Until recently, ABO-incompatible living donor kidney transplantation was regarded as an absolute contraindication. However, this kind of kidney transplantation has been used in the last years, with good results.
Objetive0ur objetive is to show the results obtained with this technique in our hospital.
MethodsForty-eight patients with a mean age of 50,9±10,9 years were included. Follow-up 44,6±30,9 months. Conditioning: rituximab 375mg/m², tacrolimus, mycophenolate mofetil or mycophenolate sodium, prednisone, plasmapheresis/immunoadsorption and intravenous immunoglobulin. Accepted IgG and IgM titers for transplantation ≤ 1:8.
ResultsPreprocess IgG titer 1:124±1:40, IgM titer 1:77±1:55. After 6±3 sessions, IgG decreased to <1:8 in 47 patients and to <1:16 in one. IgM was < 1:/8 in all cases. Twenty-five patients (50 %) presented hematoma, reintervention in 7 (14.6 %); 29 (60 %) required transfusion. Acute rejection occurred in 5 cases (8.7 % at 5 th year). CMV infection 9 (19,7 % at 5th year), BK viremia 5 (12.4 % at 5th year), post-transplant diabetes in 10 (23.4 % at 5th year), and lymphocele in 3 (6,4 % at 5th year). Patient survival was 97 % at 5th year and graft survival 95,7 % at 1st and 93 % at 5th year. Causes of graft loss: thrombosis (n=1) and mixed rejection (n=1) and death (n=2). Serum creatinine levels were 1.43±0.5mg/dl at 1st and 3rd year and 1,3±0,3mg/dl at 5th year. Proteinuria was 0.2±0.2g/24h at 1st and 5th year.
ConclusionsABO incompatible living donor kidney transplantation after conditioning with rituximab, plasmapheresis/immunoadsorption and immunoglobulins is a valid option offering excellent outcomes.as survival and acute rejections without increasing infectious complications. An increased tendency towards postoperative bleeding is observed.
El trasplante renal de donante vivo ABO incompatible era considerado una contraindicación absoluta. Desde hace años, se realiza con buenos resultados.
ObjetivoNuestro objetivo es mostrar los resultados de este tipo de trasplante realizado en nuestro hospital.
MétodosEstudiamos 48 pacientes, edad media 50,9±10,9 años. Seguimiento 44,6±30,9 meses. Acondicionamiento: Rituximab (RTX) 375mg/m2, tacrolimus, micofenolato mofetil o micofenolato sódico, prednisona, plasmaféresis/inmunoadsorción e inmunoglobulina intravenosa. Títulos de isoaglutininas aceptados para trasplantar: IgG e IgM inferiores a 1:8.
ResultadosIsoaglutininas pre-proceso: IgG 1:124±1:140, IgM 1:77±1:55.Tras 6±3 sesiones IgG descendió a <1:8 en 47 pacientes, a <1:16 en uno; IgM fue < 1:8 en todos. 24 pacientes (50%) presentaron hematoma, reintervención en 7(14.6%), 29(60%) necesitaron transfusión. Rechazo agudo 8.7% al 5º año). CMV 9(19,7% al 5º año), Viremia BK 5(12.4% al 5º año), diabetes post-trasplante 10(23.4% al 5º año), linfocele 3(6,4% al 5ºaño). Supervivencia paciente 97,1% al 5º año y del injerto 95,7% al año y del 93% al 5 año. Pérdida de injerto: trombosis (n=1), rechazo mixto(n=1) y éxitus (n=2). La creatinina al año y a los 3 años 1,4±0,4mg/dl y 1,3± al 5º año. La proteinuria al año, 3º y 5º fue 0,2±0,2g/24horas.
ConclusionesEl trasplante renal de donante vivo ABO incompatible tras acondicionamiento con rituximab, plasmaféresis/inmunoadsorción e inmunoglobulinas es una opción válida y ofrece excelentes resultados de supervivencia, con baja incidencia de rechazo agudo sin aumento de complicaciones infecciosas. Se evidencia una mayor tendencia al sangrado postoperatorio.
ABO incompatibility was considered as an absolute contraindication for renal transplantation in the EDTA guidelines in 2000.1 Since 1987, first in Japan, due to the shortage of deceased donors, and later in Europe, United States and lately in Korea, Australia and Spain, ABO incompatible live donor kidney transplantation (LDKT ABOi) This a technique commonly used that serve to alleviate the donor deficit. These results have changed the guidelines, which exclude the LDKT ABOi as an absolute contraindication.2
There are several conditioning protocols that vary in the form, doses of medication or in the methods of clearance of antibodies3 and, therefore, make it difficult to draw conclusions with a high level of evidence and advise which is the best scheme.4 In general, conditioning is based on depleting antibodies (isoagglutinins), either by their removal by plasmapheresis (PFS) or immunoadsorption (IA), or by the use of depleting agents of B lymphocytes such as rituximab (RTX), which replaces splenectomy. The results show very high percentages of survival for both the patient and the graft, the rate of complications being more variable, both for acute rejection and for infections or neoplasms.
The objective of the study is to analyze the prognosis of patients who received an ABOi LDKT in our hospital from 2008 to 2018 and compare the results with other series.
MethodsFrom January 1981 to June 2018, the University Hospital of A Coruña has performed 3180 kidney transplants; 273 were from a living donor, of which 48 were performed with an incompatible ABO donor and 23 with an incompatible HLA donor.
The study included all patients who received an ABOi LDKT, and 3 patients who, in addition to ABOi, were HLA incompatible. Patients previously accepted and signed the ABOi LDKT protocol after receiving information from the services involved in the kidney transplant.
The protocol used in our hospital consists of 3 phases: conditioning phase, kidney transplant itself and follow-up.
