Haemoglobin and myoglobin are haem proteins that play a key role as they help transport oxygen around the body. However, because of their chemical structure, these molecules can exert harmful effects when they are released massively into the bloodstream, as reported in certain pathological conditions associated with rhabdomyolysis or intravascular haemolysis. Once in the plasma, these haem proteins can be filtered and can accumulate in the kidney, where they become cytotoxic, particularly for the tubular epithelium, inducing acute kidney failure and chronic kidney disease. In this review, we will analyse the different pathological contexts that lead to the renal accumulation of these haem proteins, their relation to both acute and chronic loss of renal function, the pathophysiological mechanisms that cause adverse effects and the defence systems that counteract such actions. Finally, we will describe the different treatments currently used and present new therapeutic options based on the identification of new cellular and molecular targets, with particular emphasis on the numerous clinical trials that are currently ongoing.
La hemoglobina y la mioglobina son hemoproteínas que juegan un papel fundamental en el organismo ya que participan en el transporte de oxígeno. Sin embargo, debido a su estructura química, estas moléculas pueden ejercer efectos deletéreos cuando se liberan al torrente sanguíneo de forma masiva, como sucede en determinadas condiciones patológicas asociadas a rabdomiólisis o hemólisis intravascular. Una vez en el plasma, estas hemoproteínas se pueden filtrar y acumular en el riñón, donde resultan citotóxicas, principalmente para el epitelio tubular, e inducen fracaso renal agudo y enfermedad renal crónica. En la presente revisión analizaremos los distintos contextos patológicos que provocan la acumulación renal de estas hemoproteínas, su relación con la pérdida de función renal a corto y largo plazo, los mecanismos fisiopatólogicos responsables de sus efectos adversos y los sistemas de defensa que contrarrestan tales acciones. Por último, describiremos los distintos tratamientos utilizados actualmente y mostraremos nuevas opciones terapéuticas basadas en la identificación de nuevas dianas celulares y moleculares, prestando especial atención a los diversos ensayos clínicos que se encuentran en marcha en la actualidad.
Haemoglobin (Hb) and myoglobin (Mb) are haemoproteins that play a crucial role in the body's homeostasis, by oxygenating tissues and participating in the regulation of blood pH levels. Hb has a molecular weight of 64.5kDa and is composed of four polypeptide chains known as globins.1 Each globin contains a haem group with an iron atom in its interior, which is responsible for its functional properties. Mb is a smaller protein with a molecular weight of 17kDa, which is formed by a single globin. In physiological conditions, both Hb and Mb are found inside erythrocytes and muscle cells, respectively. However, in certain pathological conditions, these molecules are released into the blood stream and may enter and accumulate in the kidneys, where they are cytotoxic, especially for the proximal tubule epithelium. In fact, the renal accumulation of haemoproteins may induce acute kidney injury (AKI) and chronic kidney disease (CKD). In recent years, new mechanisms have been identified which are involved in kidney damage linked to these molecules, which have helped to develop experimental treatments that have already yielded positive results in recently published studies or in ongoing clinical trials, as described in more detail below.
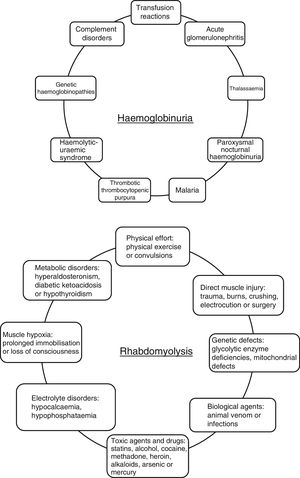
Origin of the renal accumulation of haemoproteinsMb builds up in the kidneys as a result of severe muscular damage (rhabdomyolysis), whereas Hb accumulates due to the intravascular haemolysis of red blood cells or the rupture of red blood cells that cross the glomerular membrane in diseases with glomerular haematuria, such as IgA nephropathy (IgAN), lupus or Alport syndrome. In this review, we will focus on myoglobinuria and haemoglobinuria due to space limitations.
