | 1504 | Any type of fracture: Hazard ratio 1.94 [1.72–2.18] |
| Dey et al.23 | Registry | 1081 | Incidence of symptomatic fracture 37.6/1000 people/year |
Evaluation of bone health in the renal patient is key to estimate the risk of fracture. Laboratory measurements such as bone remodeling markers, or invasive procedures such as bone biopsy are available for assessment of bone health, but it is not clear which diagnostic tests best predict the risk of fracture. Unlike in the general population, risk scales or DXA have a much more limited predictive power.
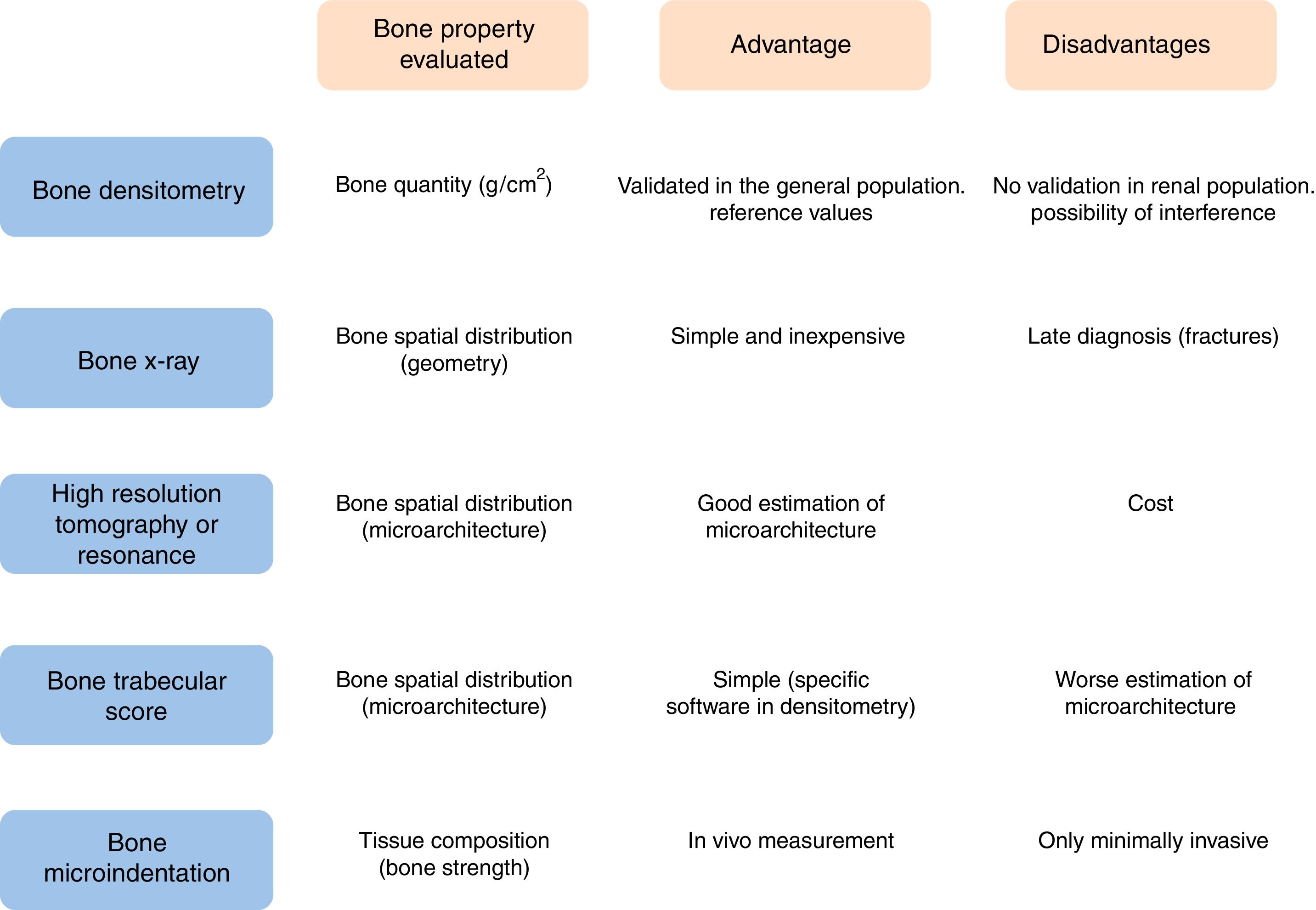
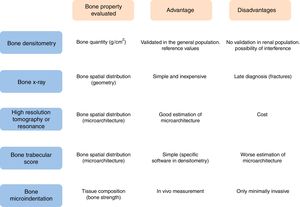
To establish the accurate diagnosis of bone disease, it is necessary to understand the different properties contributing bone resistance. Bone quantity is determined by BMD and expressed as grams of mineral per bone area (or volume if measured by computed tomography). In a given individual, it is determined by the maximum bone mass achieved and the rate of bone loss. Other aspects of bone quality also contribute to the bone mechanical strength: the spatial distribution of bone at the macroscopic (geometry) and microscopic (microarchitecture) level, and the composition of the bone tissue itself. The spatial distribution of bone can be determined by different imaging techniques, from simple radiography to high-resolution tomography or magnetic resonance.24 The higher the image resolution, the better the estimation of microscopic bone structure. In addition, the physical, chemical and biological components of the bone tissue are equally relevant for bone strength and mechanical performance of the bone, including characteristics of collagen,25 degree and homogenization of mineralization26 and non-collagenous proteins.27
There are different techniques that provide information on the different bone properties that help to make the diagnosis of bone disease after kidney transplantation.
