Introducción y objetivos: El fracaso renal agudo (FRA) es un problema muy común en los ancianos y conlleva una elevada morbimortalidad. En este estudio se analizan los factores predictores de FRA en una cohorte de ancianos y su impacto en la evolución de la función renal. Pacientes y métodos: Sobre un grupo de 80 ancianos reclutados entre enero-abril de 2006, se estudian de forma retrospectiva, en 56 pacientes que continúan con vida a los 36 meses, los episodios de FRA, sus causas y la necesidad de diálisis. Resultados: 12 pacientes (21,4 %) presentaron FRA: 4 (33,3 %) con relación a insuficiencia cardiaca, 4 (33,3 %) por infección/sepsis, 2 (16,7 %) por depleción de volumen y otros 2 multifactorial (16,7 %). Ningún paciente precisó terapia con diálisis. Los pacientes con FRA eran más añosos (p = 0,017), tenían basalmente peor función renal (p = 0,031), mayores niveles de ácido úrico (p = 0,002) y menores de hematocrito (p = 0,003). Los pacientes con FRA tenían una creatinina sérica basal de 1,57 ± 0,59 mg/dl y el pico máximo de creatinina durante el FRA fue de 4,25 ± 4,26 mg/dl (p = 0,035). La función renal a los 36 meses en pacientes con FRA había disminuido (p = 0,024). En el análisis de regresión logística (variables independientes: edad, género, índice de Charlson, creatinina sérica, urea, ácido úrico, MDRD basales), la edad (riesgo relativo [RR]: 1,20; 1,01-1,43; p = 0,039), el ácido úrico (RR: 2,65; 1,11-6,30; p = 0,027) y el hematocrito (RR: 0,64; 0,43-0,96; p = 0,031) se asociaban independientemente con el desarrollo posterior de un FRA. Conclusión: el nivel basal de ácido úrico y de hematocrito son factores de riesgo independiente para el desarrollo de FRA en ancianos, que, aun siendo de carácter funcional y sin necesidad de diálisis, producen un deterioro de la función renal en el tiempo.
Introduction: Acute renal failure (ARF) is a very common problem in the elderly and is associated with high morbidity and mortality rates. This study analysed ARF predictors in a cohort of elderly subjects and their impact on the evolution of renal function. Patients and method: A group of 80 elderly individuals were recruited between January and April 2006, and 56 of these who were still alive after 36 months were retrospectively studied, examining episodes of ARF, their causes, and the need for dialysis. Results: Twelve patients (21.4%) developed ARF: 4 (33.3%) related to heart failure, 4 (33.3%) due to infection/sepsis, 2 (16.7%) due to volume depletion, and another 2 were multifactorial (16.7%). No patients required dialysis therapy. Patients with ARF were older (P=.017), had worse renal function at baseline (P=.031), higher levels of uric acid (P=.002), and lower haematocrit (P=.003). Patients with ARF had a mean baseline serum creatinine of 1.57±0.59mg/dl and peak creatinine levels during episodes of ARF averaged 4.25±4.26mg/dl (P=.035). Mean renal function at 36 months in patients with ARF had decreased (P=.024). In a logistic regression analysis (independent variables: baseline MDRD, age, gender, Charlson index, serum creatinine, urea, and uric acid), age (RR: 1.20, 1.01-1.43, P=.039), uric acid (RR: 2.65, 1.11-6.30, P=.027), and haematocrit (RR: 0.64, 0.43-0.96, P=.031) were independently associated with the development of ARF. Conclusions: Baseline levels of uric acid and haematocrit are independent risk factors for the development of ARF in the elderly. Although these episodes may be functional in nature and not require dialysis, this can still cause a deterioration of renal function over time.
INTRODUCTION AND OBJECTIVES
Structural and functional changes associated with aging kidneys, together with the presence of certain comorbidities typical of the elderly, make this population particularly vulnerable to developing acute renal failure (ARF).1
The aetiology of this condition generally involves multiple causes, such as volume depletion, processes that alter renal haemodynamics, exposure to nephrotoxic compounds, and even iatrogenic events.2
As regards prognosis, this syndrome is associated with increased morbidity and mortality rates.1,3 It has been shown that ARF is a risk factor for the development of chronic kidney disease (CKD) and the need for dialysis in the elderly.4 In a meta-analysis, Ishani et al. demonstrated that the risk of terminal nephropathy was 13 times greater in elderly individuals hospitalised with ARF than in those without ARF, suggesting that episodes of ARF could accelerate the progression of kidney disease.5
The identification of risk factors for ARF in these patients may aid in establishing strategies for avoiding the appearance of this condition, thus reducing the morbidity and mortality produced by this syndrome.