MyoglobinuriaMyoglobinuria is the presence of Mb in urine, for which the main cause is rhabdomyolysis or the rupture of skeletal muscle.2 Rhabdomyolysis may be caused by severe trauma, situations of prolonged ischaemia, metabolic disorders, intense physical activity, alcohol abuse and some toxic compounds of chemical or biological origin3 (Fig. 1). The incidence of rhabdomyolysis is not entirely clear, but it has been estimated that it could affect 7–10% of patients presenting with an AKI.3,4
HaemoglobinuriaHaemoglobinuria is the presence of Hb in urine as a result of intravascular haemolysis. This causes a renal overload of Hb, especially when there is recurring exposure to free Hb.5 Some of the main aetiological causes of haemoglobinuria include hereditary conditions, such as paroxysmal nocturnal haemoglobinuria, thrombotic thrombocytopenic purpura, haemolytic-uraemic syndrome (HUS), sickle-cell anaemia (SCA), cell membrane defects (elliptocytosis, spherocytosis, etc.), enzymatic defects (glucose-6-phosphate dehydrogenase deficiency, pyruvate kinase deficiency), severe haemolytic anaemia caused by massive transfusion reactions, as well as other causes acquired from HUS and thrombotic microangiopathies of various origins6 (Fig. 1).
Haemoproteins and acute kidney injuryAKI is a common complication in patients with haemoglobinuria or rhabdomyolysis, especially if they were already suffering from kidney disease. Up to 50% of patients suffering from rhabdomyolysis develop AKI, depending on what is the causes.7,8 Therefore, rhabdomyolysis is one of the main causes of AKI (5–25%) and results in death in 2–46% of cases in the absence of dialysis.3,4 On many occasions, situations associated with intravascular haemolysis may also induce AKI.9,10
Haemoproteins and chronic kidney diseaseThe onset of kidney disease in patients with renal accumulation of haemoproteins is well documented. It has been reported that haemoglobinuria is an independent risk factor for the onset and progression of CKD in people suffering with SCA.11 Something similar occurs in paroxysmal nocturnal haemoglobinuria, a disease in which CKD secondary to the onset of renal vein thrombosis and haemoglobinuria is one of its most significant complications, which may affect 64% of patients and cause 18% of deaths.12 In the absence of treatment, the prognosis for atypical HUS (aHUS) is also poor, with a mortality rate during outbreak of 25%, and progression to CKD in over half of the patients the year after the diagnosis.13
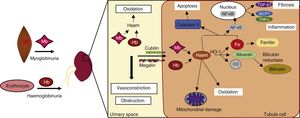
Pathophysiological mechanisms involved in haemoproteins induced renal damageThe main effect of haemoproteins on the kidneys is their direct tubule cell toxicity, regardless of what causes their release (haemo- or myoglobinuria) (Fig. 2). Under normal conditions, Hb binds to haptoglobin and forms the Hb–haptoglobin complex in the plasma.14 This complex is too large to be filtered by the glomerulus, and is therefore broken down by the spleen, bone marrow and liver. However, during intravascular haemolysis, the massive release of Hb causes haptoglobin to be consumed. As a result, Hb remains in the plasma for longer periods of time and is more likely to dissociate into dimers, which are more easily filtered by the glomerulus. Unlike Hb, Mb directly crosses the glomerular filtration membrane due to its smaller molecular size.
Once in the lumen of the tubule, the haemoproteins can be reabsorbed by the proximal tubules through the megalin/cubilin receptors complex,14 or even break down by releasing the haem group and free iron, which also have deleterious actions such as nitric oxide neutralisation, vasoconstriction and ischaemia.15 The reduced bioavailability of nitric oxide causes the deregulation of factors that control vascular tone, such as endothelin-1, thromboxane A2, tumour necrosis factor and isoprostanes.16,17 Hb and Mb are also powerful vasoconstrictors because they also react with nitric oxide, as described in diseases associated with intravascular haemolysis and rhabdomyolysis.18–20 When present in the lumen of the tubule, both Mb and Hb can precipitate and bind to the Tamm-Horsfall protein and give rise to RBC casts, which cause intratubular obstruction in the distal nephron segments.21 This obstruction is assisted by the acidic pH found in urine, which increases the stability of the links between the haemoproteins and the Tamm-Horsfall protein.22,23
Inside the tubule cells, the haemoproteins dissociate by releasing globins and the haem group, which induces oxidative stress, cell death and the production of inflammatory cytokines and fibrosis, as discussed in more detail below.