Bone X-ray can identify lesions that are characteristic of renal osteodystrophy, but these changes occur very late. Its main diagnostic value is the ability to detect asymptomatic vertebral fractures, a fact that predisposes to suffer a osteoporotic fracture of greater magnitude. This helps to select a population with a higher risk of fracture.
Among CKD patients it is not common to use the Fracture Risk Assessment Tool (FRAX), which estimates the probability at 10 years of major osteoporotic fracture (clinical vertebral, hip, forearm and humerus) applying an algorithm based on age, gender and clinical factors (with or without BMD).28 Therefore the predictive capacity of FRAX in this population is unknown. In a recent study, the FRAX score was applied to KT patients with a mean of 1.1 years after transplantation. Most of them were categorized as having a “low risk” of fracture at 10 years, showing a modest predictive power of the score since 4.6% of patients had a fracture at 10 years.29
DXA is currently the standard method to determine BMD in the general population and is used for screening for osteoporosis in CKD patients. It provides information about the total amount of mineral in the scanned bone area – usually the neck of the femur and the vertebrae – although it does not distinguish changes in bone turnover or characteristics of the bone matrix. In patients with CKD, tissue or vascular calcifications may interfere with bone measurements and give falsely elevated values. Likewise, post-transplant calcium mobilization could lower BMD values, without actually changing the bone mineral content.
In patients with CKD stages 3–5D, it is not recommended the routine performance of DXA for determination of BMD, due to its low predictive power of fractures and its inability to diagnose the type of renal osteodystrophy.2 In CKD patients, lumbar or hip BMD can be misleading and may lead to inadequate administration of antiresorptive drugs. The best location for BMD measurement is the distal radius.4 Even with these limitations, renal patients with lower BMD at the lumbar and femoral neck have the highest incidence of fractures.30
However, DXA cannot detect other bone properties of bone strength that are relevant to predict the risk of fracture, such as bone trabecular microarchitecture31 or other mechanical properties such as elasticity, trabecular spatial arrangement or the quality of the collagen matrix that are, also determinants of bone resistance.4
The bone biopsy the gold-standard diagnostic test to establish the patients bone health, but it is invasive, laborious and expensive, consequently it is used very infrequently in clinical practice. Thus given that DXA is not able to fully detect bone resistance to fractures, additional diagnostic tests are needed to establish the risk of fracture. Three diagnostic tests have emerged in recent years:
- -
High resolution peripheral quantitative computer tomography, used in patients with CKD,32 or KT33 measures the volumetric density of the cortical and trabecular region separately, and has a resolution that allows analyzing the bone microarchitecture. However, it is expensive and so far not very accessible in usual practice.
- -
The trabecular bone score (TBS), analyzes the trabecular bone microarchitecture in the lumbar spine using specific software in images obtained with DXA.31 Abnormal trabecular microarchitecture at the vertebral level has been associated with an increased risk of fracture.31 Lower TBS values have been described in the hemodialysis population34 and in Kidney transplant patients – in the early post-transplant – with respect to the general population,35 with the risk of fracture that this entails.
- -
Bone microindentation is a technique capable of directly measuring mechanical properties of bone at the tissue level. This was first described in a clinical series in 2010.36 It is based on microscopic indentation, which measures the resistance of the cortical bone to the opening of microscopic fractures or micro-fractures, a phenomenon intimately linked to the beginning of the solution of continuity in the bone that gives rise to the fracture. To date, 2 main techniques have been developed: cyclical microindentation and impact microindentation. Both are included in the generic term of reference point indentation and are based on the principle that the deeper a needle penetrates the anterior face of the tibia, the less resistant the bone tissue will be to a mechanic impact. The cyclic microindentation was used in the first studies including patients36; the study by impact microindentation, simplifies the method of measurement37–39 and makes it preferable for clinical use. The procedure is simple. It is performed on the anterior face of the tibia with an indenting handheld device, Osteoprobe® (Active Life Scientific, Santa Barbara, CA, USA). After administering local anesthesia at the puncture site, a preload of 10N of force followed by an indentation of 30N using a conical needle of 4μm is applied. The mean of 8 indentation values is transformed by a computerized algorithm. Then, 5 calibration indentations are made in a block of polymethyl methacrylate. The ratio between the value provided by the bone and by the block provides the final parameter, the bone mineral resistance index, which is expressed in absolute units.
