Recommendations for living donor kidney transplantation
More infoThis Guide for Living Donor Kidney Transplantation (LDKT) has been prepared with the sponsorship of the Spanish Society of Nephrology (SEN), the Spanish Transplant Society (SET), and the Spanish National Transplant Organization (ONT). It updates evidence to offer the best chronic renal failure treatment when a potential living donor is available. The core aim of this Guide is to supply clinicians who evaluate living donors and transplant recipients with the best decision-making tools, to optimise their outcomes.
Moreover, the role of living donors in the current KT context should recover the level of importance it had until recently. To this end the new forms of incompatible HLA and/or ABO donation, as well as the paired donation which is possible in several hospitals with experience in LDKT, offer additional ways to treat renal patients with an incompatible donor.
Good results in terms of patient and graft survival have expanded the range of circumstances under which living renal donors are accepted. Older donors are now accepted, as are others with factors that affect the decision, such as a borderline clinical history or alterations, which when evaluated may lead to an additional number of transplantations.
This Guide does not forget that LDKT may lead to risk for the donor. Pre-donation evaluation has to centre on the problems which may arise over the short or long-term, and these have to be described to the potential donor so that they are able take them into account. Experience over recent years has led to progress in risk analysis, to protect donors’ health. This aspect always has to be taken into account by LDKT programmes when evaluating potential donors.
Finally, this Guide has been designed to aid decision-making, with recommendations and suggestions when uncertainties arise in pre-donation studies. Its overarching aim is to ensure that informed consent is based on high quality studies and information supplied to donors and recipients, offering the strongest possible guarantees.
Esta guía de recomendaciones para el TR de donante vivo (TRDV) es un documento elaborado con el patrocinio de la Sociedad Española de Nefrología, la Sociedad Española de Trasplantes y la Organización Nacional de Trasplantes que actualiza la calidad de la evidencia disponible para ofrecer el mejor tratamiento de la insuficiencia renal crónica cuando se disponga de un donante vivo potencial. El objetivo principal de esta guía es proporcionar a los profesionales con responsabilidad en los estudios previos del donante vivo y del receptor trasplantado, las mejores herramientas para tomar decisiones en beneficio del donante vivo y del receptor del trasplante.
Además, en el contexto actual del TR, el donante vivo debe recuperar el protagonismo que alcanzó en un pasado reciente. Para ello, las nuevas modalidades de donación HLA y/o ABO incompatible, así como la donación cruzada disponibles en diversos centros con experiencia en TRDV, son oportunidades adicionales para el tratamiento de enfermos renales que tienen un donante incompatible.
Los buenos resultados en supervivencia del paciente y del injerto están ampliando las circunstancias de aceptación de donantes vivos de riñón, incluyendo donantes de mayor edad y otros con algunos condicionantes que incluyen antecedentes o alteraciones límite que, cuando son evaluados con criterios objetivos, pueden aportar un numero adicional de trasplantes.
No se ha obviado en esta guía que el TRDV puede representar algún riesgo para el que dona. Estos problemas que pueden aparecer a corto o largo plazo tienen que ser objeto principal de valoración previa a la donación y presentados al potencial donante para que en ejercicio de su autonomía los asuma o rechace. La experiencia acumulada en los últimos años ha permitido avanzar en el análisis de riesgos para preservar la salud de los donantes, aspecto que debe estar siempre presente en los responsables de programas de TRDV cuando se procede al estudio de idoneidad de un potencial donante.
Finalmente, esta guía ha sido estructurada para facilitar la toma de decisiones con recomendaciones y sugerencias ante incertidumbres derivadas de los resultados en los exhaustivos estudios predonación. Y todo ello, con el objetivo de que el consentimiento informado que debe certificar la calidad de los estudios y la información proporcionada a donante y receptor, alcancen las mayores garantías posibles.
In 2010 the Nefrología Journal published a supplement containing the Recommendations of the Spanish Society for Nephrology (SEN) and the Spanish National Transplant Organization (ONT) for living donor kidney transplant (LDKT).1 Four year later our country achieved its record, with 423 LDKT.