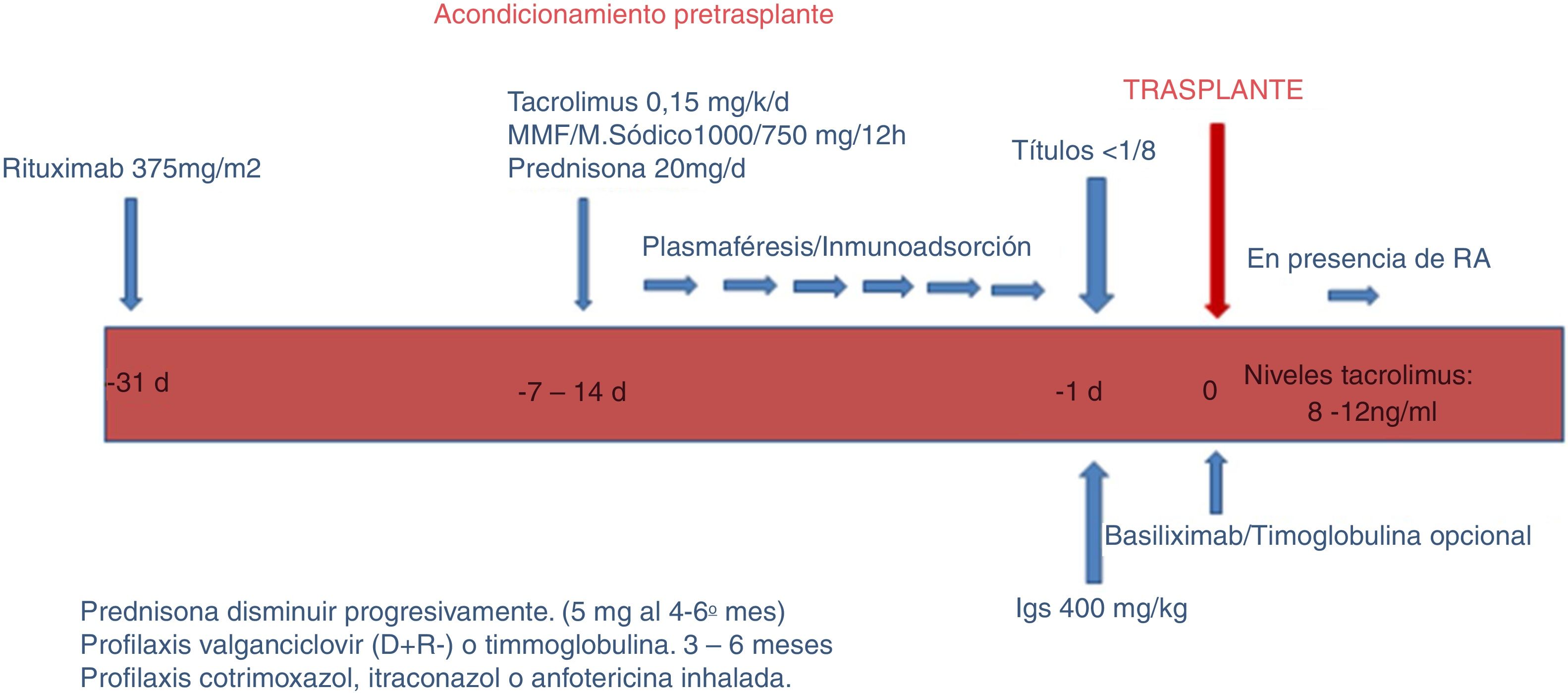
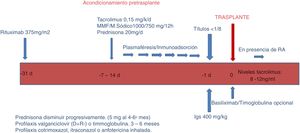
a) Conditioning phase (Fig. 1).
In this phase, the objective is to reduce the ABO antibodies (isoagglutinins) until reaching a titer equal to or less than 1: 8, using RTX, apheresis, immunosuppression and immunoglobulins.
- 1
RTX One month before the transplant, patients receive a single dose of RTX 375mg/m2 (MabThera ® ; Roche, Grenzach-Wyhlen, Germany).
- 2
Aphaeresis. Sessions of PFS, IA or both were performed, depending on the title of pre-transplant isoagglutinins. Conventional plasma separation filters were used for PFS; for IA, columns Glycosorb ® -ABO (Glycorex Transplantation, Lund, Sweden). In two of 3 patients, who were also sensitized to HLA, IA was performed with TheraSorb ® columns (Miltenyi Biotec, Bergisch Gladbach, Germany).
Titration of isoagglutinins; for IgM, it was used the saline in gel microtranscard the technique (Reverse Diluent cards, Ortho-Clinical Diagnostics, Raritan, NJ, USA); for IgG, it was estimated by an antiglobulin technique in gel micro card (Anti-C3d, Polyspecific, Ortho-Clinical Di agnostics). Isoagglutinin titres (IgG and IgM) were analyzed before and after each apheresis session. The plasma volume was increased or the apheresis technique was changed (from PFS to IA) depending on the decrease in titres.
- 3
Immunosuppression. Depending on the duration of apheresis, patients initiate immunosuppressive treatment one or 2 weeks before transplantation with rapid or delayed-release tacrolimus (0.15mg / kg) to reach levels of 8−12ng / ml, mycophenolate mofetil (CellCept ®, Roche, Grenzacch-Wyhlen, Germany) or mycophenolate sodium (Myfortic ®, Novartis, Barcelona, Spain) in dose 2g or 1440mg / day, respectively, and prednisone at a starting dose of 20mg /day.
- 4
Immunoglobulins. The day before transplantation single dose of immunoglobulins (Flebogamma was administered® 5 %, Instituto Grifols, Barcelona, Spain) 400mg / kg was administered. In 3 patients HLA incompatible, it was use specific gammaglobulin anti-CMV (Cytotect ®, Biotest Pharma GmbH, Germany; 100mg/kg) after each apheresis session.
b) Renal transplant phase itself.
With regard to induction, since 2015 we have used basiliximab (Simulect ®) in patients with major HLA incompatibility, or inadequately low levels of tacrolimus. Currently it is part of our induction protocol in LDKT. We use two doses of 20mg administered the day of transplantation and the fourth day after.
The surgical technique in the donor was laparoscopic nephrectomy and in the recipient, renal transplantation through incision in the right iliac fossa with usual technique and leaving double J stent after ureteral reconstruction.
In the immediate postoperative period, we performed hemodynamic controls and renal function, monitoring the surgical wound and postoperative complications. It was performed a renal doppler ultrasound within 12h after transplantation. Isoagglutinin titers were determined once in the first week or when there is impairment of renal function.
Immunosuppression is adjusted according to tacrolimus levels (8−12ng / ml the first month, between 8 and 10ng / ml between the first and third month and between 6 and 8ng / ml from the third- sixth month) and everolimus (3 and 8n g / ml). The doses of sodium mycophenolate are 720mg every 12h and mycophenolate mofetil 1000mg every 12h the first month. After an induction dose of 250mg of methylprednisolone (if basiliximab is employed) is continued 20mg daily, that are progressively reduced to 5mg / day from the fifth-sixth month. Steroids are not suspended by protocol. Patients receive prophylaxis with cotrimoxazole during 6 months and itraconazole for 4–5 months (lately inhaled amphotericin B replaces itraconazole). Patients with serology CMV D + / R - receive prophylaxis with oral valganciclovir for at least 3 months. With a delayed graft function, a renal biopsy is performed.
c) Follow up.
Patients are checked every week during the first month, every 2 weeks for the first 2 months, monthly until 6 months and then every 2–3 months until the end of the year. Subsequent revisions are made every 3 months. The removal of the double J is performed on an outpatient basis by the urology department one month after the transplant.
In the revisions there is monitoring of vital signs, renal function and immunosuppression. And it is also performed a prevention control of cardiovascular disease, neoplasms, opportunistic infections, dietary measures and the study of calcium-phosphorus metabolism. If renal function is impaired, renal echo-doppler, immunosuppression adjustment and renal biopsy are performed if the cause of the deterioraion deterioration is not identified.