The propensity to bone fractures is the result of the deterioration of BMD, microarchitecture and bone tissue properties, which can occur individually or in combination and in different proportions depending on each pathophysiological situation. Therefore, for a complete calculation we must contemplate the 3 components.
Impact microindentation has been applied in the study of other patient populations in which the risk of fracture can only be partially established with DXA.37–39 There are situations, such as in elderly women, where microindentation does not seem to add value to the prediction of risk of fracture,40 perhaps because it is a population in which BMD and microarchitecture, are already very deteriorated playing a predominant role in the loss of bone strength. By contrast, in women with type 2 diabetes, where both BMD and trabecular microarchitecture are preserved while the risk of fracture is clearly high, the bone mineral resistance index was the most abnormal component of bone strength.39 Therefore, microindentation can be a complement to the existing bone analysis methods, particularly in populations where BMD does not satisfactorily explain the propensity to fracture.
The best method to estimate the risk of fracture in a patient with CKD is unknown. An improvement of the diagnostic methods to determine the risk of fracture is crucial as a first step for the indication of any preventive treatment (Fig. 1).
Bone health in the long-term kidney transplant patientIn our group, we aimed to analyze the bone health of a cohort of long term kidney transplant patients, taking into account different bone properties that contribute to bone strength.41 So we performed a case–control study, in KT patients of more than 10 years of follow up and healthy controls without kidney disease or any relevant factor that may affect bone health. The transplant patients had a lower BMD than healthy controls. However, the trabecular microarchitecture estimated by TBS and the bone tissue quality measured by microindentation were comparable in both groups.
In renal transplant patients, the TBS is lower than in the general population.35 However, this study analyzed TBS in the early post-transplant, this is in contrast with our KT patients of more than 10 years of evolution that showed values of TBS similar to controls suggesting a recuperation of bone health after years post transplantation.
Additionally, we performed bone microindentation tests for the first time in subjects with kidney disease and our patients presented bone strength values similar to those of healthy controls. This indicates that the bone affectation in this transplanted population of long evolution is no longer very relevant since values of bone quality of these receptors are similar to the general population. In addition, these results are in agreement with studies showing that bone changes in the post-transplant period are temporary and revert with a fast reduction or suspension of glucocorticoids.16,42 In this regard, it is noteworthy that 80% of our cohort did not have glucocorticoids as a base immunosuppressive treatment at the time of the study.
Therefore, in general terms, the results obtained showed bone normalization in the late post-transplant, despite the fact that the BMD values were lower than those of the healthy controls. As far as we know, this is the first study that assesses bone health in KT, taking into account all its different components: bone quantity, trabecular microstructure and bone tissue quality. Therefore this is the first study performed in renal patients which includes measurement of bone quality by means of microindentation which evaluates mechanical properties of the bone providing additional information about bone resistance using a method that is simple and clinically viable. However, longitudinal studies with an appropriate number of patients will be necessary to determine the value of bone microindentation to predict the risk of fracture in this population, and to define the value of microindentation to monitor bone mineral resistance index in response to different therapeutic strategies.
ConclusionsThe predisposition to fracture is determined by bone resistance which results from several bone properties that complement each other and can be evaluated separately with different diagnostic tests. To estimate risk fracture adequately, both bone quantity and quality must be evaluated. Among renal patients, those with kidney transplant require complete evaluation of bone characteristics so optimal preventive intervention is applied. Results from TBS and bone microindentation have been associated with an increased risk of fractures in other populations, although additional prospective studies including large number of patients and longer follow-up will confirm the clinical usefulness of these techniques and their ability to predict bone fractures.
Key concepts
- •
Kidney transplant patients have a higher risk of fractures than the general population.
- •
Bone resistance involves various bone properties such as the amount of mineral, architecture and bone tissue quality.
- •
It is unknown whether techniques used to detect risk of fracture, such as bone densitometry, have the same validity in the transplant population.
- •
Other techniques, such as the bone trabecular score or bone microindentation, are proposed to complement a comprehensive bone evaluation of our patients.
The DPA institution has received funds for research scholarships from AMGEN and BIOIBERICA. ADP is a shareholder of Active Life Scientific. The rest of the authors declare no conflict of interests.
This work has been in part sponsored by a Research Aid of the Spanish Society of Nephrology. JP is a researcher of the FIS-FEDER projects PI13/00598 and PI16/00619 (Carlos III Health Institute) and coordinates a group within REDinREN (RD16/0009/0013). The microindentation technique is financed in part by CIBERFES, Instituto Carlos III (FEDER funds).
Please cite this article as: Pérez-Sáez MJ, Prieto-Alhambra D, Díez-Pérez A, Pascual J. Avances en la valoración de la salud ósea en el trasplantado renal. Nefrologia. 2018;38:27–33.