LDKT is now a consolidated treatment, and it is the first therapeutic option which should be offered to patients with end stage renal disease (ESRD). This is so for paediatric as well as adult cases in terms of survival and quality of life.
Now is therefore a good time to update the 10 year-old recommendations document. This Project was undertaken under the auspices of the SEN, the ONT and the Spanish Transplant Society (SET), with the core aim of expanding the use of LDKT. It includes all possible types of LDKT: paired donation, ABO incompatible donation, donation for highly sensitized recipients, altruistic donation and chain donation, etc. All of these procedures complement deceased donor kidney transplant (KT), and together with direct LDKT they ensure the maximum number of transplants, regardless of their complexity.
This document analyses donor safety several times. The health risk for someone who donates one of their kidneys must be minimized, carefully assessed and described to the donor, so that they are able to decide completely independently. Donor well-being is non-negotiable, and they are therefore recommended to follow a healthy lifestyle after donating, with a life-long follow-up.
This document is not a Guide like those published in the United Kingdom,2 North America3 or Europe.4 It is a hybrid between a Review, an Update and a Clinical Practice Guide, where Clinicians are able to find recommendations or the opinions of LDKT experts when the evidence is either weak or non-existent. Unfortunately the majority of recommendations in LDKT lack high-quality evidence, so that a large number of recommendations figure as suggestions with low or very low evidence quality (C, D), so that for reasons of simplicity and utility we decided to simply underline evidence quality.5 Expert recommendations and suggestions that are not graded (NG) have also been included when it was not possible to show any evidence, as well as in the case of legal requirements.
In any case, this LDKT update monograph should be highly useful for Clinicians working in the field of donation and transplant, so that they are always able to find the best resources and offer solutions to complex problems in their clinical practice.
The methodology followed for this update commenced with the identification of the most recent and important developments in LDKT since the publication of the 2010 Recommendations. This is the case for paired KT; ABO and HLA incompatible transplants; expanded criteria donors, and donor risk analysis.
The sections were distributed among the teams which had published outstanding works in each specific field. The teams were asked to undertake a systematic review of the bibliography, with the aid of evidence already underlined in documents published by the British Transplantation Society (BTS) and KDIGO2,3 before finishing with a study of the literature published in recent years.
The three coordinators also prepared a list of the minimum subjects to be covered in each section, to ensure that they were completely up-to-date. Each manuscript was reviewed by a group of Special Editors after it had been examined by the coordinators. This group was composed of important KT clinicians in Spain, and they edited the documents before agreeing the final versions with the authors.
This process took 18 months, and those involved also had to manage the work overload due to the Covid-19 pandemic. We would therefore like to offer our most sincere thanks to all of the authors who helped to prepare this document.
1The current situation of living donor renal transplantation in Spain and other countriesLiving donor KT (LDKT) is the therapy which gives the best results in patients with end stage renal failure, in terms of patient and graft survival as well as quality of life.6 Ever since the first successful LDKT between humans took place in 1954,7 advances in immunosuppression, the development of minimally invasive surgical techniques for donor nephrectomy, and agreement on how to evaluate, select and care for living donors8 mean that this treatment is now considered systematically for end stage renal failure patients.2,9
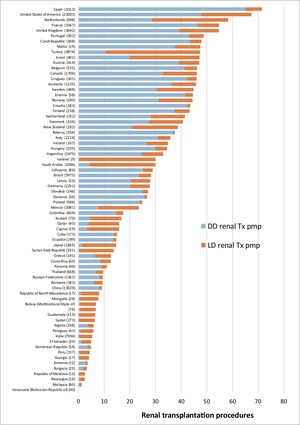
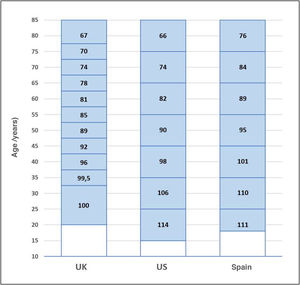
According to Global Observatory on Donation and Transplantation data, every year approximately 90,000 renal transplants are performed, of which approximately 40% come from living donors.10Figure 1 shows kidney transplantation (KT) activity per country in 2018, differentiating between deceased donor and living donor procedures. When transplantation activity is compared, countries with a lower human development index (HDI) are found to base their KT scheme on living donors, although their rates per million of population (pmp hereunder) are lower than the rates in countries with a higher HDI, which perform KT from deceased as well as living donors.11 Nevertheless, major differences are also found in LDKT pmp activity between countries at similar socioeconomic levels, as is the case within the European Union.