The variables evaluated were: demographic (age, sex, modality and time on dialysis, family kinship, blood group, presence of HLA antibodies); derived from the technique (apheresis class: PFS, IA, both ; isogglutinin titer [IgG and IgM] and the decrease in relation to the technique, number of sessions used and plasma volume purified per session, pre-transplant coagulation according to the technique: platelets preprocess and pre-transplant, prothrombin time, partial thromboplastin activated time, fibrinogen levels). Renal and patient survival were studied; renal function was measured according to creatinine in mg / dl and proteinuria in g / 24h; surgical complications: thrombosis, hematoma-hemorrhage, need for reintervention; Infectious complications: CMV and BK (PCR>700 copies). Delayed graft function (need for dialysis or no decrease in creatinine during the first week after transplantation), acute rejection demonstrated by biopsy according to Banff 2017 classification 5, isoagglutinin rebound as per the title in the first post-transplant week.
Statistical analysisStudent's t and ANOVA were used to compare quantitative variables; the Mann-Whitney test and the Kruskal-Wallis test when the distribution was not normal; Chi square and Fisher's test for qualitative variables. Survival was calculated according to Kaplan-Meier and log rank test. Multivariate study : Cox regression. SPSS program (version 15.0.1, SPSS, Inc., Chicago, IL, United States).
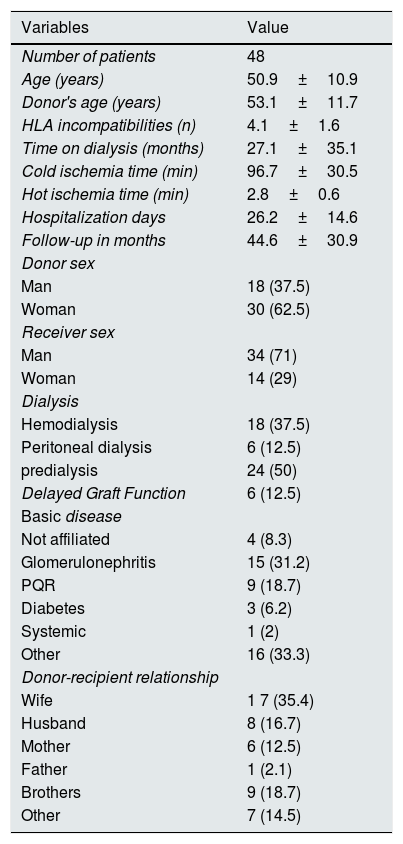
ResultsWe studied 48 patients (34 males) with a mean age of 50.9±10.9 years, 18hemodialysis, 6 peritoneal dialysis and 24 predialysis (time on dialysis: 27.1±35.1 months). Incompatibility of groups was: A-0 (n=35), B-0 (n=6), AB-0 (n=2), ABB (n=2), AB (n=2), BA (n=1). The most frequent degree of kinship between donor and recipient was between spouses (n=25; 52.1 %). The follow-up period was 44.6 months (3–117), with a median of 30.97. Other baseline and demographic characteristics are shown in Table 1.
Demographic data.
| Variables | Value |
|---|---|
| Number of patients | 48 |
| Age (years) | 50.9±10.9 |
| Donor's age (years) | 53.1±11.7 |
| HLA incompatibilities (n) | 4.1±1.6 |
| Time on dialysis (months) | 27.1±35.1 |
| Cold ischemia time (min) | 96.7±30.5 |
| Hot ischemia time (min) | 2.8±0.6 |
| Hospitalization days | 26.2±14.6 |
| Follow-up in months | 44.6±30.9 |
| Donor sex | |
| Man | 18 (37.5) |
| Woman | 30 (62.5) |
| Receiver sex | |
| Man | 34 (71) |
| Woman | 14 (29) |
| Dialysis | |
| Hemodialysis | 18 (37.5) |
| Peritoneal dialysis | 6 (12.5) |
| predialysis | 24 (50) |
| Delayed Graft Function | 6 (12.5) |
| Basic disease | |
| Not affiliated | 4 (8.3) |
| Glomerulonephritis | 15 (31.2) |
| PQR | 9 (18.7) |
| Diabetes | 3 (6.2) |
| Systemic | 1 (2) |
| Other | 16 (33.3) |
| Donor-recipient relationship | |
| Wife | 1 7 (35.4) |
| Husband | 8 (16.7) |
| Mother | 6 (12.5) |
| Father | 1 (2.1) |
| Brothers | 9 (18.7) |
| Other | 7 (14.5) |
Data are expressed as mean±standard deviation on (%).
No adverse effects were recorded after administration of RTX. PFS was the apheresis technique most frequently used: 32 patients received PFS, 10 received IA and 6, both procedures. The preprocess IgG titles were: 1: 0 (n=2), 1: 2 (n=1), 1: 8 (n=4), 1:16 (n=2), 1:32 (n=6), 1:64 (n=13), 1: 128 (n=10), 1: 256 (n=6), 1: 512 (n=4). The preproces IgM titles were: 1: 0 (n=2), 1: 2 (n=1), 1: 4 (n=1), 1: 8 (n=4), 1:16 (n=9), 1:32 (n=14), 1:64 (n=8), 1: 128 (n=4), 1: 256 (n=4), 1: 1.024 (n=1).
After 6±3 sessions IgG titer was reduced to < 1: 8 in 47 patients and < 1:16 in a patient who had a titre 1:16 the same morning of the transplantation procedure, being 1: 8 the day before. Knowing the variation of the title and the experience of other groups in accepting until 1:32, we proceeded to the transplant. The IgM titer was < 1: 8 in the 48 patients.