Oxidative stressHaemoproteins present various redox forms and are an endogenous source of reactive oxygen species.24 When haemoproteins are captured by tubule cells, the haem group is oxidised from Fe2+ to Fe3+ and produces hydroxyl radicals.25 In the presence of peroxides, Fe3+ oxidises to Fe4+ and generates hydroperoxyl radicals, which are highly reactive and contribute to the formation of new reactive oxygen species in the kidneys.26,27 All of these radicals promote the lipid peroxidation of plasma membranes and generate malondialdehyde, which intervenes in the oxidation of proteins and genetic material.4,28,29 This process leads to the production of isoprostanes, proinflammatory cytokines and the expression of adhesion molecules, which increases inflammatory response.30
InflammationThe haem group acts as a TLR-4 agonist and induces inflammatory response by activating the transcription factor NF-kB.31,32 After binding to the pattern recognition receptor, Hb promotes the activation of several signal transduction pathways such as c-Jun N-terminal, p38 and MAP kinases.33 Another involved pathway is mediated by the activation of the NLRP3 (nitrogen permease regulator-like 3) inflammasome, which is responsible for releasing different cytokines and chemokines involved in the monocyte/macrophage recruitment.34 The presence of proinflammatory macrophages (M1) has been reported in early phases in experimental models of AKI due to the accumulation of haemoproteins, which differ from anti-inflammatory macrophages (M2) in later phases.35,36 These M2 macrophages are found in renal biopsies of patients suffering from rhabdomyolysis, favism, paroxysmal nocturnal haemoglobinuria and outbreaks of macroscopic haematuria associated with IgAN.37–39
Cell deathThere have been reports of several types of cell death in the epithelial tubule of patients and in experimental models associated with the accumulation of haemoproteins.4,39–43 Necrosis and apoptosis are the types of death that have been studied at a greater depth.34,44–46 The molecular mechanisms causing death by apoptosis are associated with mitochondrial dysfunction and an increase in pro-apoptotic proteins (BAX and BAD), as well as the activation of caspase-3, the main effector caspase,34,47 and endoplasmic reticulum stress proteins.48 Other types of cell death have been described in these diseases, such as pyroptosis (cell death mediated by caspase-1 which leads to DNA fragmentation and cell lysis) and ferroptosis (iron-dependent cell death). Caspase-1 activation has been observed in experimental rat models of rhabdomyolysis,34 whereas the use of ferroptosis inhibitors in these rats reduced cell death of proximal tubules.49 Lastly, the accumulation of haemoproteins and their derivatives may induce autophagy as a defence mechanism.46,50,51
FibrosisRenal fibrosis is another mechanism involved in renal damage caused by haemoproteins. In fact, patients with SCA present with renal fibrosis and increased TGF-β in urine, which is one of the main profibrotic mediators.52 Even though fibroblasts and tubule cells play a very important role in the production of extracellular matrix proteins, recent studies show that macrophages may increase profibrotic response due to the production of mediators such as CTGF and TGF-β during rhabdomyolysis.35,36
Renal tubules are considered as the main sites of Hb toxicity. However, the presence of proteinuria has been reported in experimental models of recurring exposure to haemoproteins.53 There have also been reports of the presence of focal segmental glomerulosclerosis in experimental models of SCA54 and in patients with chronic and recurring haemolysis, such as paroxysmal nocturnal haemoglobinuria, HUS and SCA.55 These patients develop proteinuria56 and suffer from a chronic reduction of glomerular filtration.12,57 These data suggest that there is a link between intravascular haemolysis and glomerular dysfunction. The physiopathological mechanisms, however, are not clear. There are indications that the haemodynamic changes linked to this disease may be responsible for proteinuria and progressive renal damage; however, there is no definitive proof for this theory.58 Given that focal segmental glomerulosclerosis entails a loss of podocytes, these cells may also suffer from haemoprotein-mediated injury. In this sense, unpublished data from our group show that podocytes are capable of capturing Hb, which induces oxidative stress and causes these cells to die, as well as a loss of proteins involved in the glomerular filtration process such as synaptopodin and nephrin.