Spain has a high rate of deceased donor transplantation and more than 3,000 KT procedures per year. LDKT here is now a consolidated treatment option due to its benefits for patients, as well as the transplantation system as a whole, with low risk for donors. In this chapter we review the reasons which justify the LDKT scheme in our country, covering important aspects of donor protection. We describe the situation of the scheme in Spain, together with the challenges which our system has to face in the current scenario.
The need for living donor KTThe LDKT scheme is needed for several reasons. Firstly, this is due to the benefits it offers recipients in comparison with deceased donor KT. LDKT is also necessary if we are to progress towards transplantation self-sufficiency, as it would be hard to meet the needs for KT in our population given the gradual change in the profile of deceased donors. Finally, the decision to implement this scheme is based on a substantial improvement in the procedure for donors.
Living donor KT resultsLDKT is associated with better long-term results than deceased donor KT, regardless of the genetic relationship between the donor and recipient6,12 or whether it is a first or second transplantation.13 European Registry of Renal Replacement Therapy data (ERA-EDTA) show a renal graft survival rate (adjusted for age, sex and cause of primary renal disease) of 86.7% at 5 years for living donor recipients, as opposed to 81.4% for deceased donor recipients. Patient survival rates at 5 years (adjusted for age, sex and cause of primary renal disease) are 94.6% and 92.1%, respectively.14 American Registry data show very similar results to those of the European Registry.15
Different reasons have been suggested for the better results obtained with LDKT, including good living donor basal health, with less associated pathology than deceased donors.1 However, no significant differences are found in mid-term survival or the incidence of acute rejection when the results of deceased donor KT are compared with those of standard living donors in patients with similar characteristics. Nevertheless, there is a significant difference in graft function delay.16 These differences in favour of living donation are significant when the overall results of LDKT are compared with those corresponding to deceased donors, without differentiating deceased donor type (brain death or asystole, and standard or expanded criteria).6,13,17
On the other hand, LDKT is performed more often than deceased donor transplantation before the patient commences renal replacement therapy with dialysis 18. It has to be underlined that long-term dialysis and its associated comorbidity have repeatedly been identified as factors which have a negative association with transplanted graft and patient survival, regardless of chronic renal failure aetiology 19,20.
Based on current evidence, the better results achieved with LDKT are therefore fundamentally due to transplant recipient characteristics and degree of comorbidity, the delay until transplantation and donor profile.
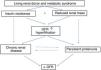
Changes in deceased donor profile and the waiting listRoad and industrial safety laws have changed fundamentally in Spain over the last 20 years, and there has also been a continuous improvement in medical care. These factors have given rise to a fortunate decrease in the mortality of the population, although this has also caused a gradual fall in potential donations due to brain death 21.
Thanks to its transplant coordination network and medical and surgical teams, our system has adapted to this new scenario. However, although this has increased the number of transplantations, it has also involved a substantial change in donor profile.
The changes in end-of-life care for critical patients have continued, with the unprecedented development of controlled asystole donation programmes 16,22. Successful organ transplants from elderly and very elderly donors now occur regularly, adapting to potential older donors with associated comorbidities. The implementation of projects such as the one for non-standard risk donors 23,24 and the preparation of clinical guides 25–28 to ensure appropriate evaluation of the risk-benefit of transplanting organs from donors with different pathologies, have made it possible to gradually broaden the degree of acceptance and subsequent use of organs from more complex donors.
It is within this context that Spain achieved its highest ever rate of KT and the highest in the world, with 72.8 transplants pmp in 2019 29. Nevertheless, in spite of the fact that the transplantation waiting list shortens year after year, at the end of 2019 it still stood at 3.933 patients, so we are still a long way from becoming self-sufficient. When examining the negative effect of the time spent in dialysis on post-transplantation survival, we find that only 5% of ESRD patients were transplanted before starting treatment with dialysis 30.