The objectives of our study were to: a) evaluate which factors serve as predictors for the development of ARF in elderly individuals, and b) evaluate the impact that ARF has on the evolution of renal function.
PATIENTS AND METHOD
Patients
Of the 80 patients in the study of elderly patients with CKD at the Hospital General de Segovia (randomly recruited in external consultations in geriatrics and general nephrology between January and April 2006, during a period of clinical stability),6,7 we examined 56 patients who were still alive 36 months after recruitment and who had developed at least one episode of ARF during the period in which they were hospitalised.
Method
Ours was a retrospective, observational study. We defined ARF as an increase in serum creatinine >0.5mg from the previous measurement. We registered the peak serum creatinine value measured during the ARF episode. We also examined the causes of ARF, as well as the need for renal replacement therapy. Glomerular filtration rate (GFR) was estimated using the abbreviated MDRD (Modification of Diet in Renal Disease) formula.8
We registered baseline comorbidity using the Charlson index, without including age as a factor.9
Statistical analysis
We performed all statistical analyses using SPSS statistical software, version 15.0. All data were expressed as percentages, means, and standard deviations. We compared means using Student’s t-tests if the data distribution was normal, and Mann-Whitney U-tests for non-parametric data. We compared proportions using Fisher’s chi-square test. With the objective of evaluating the impact of ARF on parameters of renal function over time, we used a linear model for repeated measures. In order to analyse the variables that serve as predictors for ARF, we performed a logistic regression analysis. The statistical significance level was set at 95% (P<.05).
RESULTS
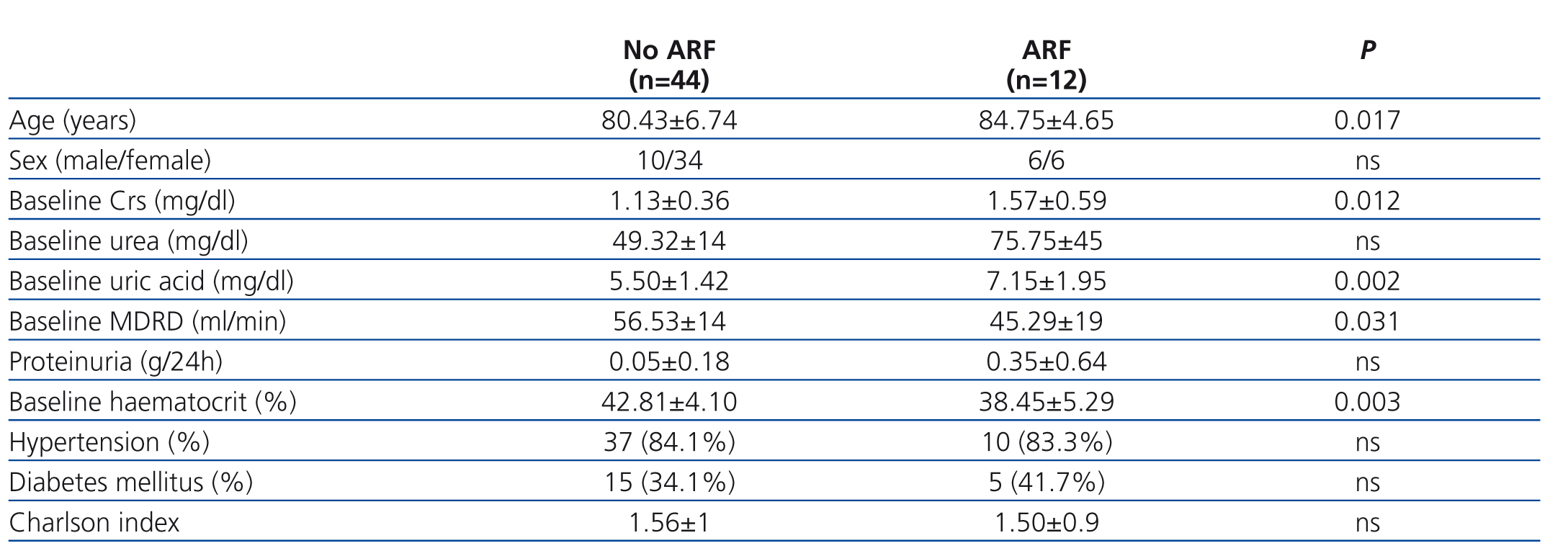
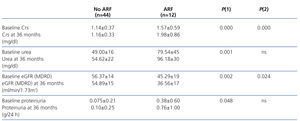
Of the 56 patients who completed the 36 month follow-up period, 12 (21.4%) developed at least one episode of ARF during hospitalisation. Table 1 summarises baseline comorbidity values, socio-demographic characteristics, renal function parameters, and haematocrit, and compares values between patients with and without ARF. Patients who developed ARF were significantly older, had worse baseline renal function, higher uric acid levels, and lower haematocrit. There were no significant differences in terms of comorbidity, history of ischaemic heart disease, heart failure, or use of diuretics during the baseline period when comparing between the two groups.