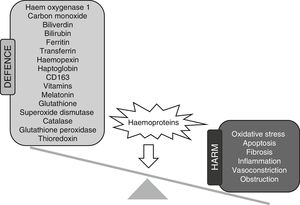
Defence mechanisms against the renal toxicity of haemoproteinsThere are two types of defence mechanisms that work against the harmful effects of haemoproteins: direct and indirect. The direct mechanisms promote the catabolism of haemoproteins and by-products, whereas the indirect mechanisms reduce oxidative stress resulting from the presence of these molecules, thus eliminating the reactive oxygen species or repairing the possible damage caused (Fig. 3). Below is an analysis of each of these defence mechanisms relating to renal damage caused by haemoproteins.
Direct mechanismsHaptoglobinHaptoglobin (Hp) is a glycoprotein found in high concentrations in plasma (0.3–3g/l) and is mainly secreted by hepatocytes, although it is also synthesised in other tissues such as kidneys. Hp irreversibly binds to Hb and impedes its filtration in the kidneys59 and its translocation to the endothelium,60 which counteracts its harmful effects.61 Hp can also bind to Mb, but with less affinity that it does to Hb.62 The Hb–Hp binding promotes the interaction and subsequent internalisation of this complex through the CD163 receptor of the membrane, which is present in monocytes and macrophages.63 Hp levels are highly reduced in patients with chronic haemolysis, such as SCA,64 because Hp breaks down after being endocited.65 The importance of this protein for Hp has been reported in gene knockout studies in rats, which are more sensitive to damage from haemolysis.59 Studies in animal models of SCA and rhabdomyolysis have shown that the administration of Hp reduces vaso-occlusion,31 oxidative stress66 and renal damage.60,67,68
CD163CD163 is a receptor found on the surface of circulating monocytes and macrophages, whose main function is Hb clearance in tissue.63 CD163 has a high affinity for Hb–Hp complexes, although it can also bind to free Hb.69 The macrophages that express CD163 have reduced hydrogen peroxide release and important anti-inflammatory functions through the production of IL-10 and HO-1 stimulation.70 Our group has observed an increase in the macrophages expressing CD163 in renal biopsies of patients with massive haemolysis, such as paroxysmal nocturnal haemoglobinuria37 and favism.39 The renal expression of CD163 was higher in areas where iron had accumulated and where oxidative stress markers were found. We have recently written about the presence of CD163 in the kidneys of patients and experimental models of rhabdomyolysis.35 Since the anti-inflammatory and antioxidant functions of CD163 are well known, these data suggest that CD163 could play a nephroprotective role in response to the renal accumulation of haemoproteins.
Haem oxygenaseHaem oxygenase (HO) is one of the main defence mechanisms in situations of renal overload of Mb and Hb. HO is the enzyme responsible for breaking down the haem group, and thus releasing biliverdin, Fe2+ and carbon monoxide,71 which are powerful anti-inflammatory and antioxidant molecules that enhance the beneficial effects of HO.72,73 There are three isoforms of HO (HO-1, HO-2 and HO-3) which differ in their tissue distribution, regulation and function. Unlike other isoforms, the expression of HO-1 is induced in conditions of oxidative stress, and is expressed in many tissues, including the kidneys.74 The renal expression of HO-1 is increased in experimental models of haemoglobinuria and rhabdomyolysis, as well as in patients with intravascular haemolysis.66,75,76 The deficiency of this enzyme in patients with intravascular haemolysis increases tubular and glomerular damage.77 Similarly, animals used in gene knockout studies for HO-1 have shown greater sensitivity to rhabdomyolysis, higher levels of creatinine and higher mortality rates.78
TransferrinTransferrin (Tf) is a glycoprotein that is mainly secreted by the liver, and which binds to free iron to mitigate its adverse effects.79 Depending on the iron concentration, the tubule cells express the Tf receptor (TfR1), which plays an essential role in the metabolism of this molecule in the kidneys.80 Its expression is regulated by the iron-regulatory proteins 1 and 2, which are highly expressed in the proximal tubules and which act as sensors of iron levels.81–83 Under normal conditions, approximately 30% of the Tf iron-binding sites are saturated. However, these levels increase in the presence of iron accumulation disorders,84 such as severe haemochromatosis, in which case Tf saturation exceeds 60%.85,86 In addition, patients or experimental models of hypotransferrinaemia have low levels of Tf, which promotes renal overload of iron.87,88