The increase in deceased donors has occurred due to the ongoing rise in the number of progressively older donors, including those in controlled asystole. This makes it unlikely that young patients with end stage renal failure will be transplanted after only a short delay. Although this population segment may benefit more clearly from living renal donation, this therapy is suitable for patients in any age band.
It has to be pointed out that the scarcity of young donors also affects the availability of other organs, such as the pancreas. Very briefly, diabetic patients compete against potential young recipients for young kidneys. They all seek an appropriate donor in terms of age, especially in the case of highly sensitized patients. LDKT may help to resolve this situation by occasionally offering the possibility of pancreas transplant.
Improving living donor safetyAlthough the benefit of LDKT for patients is clear, this therapy could not be used without the sine qua non condition of guaranteeing donor safety. Each chapter of this clinical guide therefore takes into account the possible consequences for donors and their subsequent quality of life.
The standardization of the evaluation and care of living donors 1,31, as well as the use of increasingly less invasive nephrectomy techniques 32, have the primordial aim of protecting donor health 1,8,9.
Although performing a nephrectomy in a healthy individual is not innocuous, the risk of immediate mortality associated with living renal donation is estimated to stand at 0.03%, and this has not varied in the last 15 years 33. The introduction of laparoscopic nephrectomy has led to a considerable improvement in the immediate postoperative period, and donors recover and recommence their social and professional lives sooner. Although the probability of a living renal donor developing renal disease over the long-term is lower than the figure for the general population (where comorbidity is a factor), it is still higher than it would have been if the donor had not been subjected to nephrectomy 34–37. Age, African American, obesity and familial genetic diseases have been shown to be risk factors in long-term donor evolution. It is therefore necessary to evaluate donor risk and take these factors into account, in the information supplied to donors and recipients as well as in donor care after the procedure 9.
Donor protectionA LDKT scheme has to be based on the fundamental principle of complete protection of the living organ donor. The basic principles of this protection are contained in different international legal documents: the WHO Guiding Principles on Human Cell, Tissue and Organ Transplantation, the Council of Europe Convention on Human Rights and Biomedicine, as well as its Additional Protocol concerning Transplantation of Organs and Tissues of Human Origin, and Directive 2010/53/EU of the European Parliament and the Council, of 7 July 2010, on the Standards of Quality and Safety of Human Organs intended for Transplantation38–40. In Spain these principles are expressed in Law 30/1979, of 27 October, on organ harvesting and transplantation, and in Royal Decree 1723/2012, of 28 December, which governs the Activities of Obtaining, Clinical Use and Territorial Coordination of Human Organs intended for Transplantation, establishing Quality and Safety Requisites 41. Apart from this national and international legislative framework there are also national professional standards 1, such as this Guide, and international ones, especially the KDIGO guides 9.
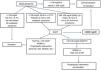
The protection of a living donor starts with the appropriate evaluation and selection of a potential candidate, based on medical and psychosocial criteria 8. Prospective donors should be subjected to an exhaustive standardized evaluation of their potential as such to assess the risk that donation would involve for their state of health–as well as for the potential recipient of the donated organ. Risk assessment covers aspects of psychosocial as well as physical health, enabling the identification of absolute or relative contraindications to donation.
The public health system acquires a responsibility towards living donors, because of the complications they may develop over the short, medium or long-term. It is therefore obligatory to ensure living donors have continuous medical protection and care to preserve their residual renal function (in the case of renal donors) while treating any possible complications deriving from donation. Within the European Union it is also obligatory to record information about each living donor in national registries designed to show their basal clinical and demographic characteristics and evolution, including any complications associated with the donation process. Apart from recording this information, it is also obligatory for medical workers to notify the National Biovigilance System 42 of any adverse events (donation incidents that place a living donor at risk, even if no harm was caused) and adverse reactions (incidents that harmed a living donor).