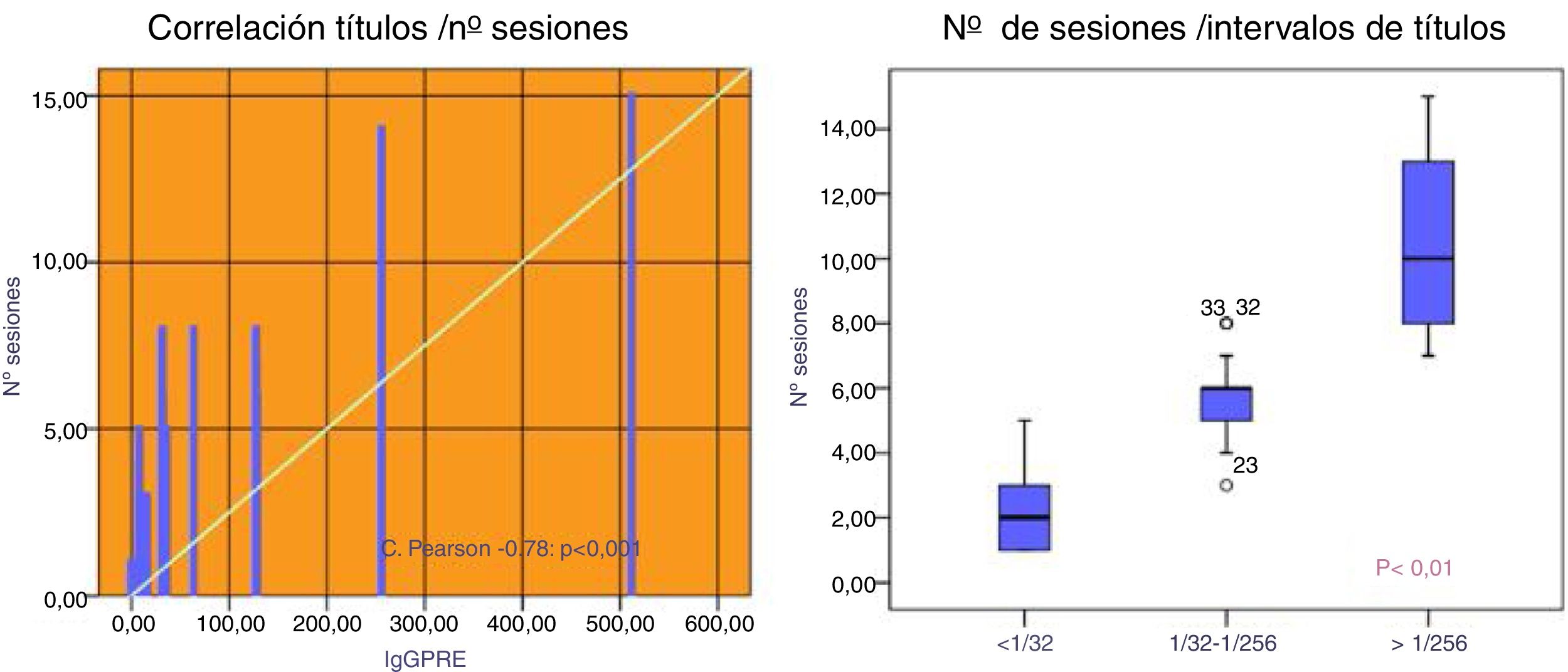
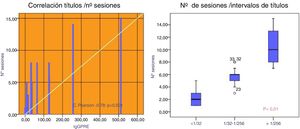
Patients with an initial IgG titer < 1:32 needed 2.3±1.5 sessions mostly PFS (86 %). Those with a title 1: 32-1: 256 required 5.6±1.2 sessions being used PFS in 65 % of them. Patients who had hightiters of 1: 256 10.3 needed 10.3±2.9 sessions, using IA in 100 % of cases (75 % isolated and 25 % combined with PFS), p<0.001 (Fig. 2).
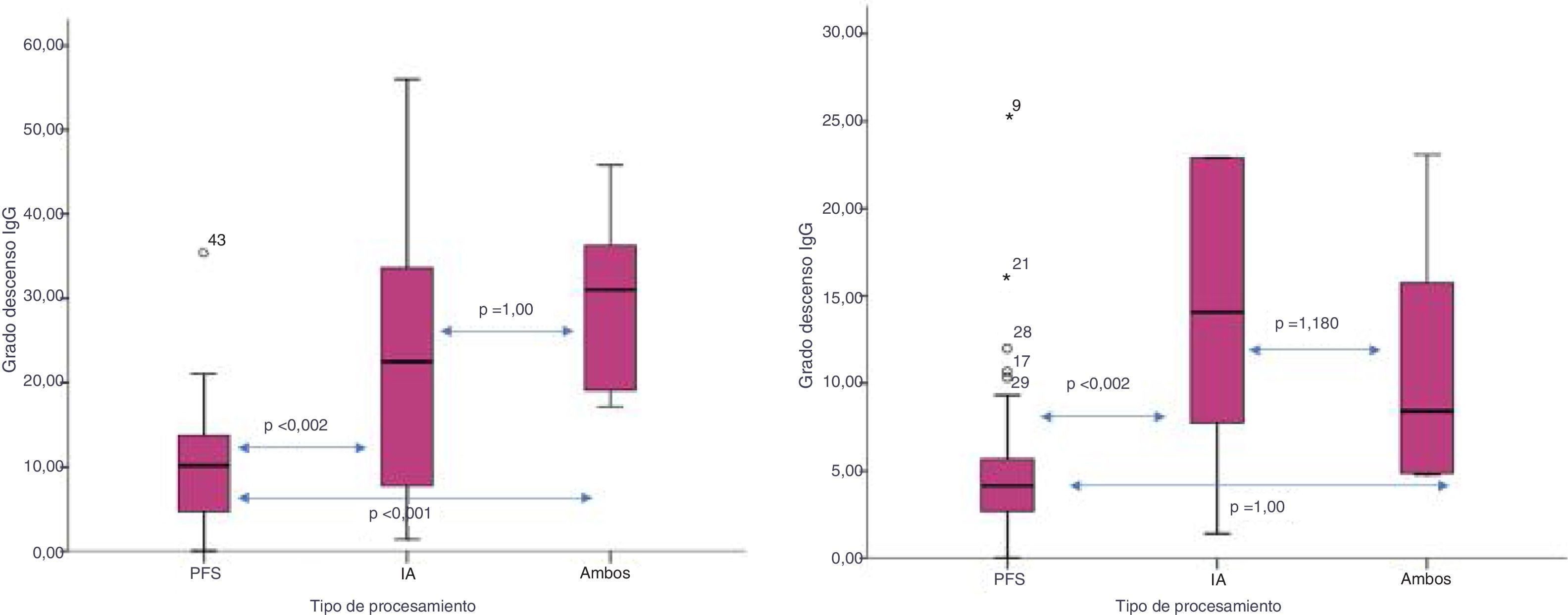
The degree of decrease in IgG isoagglutinins (IgG pre minus IgG post/ number of sessions) was higher with the isolated or combined used of IA versus PFS: 24.9±17 vs. 30±10.7 vs. 10.6±7.6, respectively (p<0.002). This decrease was also greater for IgM using IA versus both techniques or PFS: 27.2±35 vs. 18.8±7 vs. 5.3±5, respectively (p=0.002) (Fig. 3).
Three patients were sensitized to HLA. Two had a mean fluorescence intensity (MFI) against class I of 9100 and 5300 and an MFI against class II 15,600 and 2,500, respectively. Another patient presented sensitization only against class I, with an MFI of 3900. The IgG isoagglutinin titres in the first 2 patients were 1:64 and 1: 128, respectively, and those of IgM, 1:32. The third patient presented IgG 1: 128 and IgM 1: 256. Two patients received IA with TheraSorb ® columns and one patient received PFS. The IgG isoagglutinin titers decreased to 1: 4, 1: 8 and 1: 2, respectively, and the IgM titers, to 1: 2 in all. Post- transplant MFIs were < 1000 (150–600) for both class I and classII.
The number of post-transplant PFS sessions was 1.4±0.38, although since 2013 we have not performed post-transplant apheresis.
There was no rebound of isoagglutinin posttransplant; the posttransplant IgG titer was 1:3.6±1: 4 (0–16) and the IgM titer was 1: 2.3±1: 4, 8 (0–32). The post-transplant titer was higher depending on the preconditioning titles; it did not show statistically significant differences for IgG, but for IgM the differences were significant: 1:10±1:14 for IgM > 1: 256 vs. 1: 2.5±1: 2.6 for 1: 32-1: 256 vs. 1:1±1: 1.3 for < 1:32 (p=0.008).