HaemopexinHaemopexin (Hx) is a plasma protein that complexes to the haem group for its subsequent internalisation and hepatic clearance through its binding to the LDL receptor-related protein-1 (LRP1) receptor.89–91 In haemolysis, the Hb oxidises and releases the haem group into the bloodstream to later bind to the serum albumin, which transfers the haem group to the Hx and releases the complex in the liver. Once in hepatocytes, the Hx–haem complex breaks down in lysosomes, although a small amount of Hx is recycled and returned to the bloodstream. Therefore, in patients with haemolytic events, the plasma concentrations of serum Hx are reduced92–95 as it builds up in the renal cortex and increases its levels in urine.96 Hx plays a protective role against the harmful effects of the haem group.31,32,97 Rats used in gene knockout studies for Hx have a poor recovery of renal function after suffering an intravascular haemolysis event, because they have a greater renal accumulation of iron and, therefore, higher oxidative stress levels.98,99
FerritinFerritin is a protein consisting of 24 subunits, forming a hollow spherical structure.100 Its primary function is to store iron, so it has a protective capacity against the toxicity caused by iron and haemoproteins. After the HO-1-catalysed reaction, the iron is released from the haem group and is stored inside the ferritin.101 The expression of ferritin is regulated by the concentration of iron and the HO-1 activity.102 This protein plays a crucial role in diseases related to haemoproteins, as ferritin-deficient rats show notable renal damage,103 and it is a good serum marker for SCA.104 Plasma levels of Tf are also higher in ferritin-deficient rats that are subjected to rhabdomyolysis.103
Nrf2Nrf2 is a transcription factor that controls the expression of several antioxidant genes such as HO-1 and ferritin.105,106 Under normal conditions, Nrf2 is found in the cytoplasm bound to its repressor Keap1, which is susceptible to changes in the redox state and is subjected to proteolytic degradation through the proteasome. In the presence of oxidative stress, Nrf2 is released from Keap1 and translocates to the nucleus, where it activates the expression of antioxidant genes.107–112 The activation of Nrf2 has a positive effect against renal damage linked to the accumulation of haemoproteins in experimental models and patients with haemolytic anaemia.113–115
Indirect mechanismsThis second group is composed of antioxidant molecules and various antioxidant enzymes.
Non-enzymatic mechanismsThere are many molecules in the body that have an antioxidant action, such as vitamins, melatonin and bilirubin. These molecules neutralise free radicals and are involved in the protection against renal damage caused by haemoproteins.
Vitamins are an important antioxidant group. One such example is vitamin C, which reacts with the superoxide anion and lipid peroxides, thus reducing the oxidative stress induced by the in vitro116 and in vivo117 RBC lysis. Similarly, treatment with vitamin C was effective in a context of AKI caused by haemoglobinaemia in a patient with glucose-6-phosphate dehydrogenase deficiency.118 The levels of vitamin C decrease after the development of rhabdomyolysis, and its administration has partially reduced histological disorders and renal function in experimental models of rhabdomyolysis.119 Vitamin E is another important vitamin because it plays a significant role in maintaining the redox balance and the integrity of cell membranes, acting on peroxyl and hydroperoxyl radicals. The administration of vitamin E inhibited the RBC lysis of patients suffering from paroxysmal nocturnal haemoglobinuria, which suggests that this vitamin is an effective treatment for these patients.120,121 Vitamin E has not been as effective as vitamin C, however, in the treatment of rhabdomyolysis.122
Melatonin is a hormone secreted by the pineal gland which has several antioxidant properties, as it neutralises free radicals such as hydrogen peroxide, the hydroxyl radical, peroxynitrite and the superoxide anion. This molecule also stimulates the expression of other antioxidant molecules, such as superoxide dismutase, glutathione peroxidase and glutathione reductase. Several studies have shown that this hormone plays a protective role in models of AKI caused by rhabdomyolysis or intravascular haemolysis by reducing tubular necrosis and lipid peroxidation associated with these conditions.117,123