Determining the validity of the consent to donate is a basic part of evaluating a living donor. A valid consent must be freely given, informed and clearly expressed. When evaluating a living donor it is fundamental to elucidate the underlying reasons for the donation, and to evaluate the legitimacy of the relationship between the potential donor and the recipient. Information about the process should be broad in scope, structured and comprehensible. The possibility of discontinuing the process at any time and without the need for any explanation whatsoever should be expressed. The living donor advocate plays a fundamental role in determining the validity of consent: this professional is unconnected with the individuals who are going to perform the harvesting and engraftation of the organ, and they work to ensure the complete protection of the person. In our country this role is fulfilled by the intervention of an independent medical professional, while the Ethics Committee of the hospital in question also participates, and the potential donor has to appear before the Examining Magistrate. This three-filter system established by our legislation is probably one of the strongest safeguards in the world within this context. The donation procedure must be halted if situations which lead to suspicions of human trafficking or other comparable scenarios are identified. The relevant authorities should also be notified, to activate investigations and prosecute any possible crimes.
The protection of living donors also involves the elimination of disincentives against donating organs while alive. These include reimbursing any expenses incurred by the donor and compensation for loss of earnings due to the assessment, surgical operation and subsequent recuperation. Another example of measures to be applied would be protection against loss of employment due to time off work for diagnostic tests or the act of donation itself.
The evolution of living donor KT in Spain and how it compares with other countriesIn spite of the strong international consensus in favour of LDKT, this treatment did not become widespread in Spain until the early years of the 21st century. This was partially because of the success of the Spanish model of donation and transplantation 43, and partially because clinicians did not wish to perform a nephrectomy on healthy individuals 44,45.
Given the agreement reached in the Amsterdam Forum, the international stance regarding donor protection and the good results obtained with this therapy in major international registries 8,33,46,47, LDKT started to be advocated in different areas (transplantation teams, scientific societies, patient associations, regional transplantation coordination bodies and the ONT) 48. Work was undertaken to raise awareness of this therapy among patients and their families: the benefits it offers patients, the type of assessment donors are subjected to and the possible consequences of donation for them 1. Major efforts were also made to train the clinicians involved in this activity.
The implementation of a national registry was fundamental in Spain to show the possible consequences of living donation. This registry includes data on the main clinical and epidemiological characteristics of living renal donors, the nephrectomy techniques used, their complication rates and the possible clinical implications of renal donation over the short-, medium- and long-terms. This registry started working in 2010 and, thanks to the commitment of administrative bodies and clinicians, it now contains information on 96% of living renal donors. It makes it possible to extract reliable conclusions, so that it is used to assess donor risk as well as supplying information they are given about the procedure.
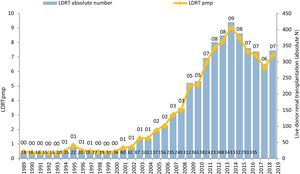
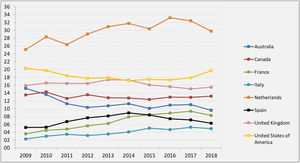
These measures have driven the emergence of new LDKT schemes and increased activity in existing ones. While 61 LDKT were performed in 12 hospitals in 2004, more than 300 procedures in 33 accredited hospitals were performed in 2019, corresponding to an activity of 7.1 transplantations pmp 29 (Figure 2). Although this is good news, it is important to reflect that, in spite of the increase in activity, we are still a long way from achieving the aim of preventing many patients from having to enter dialysis. When LDKT over recent years in Spain is compared with the situation in other nearby countries or ones in similar sociodemographic and economic circumstances, we find a lower rate of activity than is the case in the Scandinavian countries, the Netherlands, the United Kingdom, the United States (U.S.A.) or Canada 49. A slight fall in recent years is a striking finding in the U.S.A., although this has been analysed by the system there and has now been reversed (Figure 3).