Elapsed since the last session of apheresis time to transplantation was 29.8h. The time was shorter when we used IA: 12.2±11.6h versus 34.6±12.6h when we used PFS (p=0.001), or 33.6±18.9h when we used both techniques (p=0.01).
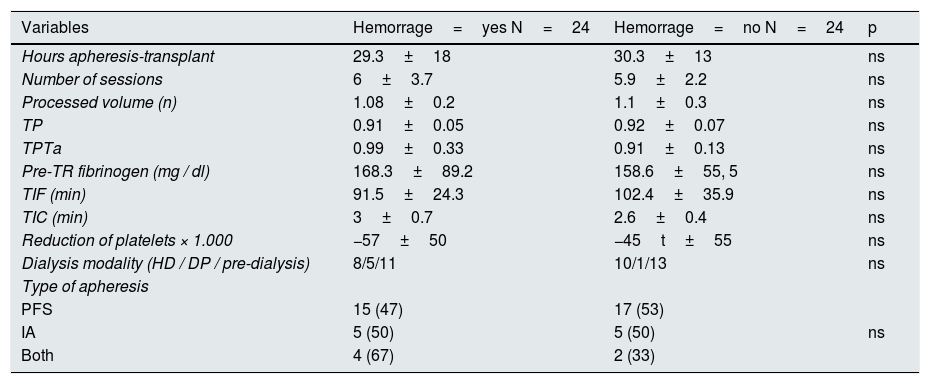
The time of cold ischemia was 96.7±3min and the hot ischemia time was 2.8±0.6 mi n. Twenty-four patients (50 %) had hematoma / postoperative bleeding - of which 7 (14.6 %) had to be reoperated - and 29 (60.4 %) needed transfusion. The reoperation was performed in 6 patients who had received PFS and in one patient with both techniques. The need to transfuse occurred in 19 patients who had received PFS, in 6 with IA and in 4 with both techniques. The presence of hematoma occurred in 15 patients who received PFS, in 5 with IA and in 4 with both techniques (without statistical significance). We found no differences in the number of platelets, TP, TPTa, fibrinogen, type of apheresis, number of sessions, time on dialysis or time since the last apheresis session until the intervention. According to the apheresis technique used, IA had greater pre-transplant thrombocytopenia than PFS : 104±23 vs. 170±45×103 (p<0.001) or with both techniques 149±13×10 3 (p=0.09) and with IA pre-transplant fibrinogen levels were higher than with PFS : 210±80 vs. 156±73 and higher than with both procedures 141±27 (but without statistical significance). The sum of thrombocytopenia associated with IA and hypofibrinogenemia associated with PFS could explain the incidence of hematoma, although it was not statistically significant (Table 2). The incidence of hematoma / bleeding is similar to the observed in the kidney transplant HLA incompatible but higher than in patients receiving a ABO compatible live donor kidney transplant, which in our group is 8 %.
Postoperative hemorrhage.
| Variables | Hemorrage=yes N=24 | Hemorrage=no N=24 | p |
|---|---|---|---|
| Hours apheresis-transplant | 29.3±18 | 30.3±13 | ns |
| Number of sessions | 6±3.7 | 5.9±2.2 | ns |
| Processed volume (n) | 1.08±0.2 | 1.1±0.3 | ns |
| TP | 0.91±0.05 | 0.92±0.07 | ns |
| TPTa | 0.99±0.33 | 0.91±0.13 | ns |
| Pre-TR fibrinogen (mg / dl) | 168.3±89.2 | 158.6±55, 5 | ns |
| TIF (min) | 91.5±24.3 | 102.4±35.9 | ns |
| TIC (min) | 3±0.7 | 2.6±0.4 | ns |
| Reduction of platelets × 1.000 | −57±50 | −45t±55 | ns |
| Dialysis modality (HD / DP / pre-dialysis) | 8/5/11 | 10/1/13 | ns |
| Type of apheresis | |||
| PFS | 15 (47) | 17 (53) | |
| IA | 5 (50) | 5 (50) | ns |
| Both | 4 (67) | 2 (33) | |
Data are expressed as mean±standard deviation on (%).
Patients received rapid-release tacrolimus (n=38) and delayed-release tacrolimus (n=10), mycophenolate mofetil (n=23), mycophenolate sodium (n=23) and everolimus (n=2). All patients received steroids from the begining. During the follow-up, 15 patients modified their immunosuppression due to: toxicity (n=6), malignancies (n=2), infection (n=2) and others (n=5). The change from anging anticalcineurin to mTOR inhibitor it was performed by suspending calcineurin once the i-mTOR reached levels within the therapeutic range (3−8ng / ml), usually after 4 days. The exchange of mycophenolic acid derivatives for another or for i-mTOR was performed without delay. Steroid withdrawal was performed in 9 patients due to adverse effects. No adverse reactions were recorded due to the administration of immunoglobulins.
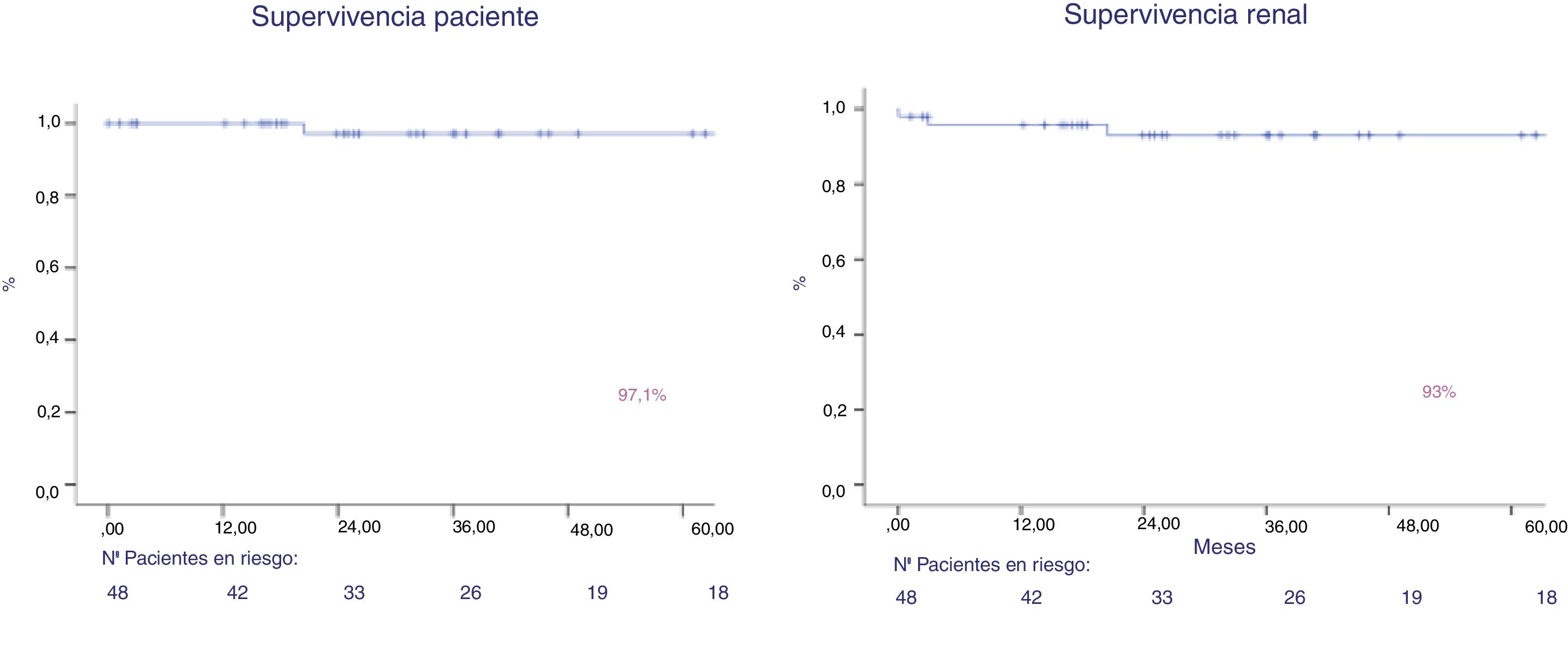
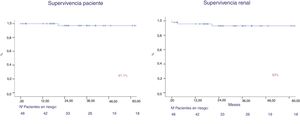
After a follow-up of 44.6 months, patient survival was 97 % and graft survival (death was not censored) was 93 % at year 1 and 5 (Fig. 4). Two patients lost the graft, due to venous thrombosis and mixed rejection. Two patients lost their graft due to death. Both maintained the graft function until death.