As regards the causes of ARF, four patients (33.3%) developed ARF in association with heart failure; 4 cases (33.3%) presented deterioration of renal function due to an infectious process/sepsis; 2 (16.7%) were caused by volume depletion associated with drug use (levofloxacin/opiates), and another 2 cases (16.7%) were caused by multiple factors. No patients required renal replacement therapy with dialysis. Three patients died during the episode of ARF (two of multiple origin and another related to sepsis).
Patients with ARF had a mean baseline serum creatinine value of 1.57±0.59mg/dl, and a maximum peak serum creatinine reached during the episode of ARF of 4.25±4.26mg/dl (P=.035).
We observed no significant differences in terms of level of control of blood pressure (BP) values after 36 months in our study patients (mean systolic BP: 130.02±16mm Hg without ARF vs. 136.16±12mm Hg with ARF; mean diastolic BP: 71.75±10mm Hg without ARF vs 67.83±7mm Hg with ARF).
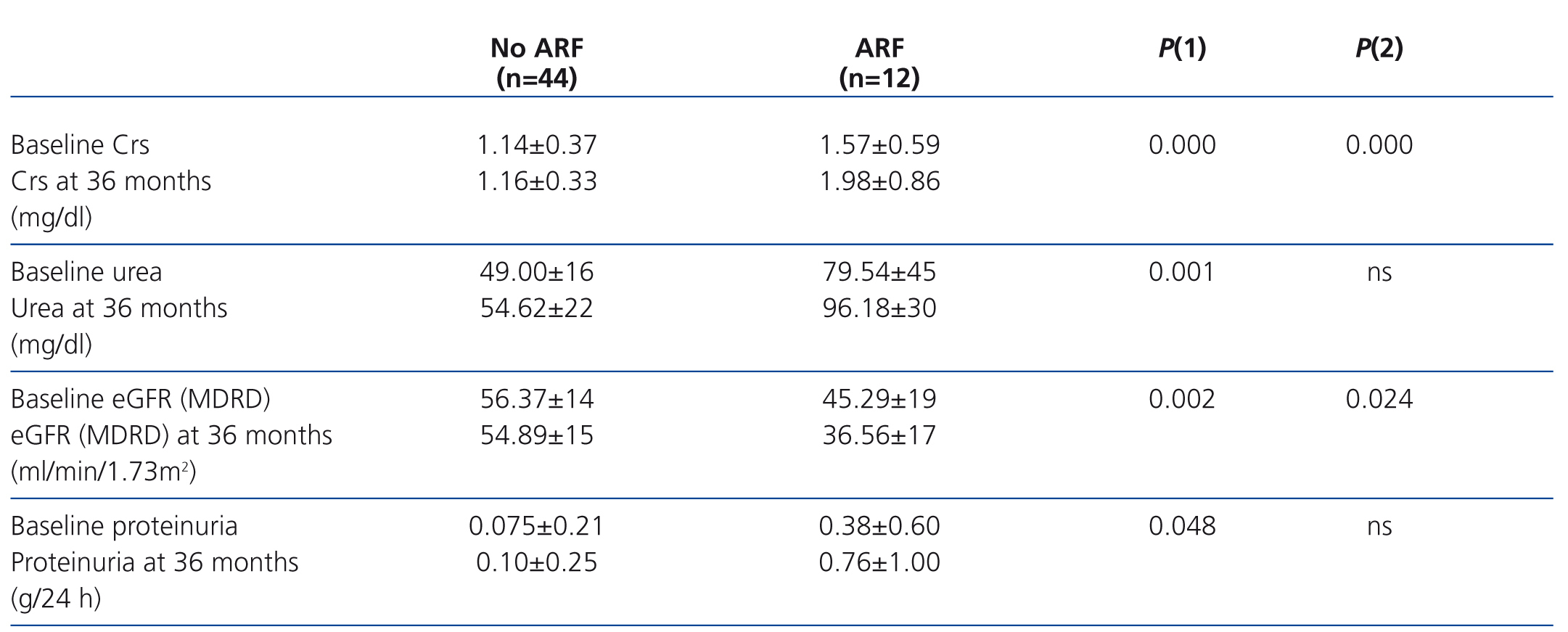
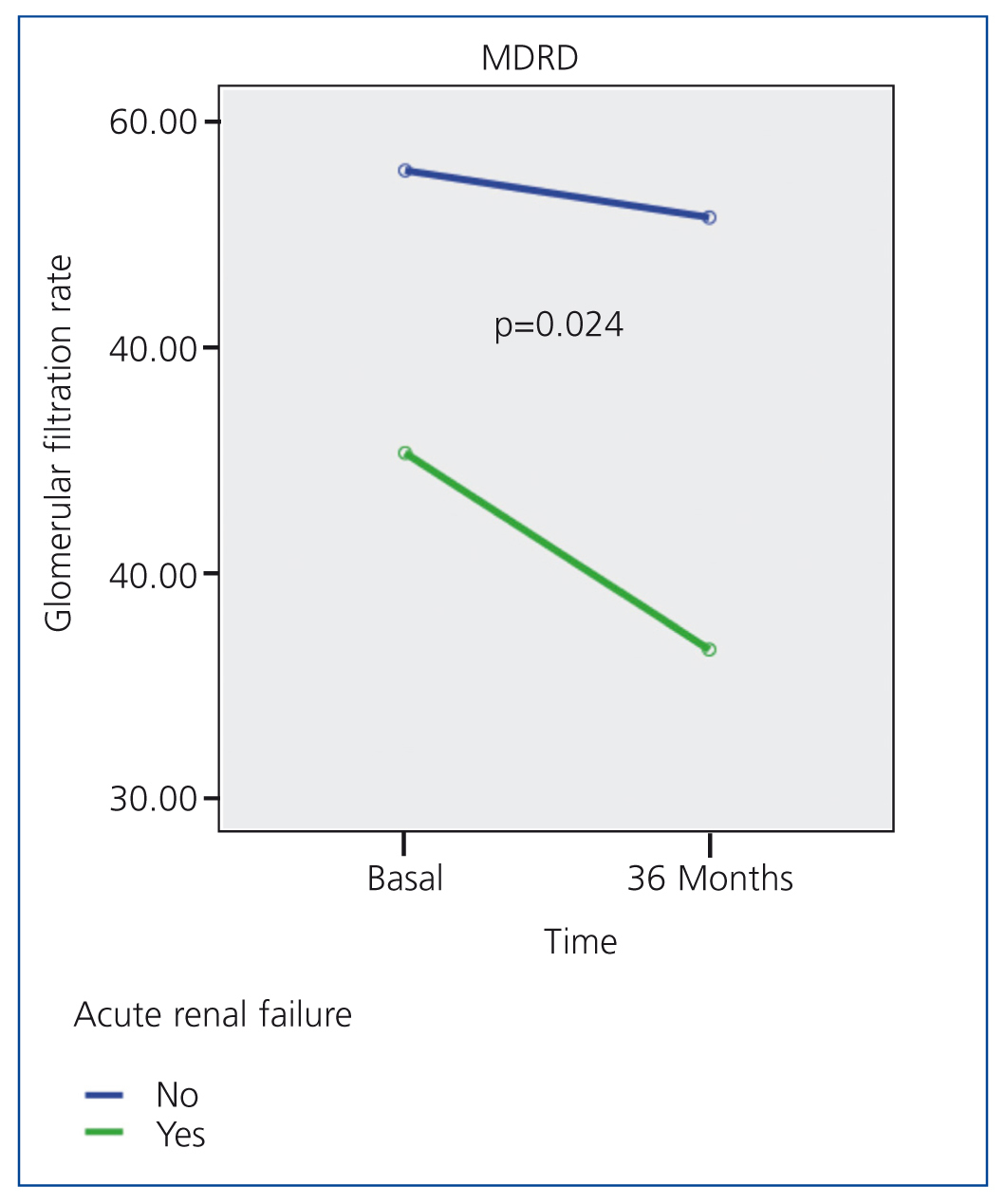
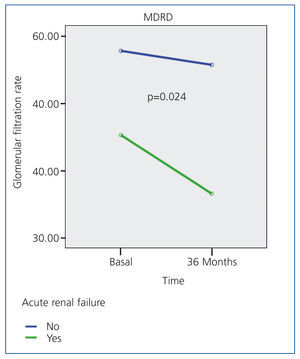
Table 2 summarises the impact of the episode of ARF on renal function parameters during the follow-up period. Figure 1 displays how patients with ARF suffered a greater deterioration in MDRD-estimated glomerular filtration rate during the 36-month study period.