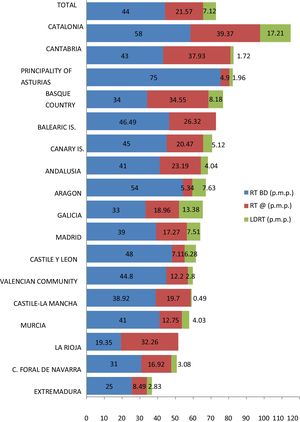
Nor has transplantation activity increased uniformly throughout Spain. There are large differences between the numbers of LDKT procedures pmp performed in the different Autonomous Communities, leading to unequal access to this therapeutic option (Figure 4). Several of the Communities with the highest rates of LDKT pmp also have high rates of deceased donor KT pmp. LDKT activity in Catalonia and Galicia is similar to that registered in a country as active in this field as the United Kingdom 49.
Adapting the living donor KT scheme to new needsStandard LDKT takes place between a donor and recipient who are blood group and HLA compatible. One of the advantages of living as opposed to deceased donation is that as it often takes place between blood relatives, there is a higher probability that donor and recipient will share a higher number of antigens. Nevertheless, this fact has not prevented LDKT taking place between genetically unrelated individuals, with excellent results 50. This favours the increasing use of transplantation between couples, friends and unrelated individuals, as occurs in paired and altruistic renal donation programmes 51.
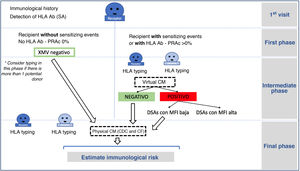
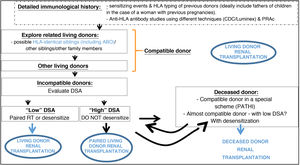
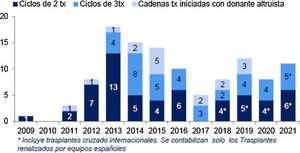
The increase in living donation has led to a rise in the number of potential donor assessments which detect incompatibility with the recipient. In fact, from 30% to 40% of potential donors who are evaluated are found to be incompatible with their recipient. This may be due to blood group incompatibility, the presence of hemagglutinins against the donor's blood group, or the detection in the recipient of specific antibodies against the HLA of their donor. Different strategies have been used to overcome incompatibility: some of them are based on desensitizing the recipient so that direct incompatible transplants can be used (ABO or HLA incompatible) 52–54 while others use donor interchange within a pool of incompatible pairs, known as pooled or paired transplantation 51,55,56. Both strategies have been used in Spain for more than 10 years, enabling LDKT for approximately 50 patients per year 29 who would otherwise have remained on the waiting list for a deceased donor.
Adapting the LDKT scheme is not solely based on overcoming incompatibility, as it also involves broadening donor acceptance criteria in terms of cardiovascular risk factors, as well as donor and recipient age. Respecting the former, and always with donor protection as the core aim, the number of donors with pharmacologically-controlled arterial hypertension (hypertension) has risen over recent years. This is also the case for patients with obesity (defined as a BMI≥30 Kg/m2), and these donors now amount to 10% and 16% of live renal donors, respectively 57.
Respecting age, according to Spanish Registry of Renal Patients (REER) data, annual post-transplantation mortality is lower than that of those patients of all age groups who remain in dialysis 30, so that LDKT is also an excellent option for the oldest group of patients. In fact, in Spain 16% of LDKT recipients are above 60 years old, as are 21% of donors, with good post-donation evolution 57.
Optimising the living donor KT schemeThe national LDKT scheme over recent years has gradually improved in quantitative terms, with an increasing number of transplantations. It has also improved qualitatively, thanks to better knowledge of the process and its results, increasingly systematic information about this therapy and the overcoming of technical obstacles against transplantations of this type. Nevertheless, several challenges still have to be faced, and these are described in the National Organ Donation and Transplantation Strategy for 2018-2022 58.
Insufficient LDKT activity and the differences between hospitals and Autonomous Communities mean that a strategy of identifying and spreading good practices is necessary. This is so for the organization and development of living donor information and assessment, as well as selection processes in terms of immunology, nephrology, urology and psychosocial aspects. Good practices should also be identified and shared regarding the surgical procedure for the donor and their care and follow-up. The ONT, the SEN and the SET have therefore led a benchmarking project which will produce specific recommendations to be adopted by hospitals and clinicians to improve critical areas within the LDKT process. It is important to always supply information about LDKT in ESRD consultations. The position in charge of coordinating LDKT activity in accredited hospitals should be identified, and ad hoc training actions for the teams and clinicians who are able to prescribe this renal replacement therapy have to be developed.