Five patients presented acute rejection (8.7 % at the fifth year): 2 borderline, 2 1A, treated with steroids and one was mixed rejection (mediated by antibodies and T cells) that did not respond to treatment with steroids, thymoglobulin, PFS, immunoglobulins and eculizumab (patient doubly sensitized HLA and ABO). Although C4d staining was positive in 3 of the 4 patients, who presented with T-cell mediated rejection, we do not consider C4d positivity a marker of rejection, since their presence, in ABOi transplantation, is usually associated with a process of accommodation. In addition, we found no increase in isoagglutinins or other histological signs of antibody-mediated rejection (BANF 2017). The patient who presented a mixed rejection (mediated by cells and HLA antibodies) showed an increase in anti-HLA antibodies (without an increase in the isoagglutinin titer) and histological features of antibody-mediated rejection.
The presence of rejection was associated to the donor's age: 66.6±11.7 vs. 51.7±0.7years (p=0.01), fewer apheresis sessions: 3.4±2.8 vs. 6.3±2.9 (p=0.04), a delayed graft function: 50 vs. 4.8 % (p=0.01) and the degree of IgG decrease : 4.9±4.5 vs. 17. 3±13.1 (p=0.04). In patients who suffered acute rejection after transplantation the IgG titer was 1: 2±3.4 vs. 1: 3.8±4 (without statistical significance). The smaller number of apheresis sessions and the modest decrease of IgG is related to the lower title of preconditioning isoagglutinins and, therefore, with the limited number of sessions required to obtain titles < 1: 8. In the Cox analysis, the absence of delayed graft function is considered a factor for not presenting rejection: Exp (B) 0.11 (95 % CI 0.01-0.78) and the donor age is risk factor for rejection: Expo (B) 1.11 (95 % CI 1.007–1.239). But, probably the low sample size prevents to draw conclusions.
Infectious complications post-transplant : CMV viremia=9 (16.7 % at the fifth year), BK viremia=5 (12.4 % at the fifth year). Patients presenting CMV were older 57.7±9.8 vs 49.3±10.7 years (p=0.03), and BK replication were more frequent in posttransplant patients with diabetes (30 vs. 5 %; p=0.05) or in those with acute rejection (40 vs. 6.5 %, p=0.07). In the Cox analysis did not reveal statistical significance. We found no association of infectious complications with mortality.
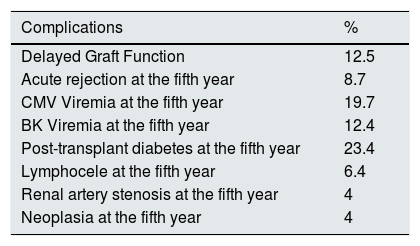
Other complications: diabetes post-transplant 10 (23.4 % at the fifth year), lymphocele 3 (6.3 %), renal artery stenosis 2 (4.2 %), neoplasms: a clear cell carcinoma in the native kidney diagnosed the seventh post-transplant year (3 %) and a cutaneous epidermoid carcinoma (Table 3).
Post-transplant complications of the ABO incompatible.
| Complications | % |
|---|---|
| Delayed Graft Function | 12.5 |
| Acute rejection at the fifth year | 8.7 |
| CMV Viremia at the fifth year | 19.7 |
| BK Viremia at the fifth year | 12.4 |
| Post-transplant diabetes at the fifth year | 23.4 |
| Lymphocele at the fifth year | 6.4 |
| Renal artery stenosis at the fifth year | 4 |
| Neoplasia at the fifth year | 4 |
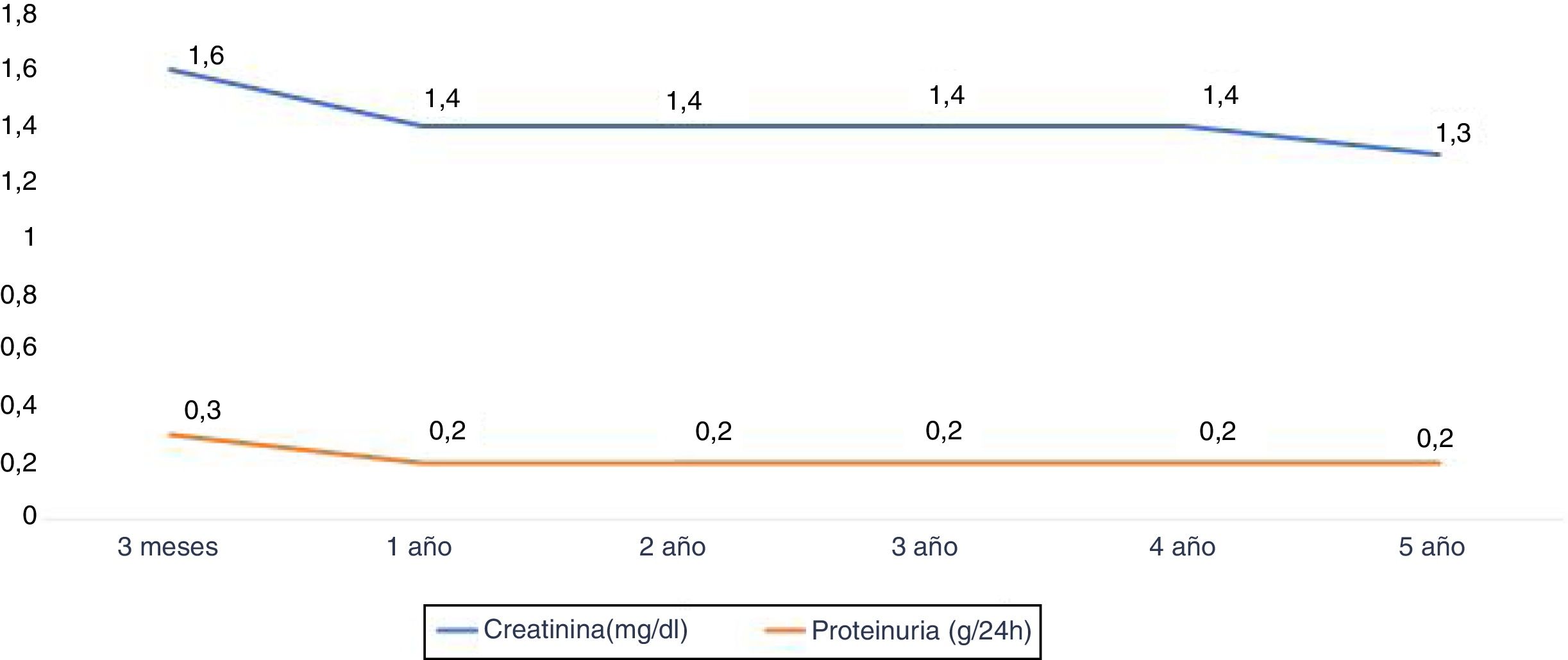
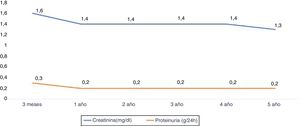
The renal function measured according to the serum creatinine (mg / dl) at year 1, 2, 3, 4 and 5 years was: 1.4±0.5, 1.4±0.4, 1.4±0. 4, 1.4±0.4 and 1.3±0.5 respectively. Proteinuria at year 1,3 and 5 years was 0.2±0.2g / 24h (Fig. 5).