In the logistic regression analysis to evaluate which factors were the best predictors for the appearance of ARF in this study population (independent variables: baseline MDRD, age, gender, Charlson index, serum creatinine, urea, and uric acid), age (relative risk [RR]: 1.20; 1.01-1.43; P=.039), uric acid (RR: 2.65; 1.11-6.30; P=.027), and haematocrit (RR: 0.64; 0.43-0.90; P=.031) were independently associated with the development of ARF.
DISCUSSION
Approximately one-fourth of all individuals in the study of elderly patients with CKD that were still alive 36 months after being recruited to the study had developed at least one episode of ARF during hospitalisation. In these cases, the primary aetiology was for the most part functional (in the context of heart failure, infectious processes, and in two cases, volume depletion associated with drug administration); in addition, these patients were treated without the need for dialysis. These results are comparable to those from previous studies.1,2
The availability of biological markers for the early detection and treatment of ARF could aid in reducing the morbidity and mortality rates associated with this syndrome.10 In our study, the multivariate analysis revealed that baseline haematocrit and uric acid values were both predictive factors for ARF.
The association between preoperative haematocrit values or low haematocrit during the intra-operative procedure in patients undergoing cardiac surgery and ARF has already been described.11
In recent years, a growing body of evidence has been compiled associating elevated uric acid levels in the blood with cardiovascular and renal pathologies.12 Studies have demonstrated an association between elevated blood uric acid levels and the incidence of CKD, as well as a reduced drop in renal function in patients with renal failure who are treated with alopurinol.13 In addition, our group has demonstrated that uric acid is an independent marker for risk of mortality in the global population of elderly individuals.14 Lapsia et al. demonstrated that preoperative uric acid levels are associated with increased incidence and risk of acute renal failure in patients following heart surgery.15 In our study, despite the limited number of elderly patients who developed ARF, the uric acid levels in baseline measurements taken from our patients upon recruitment into the study also turned out to be an independent risk factor for developing ARF. One possible explanation for the value of uric acid as a marker for ARF could be the fact that uric acid values increase during hypoxia (such as in chronic pulmonary obstructive disease, heart failure, etc.). As such, patients with greater levels of uric acid in the blood would tend to be also subjected to a greater degree of ischaemia, making them more susceptible for developing ARF.
In addition, studies have also shown that episodes of ARF accelerate the progression towards CKD.5 In our cohort of elderly patients with clinical stability upon recruitment into the study, we also demonstrated that these patients (who develop ARF) suffer a greater deterioration in renal function than those that do not, despite the fact that the episodes of ARF were functional in nature and related to other pathologies, in addition to the fact that no patients required dialysis treatment, making these the patients who would most benefit from monitoring in nephrological consultations.
To conclude, baseline uric acid and haematocrit values are independent risk factors for the development of ARF in elderly individuals. Despite the fact that these episodes of ARF are functional in nature and do not require dialysis treatment, they produce a deterioration in renal function over time.
Conflicts of interest
The authors declare that they have no potential conflicts of interest related to the contents of this article.
Table 1. Comparison of the sociodemographic characteristics, parameters of renal function, haematocrit, and comorbidity during the baseline period between elderly patients with and without at least one episode of acute renal failure during a 36-month monitoring pe
Table 2. Impact of acute renal failure on the evolution of renal parameters after 36 months
Figure 1. Evolution of MDRD-estimated glomerular filtration rate following an episode of acute renal failure