Measures to protect living donors have to be improved in our country, including those which apply to social and work-related factors that require reforms which are already being prepared. The introduction of technical improvements in the national paired KT, altruistic donation and incompatible donor schemes will also make it possible to increase the number of patients who are able to benefit from LDKT with suitable post-transplantation results.
Finally, the need to reach clinicians in an effective way is one of the reasons why the SEN, the SET and the ONT decided to prepare this LDKT recommendations document.
2Regulations governing living donor KT in Spain- •
LDKT is governed in Spain by the 30/1979 Transplantation Law, developed by Royal Decree 1723/2012 (Quality of evidence: NG).
- •
Our law permits living donor organ donation if this is compatible with life, and if the function of the organ may be compensated for by the organism in a safe manner (NG).
- •
Such donation requires the absence of any economic, psychological or social conditioning factors, and that the destination of the organ will be transplantation in a certain individual (NG).
- •
Although the regulations mention the possibility of donation between individuals related genetically, by kinship or close friendship, they do not exclude donation between individuals with no ties, on condition that this takes place voluntarily, altruistically and selflessly (NG).
- •
The donor has to be legally of age, with full mental faculties and a suitable state of health, which is to be accredited by the relevant medical certificate issued by a doctor who is not involved in the donation process, and they should be fully informed of the consequences of their decision (NG).
- •
The donor must grant their consent expressly, freely and consciously before a judge, although before this takes place they will have already signed a medical informed consent document in which they state that they have been informed of the possible risks for their person and the advantages which are expected to arise from the engraftation of the organ in the recipient (NG).
- •
Hospitals which perform LDKT must be authorized for the harvesting as well as for the transplantation of the said organ, which makes it necessary to meet the requisites demanded for the authorization of hospitals to obtain and transplant from cadaveric donors, so that framework protocols should be prepared to guarantee the quality and safety of the procedure (NG).
- •
Failure to comply with the requisites established in the transplantation regulations may give rise to administrative responsibility due to the commission of an infraction (NG).
- •
In the cases of organ harvesting without the free, informed and express consent of the living donor, or when a reward of any nature intervenes, an organ trafficking crime will have been committed and penal responsibility will be demanded (NG).
More than forty years have passed since Law 30/79 on the harvesting and transplantation of organs was passed, and it has only been modified once. The change was made by Law 26/2011, of 1 August, to adapt the regulation to the International Convention on the Rights of Persons with Disability, and which adds letter e) to article 4 in the subject of how individuals with disability are to be informed and how they are to give their consent.
In spite of the passage of time, this regulation is still a valid and secure instrument for governing the subject it covers, giving the system a high level of reliability. The ethical and legal implications deriving from living donor organ donation are unquestionable. Living donors are healthy people who are subjected to a surgical operation that is not indicated to improve their physical condition, but rather that of someone else who is sick. This intervention involves the donor losing a major organ, harm which is legally permitted on the grounds of the consent of the donor and the expected benefit for the recipient. However, this exception to the general rule of penalising those who cause severe injuries is conditioned by the need for strict compliance with certain requisites, to guarantee that consent is given freely and selflessly by the donor, after receiving exhaustive information on the risks and benefits involved.
The articles of the law establish the governing principles of the activity and distinguish two different legal regimes, depending on whether the organs in question are from a living or cadaveric donor. Each specific regime is supervised by the regulations that develop the law, complemented by the directive governing the harvesting and use of human tissues.
The basic principle is that of being free of charge, expressly forbidding any economic compensation for the donor due to organ donation, while it is also prohibited for the recipient to be subjected to any demand for payment or reward whatsoever. This declaration is understood without prejudice to adopting the necessary measures to ensure that donation will never be costly for the donor. It is therefore possible to compensate donors for any economic losses incurred as the result of the donation process, without violating the principle of being free of charge.