Glutathione, in its reduced state (GSH), is a powerful cell antioxidant that can be oxidised to glutathione disulfide (GSSG) through several enzymatic reactions. Several experimental models of myoglobinuria and haemoglobinuria, as well as studies in patients with HUS and SCA, show decreased levels of GSH in the kidneys.117,123–128 The depletion of GSH increases toxicity mediated by oxidative stress in these diseases, because, by restoring the GSH levels, treatment with N-acetylcysteine reduces histological disorders and inhibits cell death associated with these conditions.129,130
Enzymatic mechanismsThis group includes molecules with enzymatic activity that reduce the content of intracellular reactive oxygen species and, therefore, protect cells from oxidative damage. Superoxide dismutase (SOD) is able to dismutate O2− in O2 and H2O2. For the elimination of H2O2, there are other enzymes with peroxidase activity. These include catalase, glutathione peroxidase (GPx) and reduced thioredoxin (Trx). Catalase is an oxidoreductase that catalyses the decomposition reaction of H2O2 in O2 and water. GPx catalyses the decomposition of H2O2 through the oxidation of GSH to GSSG and water. The GPx, catalase and SOD activity is reduced in experimental models of intravascular haemolysis117 and rhabdomyolysis.125,128,131–134 Furthermore, the plasma levels of GPx and SOD show a negative correlation with albuminuria in patients with SCA.135 Lastly, Trx protects from renal damage associated with rhabdomyolysis through the reduction of oxidative stress and inflammation.136
TreatmentsThere is currently no specific treatment for preventing the damage induced by ferroportins in their different forms of clinical presentation. The alkalisation of urine may be beneficial by reducing the dissociation of the iron found in haemoproteins. This alkalisation can be carried out with oral bicarbonate, while monitoring the urine and serum pH levels. However, no clear benefits have been reliably proven. The use of calcium channel blockers in experimental models has shown an increase in the urinary excretion of iron by mechanisms that remain unknown, which results in a decrease in the renal accumulation of iron.137
The use of iron-chelating agents in diseases associated with the accumulation of this molecule reduces oxidative damage138 and also avoids iron deposition.139 Prophylactically administered deferoxamine reduces oxidative stress resulting from the presence of Hb.140 Iron-chelating agents reduce the toxicity produced by the massive deposition of iron in multitransfused patients,141 although recent studies question their nephroprotective capacity in haemoglobin-induced AKI.142 It should be noted that certain iron-chelating agents, such as deferasirox143 and deferoxamine,144 are potential nephrotoxic agents, requiring strict monitoring during their use.145
Prophylactic treatment with antioxidants such as N-acetylcysteine has yielded positive results in preventing tubular damage secondary to myoglobinuria or haemoglobinuria.146 Other antioxidants, such as acetaminophen, were also effective.27,147 The possible protective role of vitamin E148 and vitamin C,27 in addition to polyphenols,149 flavonoids128,150–152 and l-carnitine,153 has also been investigated, yielding disparate results, as mentioned earlier. Recent studies have also addressed the use of stem cells and have produced positive results, especially in models of rhabdomyolysis.154
Clinical trialsOngoing clinical trials for the treatment of disorders caused by haemoproteins are focused on two aspects. First, treating the underlying disease to prevent the release of haemoproteins into the plasma, and second, mitigating the damage potentially caused by haemoproteins once they are released. As such, in diseases such as aHUS and paroxysmal nocturnal haemoglobinuria, the majority of trials test drugs that act on the complement system, mainly eculizumab, or even new molecules that act in other ways. These include: CCX168 (C5aR antagonist); conversin (protein that prevents action on its C5 convertase); TT30 (ALXN1102 and ALXN1103; recombinant proteins containing Factor H domains 1–5 and which reduce the complement's convertase activity and activate Factor I); LFG316 (anti-C5 monoclonal antibody); APL-2 (C3 inhibitor) and ALN-CC5 (hepatic inhibitor of C5 synthesis) (Table 1). In aHUS, antibodies are also being developed that work against MASP-2, known as OMS 723. In SCA there are trials involving drugs such as SCD 101 and ICA-17043 (which stop red blood cells from turning into falciform cells); decitabine, vorinostat and panobinostat (to increase foetal haemoglobin); and statins, sodium nitrate and ambrisentan (type A-selective endothelin receptor antagonist) to improve endothelial dysfunction, maintain good tissue perfusion during crises and avoid the rupture of red blood cells. SCD 101 has also been used in beta thalassaemia.