DiscussionIn a meta-analysis, Lo et al.4 analyze 4810 ABOi kidney transplants performed under different conditioning protocols until 2015; the demonstrate excellent results, so the contraindication of this type of transplant, initially recommended by the European guidelines in 2000,1 has been modified.2
In our hospital, LDKT ABOi was started in 2008 and since then we have performed 48 of them. Patient survival in this study has been a 97 %. In most series it exceeds 90 %. The differences are usually related to the follow-up time, conditioning protocols, study period and infectious complications,4,6–10 although in a current review of 930 ABOi versus 89,713 ABO compatible transplants, Mustian et al.11 found an increased risk of death at year 1 of 1.81 (1.26–2.6) and at year 3 increased risk of death was 1.55 (1.21–1.98) that was related to age > 50 years and the presence of acute rejection.
Infections as a cause of mortality has been associated with the use of RXT, although this statement is controversial. Koo and Yang indicate the existence of an important heterogeneity in the use of RXT both in the number of doses and in the quantity of the doses used.3 In Japan, the use of RTX against splenectomy improves survival, but the use of 2 or more doses of RXT of 375/m 2 has been associated with an increase in mortality caused by infections in ABO compatible renal transplantation.12 Grim et al. report an increase in infections in 35 incompatible ABO and HLA patients treated with RXT and thymoglobulin, without an increase in mortality.13 Lee et al. did not find a higher incidence of infections with the different doses of RTX; however, they did find a higher incidence of rejection associated with lower doses of RTX.14
Two of our patients died, one during the postoperative period of aortic valve replacement and the other due to sudden death, with an age of 68 and 73 years, respectively, and with a functioning graft. The low mortality observed in the present study could be attributed to the use of a single dose of RTX, administration of immunoglobulins, low incidence of acute rejection, low number of infectious complications and a follow-up period of 44 months.
Graft survival in our series is 93 % in the fifth year, similar to other series. This survival depends on the follow-up time, the conditioning employed or the period of time studied.4,6–8,10,15–18 Becker et al., using IA columns of TheraSorb ®, communicate 95 % survival by the third year,19 and Galliford et al., using a steroid-free protocol from the first week had 100 % survival the first year.20 Recently, Mustian et al. found a decrease in renal survival censored for death in patients ABOI, 2.34 (1.85–2.96), associated with patient age>50 years.11
The frequency of acute rejection in our series is 8.7 % at the fifth year. In a recent meta-analysis,4 the frequency of acute rejection was 32.9 % (2.9–52.3 %). The frequency of acute rejection is variable, ranging between 4 and 44.5 %, depending on the conditioning, the type of rejection (mediated by antibodies or by T cells) or the presence of HLA incompatibility added.10,15–19,21 Data from the American registry11 find an incidence of acute rejection of 19.4 %, with a risk increase of 1.78 compared to compatible ABOs. Although it does not refer to the type of rejection, there is a strong correlation with a higher degree of HLA incompatibility and a higher percentage of PRA. In our group we found an association of rejection with a greater age of the donor, with a lower degree of reduction in the titer of isoagglutinins and with a greater frequency of delayed graft function. However, in the multivariate analysis the absence of delayed graft function was not a factor associated with the presence of rejection and the older age of the donor was a risk factor. These results should be interpreted in the context of a small number of patients and rejections, to make this statistical significance be translated to clinical significance.
CMV viremia was detected in 9 patients (19.5 %). There is also much disparity in the different series, which vary according to the period analyzed and the type of immunosuppression, ranging from 3.3 % reported by Melexopoulou et al.22 and 62.7 % by Kwon et al.,10 although the most common figures are around 21 %.9,15 Melexopoulou et al. use everolimus de novo in their protocol, adrug that prevents viral replication and may be responsible for this low incidence, as in other series.23 Recently, the TRANSFORM study demonstrated that the association of de novo everolimus with low doses of anti calcineurinics decreases the incidence of CMV and BK viral infections as compared with MMF and standard doses of anticalcineurinics.24 Höcker et al. demonstrated a significant decrease in viral replication in patients immunosuppressed with everolimus and low doses of cyclosporine or tacrolimus.25 The incidence of CMV viremia is similar to that of our HLA desensitization population and slightly higher than that of the living donor transplanted population (15 %). In our series patients who received everolimus de novo did not show CMV replication.
Five patients presented BK viremia in our series (12.4 % at the fifth year) without evidence of nephropathy. Other authors find similar incidences, ranging between 9.3 and 37 %.9,10,16,19,26 Melexopoulou et al. find 16.7 % of BK viremia, and 4 out of 5 patients have BK nephropathy. We do not know the timing at which this viremias are presented; it could be when patients were receiving everolimus or after conversion to mycophenolate, since the i-MTOR have demonstrated a lower incidence of BK in other series no ABOi24,27 by preventing virus multiplication in early stages.23 Belliere et al. demonstrated a decrease in viral load in LDKT ABOi patients converted from MMF to everolimus due to BK viremia.28 In our series, a patient treated with everolimus de novo developed BK viremia at 4.2 months post-transplant; the other immunosuppressed patients that received rapid-release tacrolimus and mycophenolate mofetil developed BK replication at 3, 4, 7 and 32 months. The incidence is slightly higher than that of our general population of ABO compatible live donor renal transplant (4 %) and there is little difference from other series.
Toyoda et al.29 studied a group of desensitized HLA patients and found no higher incidence of infections, especially viral infections, where they use RTX and immunoglobulins. They show that the depletion of T cells and secondarily macrophages and natural killer cells would make disappear a possible viral reservoir and the immunoglobulins would act as a treatment against viral particles. Muramatsu et al.30 find in these patients a great diversity of data on the incidence of infections, both CMV and BK, and it varies according to the dose of RTX, the incidence of rejection and the time of publication of the series.
The number of surgical complications observed is high: 50 % hematoma / hemorrhage, 14.6 % need an intervention and 60.4 % need red blood cell transfusion. It seems established that PFS and IA could cause a state of "bleeding" due to factor xiii deficiency (not detected in the tests that are commonly used), thrombocytopenia, hypofibrinogenemia and loss of other coagulation factors.31,32 De Weerd et al.33 reported that 29 % of patients need transfusions and reintervention in 3 patients. They associated bleeding with the number of IA sessions. Zschiedrich et al. showed surgical revisions in 21 % due to hemorrhage attributed to heparin administered in the graft reperfusion.18 Lentine et al. found an incidence of bleeding that ranging from 10 to 67 %, being higher in patients with splenectomy (25.9 %).34 They relate this complication to the factors previously mentioned and fundamentally associated with apheresis. Kim et al. found 12.8 % of postoperative bleeding related to a uremic state and the situation of pre-dialysis.35 In our series we found no differences in the number of platelets, TP, TPTa, fibrinogen, number of apheresis sessions, type of apheresis, time or dialysis situation or time since the last PFS/IA until the intervention. Nor did we find differences in the number or severity of complications according to the apheresis technique used, although we observed a greater thrombocytopenia associated with AI and lower fibrinogen levels associated with PFS, and although this association of both techniques presented a greater number of complications, the differences were not statistically significant. The factor XIII was not measured and the post-transplant heparinization pattern is the same as in the ABO compatible living donor renal transplant, where the frequency of postoperative bleeding is less than 8 %. The number of surgical complications did not cause an increase in hospitalization, which, in our series, is related to the preconditioning isoagglutinin titer.
The number of PFS / IA sessions pre- intervention was 6±3, similar to other series. In Friburgo the average is 5.7±3.6, in Heidelberg, 75–12 and in Athens, 5±3. In general, it depends on the titles preconditioning isoagglutinins, technical of apheresis and the cut-off marked to accept the transplant. Lawrence et al. needed 8 sessions of PFS to reach < 1:4; 5 patients from 51 transplant candidates could not be transplanted because they did not reach those titers. They used PFS, which does not diminish the isoagglutinin titers much as the DDP (double filtration plasmapheresis), used in Japan, or the IA, used mainly in Europe, although Parmentier et al. found greater reductions of isoagglutinin with PFS than with IA.36 Tobian et al. found a 20 % reduction of isoagglutinins with each treatment using PFS37 and Vallian et al. a 30 % reduction of IgM isoagglutinins after the first IA.38 Rostaing et al. managed to process large plasma volumes in a single IA session reducing the isoagglutinin titer and allowing to perform the transplant.39 Our group uses IA when the titles are > 1: 256, or, plus PFS, when they are greater than 1: 512. According to our results, IA or its association with PFS decreases more isoagglutinins than isolated PFS. In our hospital, we were unable to reduce the isoagglutinin titer and, therefore, we were unable to transplant 2 patients who had baseline tites of 1: 1024 and 1: 2048.
It is not established whether patients with titles < 1: 8 at the time of transplantation need to PFS or RTX. Masterson et al.40 reported 100 % survival in 20 cases without conditioning and one rejection mediated by antibodies and 4 rejections mediated by T cells. Gelpi et al.41 find a 90 % survival and a borderline rejection. Krishnan et al.42 report 2 cases with antibody- mediated rejection and subsequent renal loss. Nanmoku et al.43 compared 21 ABOi patients who received PFS with IgG 1:32 tites with a group of 11 patients who did not receive PFS with a 1: 8 tite and did not observe a higher incidence of rejection, complications or changes in survival. In our series, 3 patients with preconditioning titles <1: 8 received a session of PFS and RTX. One patient presented acute borderline rejection.
The technique that we use for the quantification of isoagglutinins is the microrcard gel. Muramatsu at al.30 reviewed the methods of quantification of isoagglutinins based on experiences in northern Europe and Japan and conclude that the tube hemagglutination technique give results with a wide range of tites and a variation of the technique; using a micro-card gel is closer to the ideal method (flow cytometry), with greater reproducibility and lower interindividual and intercenter variability. The incidence of rejection when this quantification method is used ranges between 6.5 and 27 %.16,18,19,31,32 Krishnan et al. described two patients who did not received pretransplant conditioning to present title < 1: 8 by microcard gel and they developed rejection mediated by antibodies, with a significant increase in titers. These authors demonstrated, using flow cytometry, a hyperresponse in receptors O receiving donors A1 and B.42
The frequency of post-transplant diabetes is 23.4 %, and this data is not reflected in the series. Its incidence does not differ from the population ABO compatible. The incidence of lymphocele is 6.2 % lower than other series,18,19,22 which ranges between 12 and 33 % and that some author44 associates this with MMF immunosuppression.
After a mean follow-up of 44 months, one patient developed a clear cell carcinoma in her native kidney and another developed a cutaneous carcinoma. There are limited data in the literature on the incidence of neoplasms in LDKT ABOi. Lo et al. indicate a single study45 showing 2 lymphomas and a gastric cancer. In the American registry they do not find a higher risk of cancer in ABOi than in compatible ones.46 Possibly the absence of data reflects the short follow-up time of these transplants, which conditions the results.
The limitations of our study are the small sample size, which limits some analysis (postoperative complications), the use of 2 apheresis techniques (mainly motivated by the high cost of IA), which makes the decrease in titles not to be homogeneous, and the short follow-up period. Despite the limitations, the overall results seem appropriate.
In conclusion, our series demonstrates the efficacy of TRDV ABOi T, with an excellent survival of both the patient and the graft, without any increase in the incidence of infectious complications. As reflected by other authors, it remains to be evaluated the optimal immunosuppressive treatment, the cut-off of the titles and the number of apheresis sessions necessary to achieve that cut-off in the presence of high titers, which is the apheresis technique that is most efficient, the need for conditioning in the presence of "low" basal isoagglutinin titers, doses of RXT, the use of anti-CD25 or steroid boluses at the time of transplantation.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Rivera CF, Rodríguez MC, Muñíz AL, Hermida TF, Bestilleiro RS, Saavedra CA, et al. Trasplante renal de donante vivo ABO incompatible. Estudio de 48 pacientes tras desensibilización. Nefrologia. 2019;39:612–622.