Clinical trials in diseases associated with the renal accumulation of haemoproteins.
| Disease | Mode of action | Trial treatment | Clinical trial number | |
|---|---|---|---|---|
| Treatment of underlying disease | HUS | Inhibition of complement | Eculizumab | NCT00838513 |
| CCX168 | NCT02464891 | |||
| Antibody against MASP-2 | OMS 721 | NCT02222545 | ||
| Paroxysmal nocturnal haemoglobinuria | Inhibition of complement | Conversin | NCT02591862 | |
| TT30 | NCT01335165 | |||
| LFG316 | NCT02534909 | |||
| APL2 | NCT02588833 | |||
| ALN-CC5 | NCT02352493 | |||
| Sickle-cell anaemia | Covalent modifiers of haemoglobin | SCD-101 | NCT02380079 | |
| ICA-17043 | NCT00294541 | |||
| Increase in foetal haemoglobin production | Decitabine | NCT01375608 | ||
| Vorinostat | NCT01000155 | |||
| Panobinostat | NCT01245179 | |||
| Improvement of endothelium function | Simvastatin | NCT00508027 | ||
| Sodium nitrate | NCT00095472 | |||
| Ambrisentan | NCT02712346 | |||
| Prevention of damage caused by haemoproteins | Rhabdomyolysis | Elimination of myoglobin through renal replacement therapies | Continuous therapies | NCT00391911 |
| High cut-off HicoRhabdo filters | NCT01467180 | |||
| Immunoabsorption (CytoSorb®) | NCT02111018 | |||
| Decreased oxidation | N-acetylcysteine | NCT00391911 | ||
| Malaria | Decreased oxidation | Paracetamol | NCT01641289 | |
| Beta thalassaemia | Iron-chelating agents | Deferasirox | NCT00560820 | |
| Exjade-desferal | NCT00901199 |
Once haemoproteins are released into the plasma, the trials focus on two strategies. The first strategy is to try to clear these molecules from the plasma. This has been done in several trials in patients suffering from rhabdomyolysis who require renal replacement therapy, in which the effects of continuous techniques, high cut-off haemofilters and immunoabsorption techniques (CytoSorb®) have been analysed in order to remove Mb from plasma as quickly as possible. The second strategy is based on reducing their toxic effect. To do this, efforts are being made to prevent haemoglobinuria-induced oxidation in malaria using paracetamol, and myoglobinuria-induced oxidation using N-acetylcysteine. Trials are also under way that use iron-chelating agents (deferasirox or a combination of exjade and desferal) in thalassaemia to prevent iron deposition in target organs.
ConclusionThe accumulation of haemoproteins in the kidneys is nephrotoxic. There is evidence showing the short-term adverse effects and the chronic loss of renal function. Even though several adverse effects have been reported about these molecules, we must continue to determine the pathogenic mechanisms of haemoproteins to identify new therapeutic targets and prevent their adverse effects. In this sense, podocytes may constitute new cellular targets of the harmful effects of haemoproteins. From a therapeutic perspective, the data currently available are primarily based on studies in animal models. Therapeutic measures aimed at reducing myoglobinuria or haemoglobinuria will be hugely important to prevent renal damage caused by these molecules.
FundingThis study was funded using grants from: the Spanish Society of Nephrology (Sociedad Española de Nefrología), the Spanish Health Research Fund (Fondo de Investigaciones Sanitarias, FIS) (ISCIII/FEDER) (Miguel Servet Programme: CP10/00479 and CPII16/00017; PI13/00802 and PI14/00883) and Fundación Renal Íñigo Álvarez de Toledo (FRIAT) (grants given to JAM); Fundación Conchita Rábago (grant given to MGH); REDinREN (RD012/0021), FIS PI13/02502 and ICI14/00350 (grants given to MP); and FIS/FEDER PI14/00386 and Diabetes and Associated Metabolic Diseases Networking Biomedical Research Centre (Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas asociadas, CIBERDEM) (grants given to JE).
Conflicts of interestThe authors declare that they have no conflicts of interest.
Both authors share authorship as first authors.
Please cite this article as: Guerrero-Hue M, Rubio-Navarro A, Sevillano A, Yuste C, Gutiérrez E, Palomino-Antolín A, et al. Efectos adversos de la acumulación renal de hemoproteínas. Nuevas herramientas terapéuticas. Nefrologia. 2018;38:13–26.