The second principle expresses the purpose that has to guide the whole activity being regulated: organs may only be collected and transplanted for therapeutic ends, thereby excluding purposes such as research or other equally legal benefits.
The legal regime differs depending on whether the donor is living or cadaveric: for living donors, the law demands compliance with a series of requisites which have the aim of guaranteeing the validity of the consent that the donor should give after receiving the necessary information. These are:
- a)
That the donor must be of age. Royal Decree-law 9/2014, of 4 July, which establishes the quality and safety standards for donating, obtaining, evaluating, processing, preserving, storing and distributing human cells and tissues, and passes the regulations governing their coordination and functioning for use in humans, permits obtaining cells and tissues from minors or individuals incapable of giving consent in the case of surgical residues or haematopoietic precursors, or other reproducible cells and tissues with a therapeutic indication that is or may be vital for the recipient. Under these circumstances, consent will be granted by the individual who is the legal representative of the donor (article 7.1).
- b)
That the donor must be of sound mind. That the donor was previously informed of the consequences - physical, spiritual and psychological - of their decision, as well as the eventual repercussions that the donation may have on their personal, family and professional life. Respecting cells and tissues, the regulation requires a personal interview with the donor, during which a structured questionnaire has to be completed. According to article 7.1 of Royal Decree-law 9/2014, of 4 July, the information which the doctor who will perform the harvesting or who is in charge of the same should offer the donor has to include the objective and nature of the means of obtaining the cells and tissues; its consequences and risks; the analytical tests which will be performed; data recording and protection; and the therapeutic purposes of the procedure. Likewise, the donor will be informed of the protective measures applicable to them and the expected benefits for the recipient of the use of the tissue or cell group collected. In the event of a “possible similar use” of the cells or tissue, the content of the information offered will also have to include: i) the indication that the cells and tissues obtained will be available for allogenic use in other patients, if this is therapeutically indicated, ii) information that is up-to-date, true and complete on the state of scientific knowledge respecting therapeutic or research use; iii) the processing and storage conditions in authorized establishments, and iiii) any other question associated with the therapeutic use of the cells and tissues obtained that was not medically indicated at the time they were obtained and when preservation of them commenced. Likewise, they are to be informed of the benefits which the recipient is expected to obtain from the transplantation of the donated organ.
- c)
That the donor is to grant their consent expressly, freely and consciously.
- d)
If the donor were a person with disability that fulfils the requisites described in the above sections, the information and consent document should be provided in suitable formats, following the rules set by the principle of design for all, so that they are accessible and comprehensible for their type of disability.
- e)
That the destination of the collected organ will be transplantation into a selected person with the purpose of substantially improving their life expectation or conditions.
- f)
That the anonymity of the recipient is guaranteed.
The legal regulation concludes by listing the requisites which have to be met for the person in charge of the transplant to give their consent for the same, all of which are considered from the viewpoint of the recipient. The demand for this requisite is modified by legal regulation - RD 1723/2012- as article 5 of the same stipulates that this limitation is not applicable to those directly involved in the transplantation of organs from a living donor between genetically related individuals or those related by kinship or close friendship:
- a)
That they should be fully aware of the type of intervention that will be carried out, knowing the possible risks and foreseeable advantages which, in physical and psychological terms, may derive from the transplantation.
- b)
They should be informed when so required that necessary immunological, histocompatibility studies or any others that may be necessary have been performed on the donor and future recipient by a laboratory that is accredited by the Ministry of Health, Social Policy and Equality.
- c)
That the said information is supplied using appropriate formats, following the rules set by the principle of design for all, so that it is accessible and comprehensible for individuals with disability.
- d)
That they express their consent to the performance of the transplantation in writing or another medium suitable for their disability when they are an adult who is legally responsible for their actions, or by their legal representatives, parents or tutors if they are disabled, or in the case of minors. If the recipient is a person with disability, their personal circumstances must be taken into account together with their capacity to make the said specific decision, considering the supply of support for making these decisions. In the case of persons with disability and the need for support in decision-making, they will freely decide once they have been supplied with the forms of supports and assistance necessitated by their specific circumstances.
The recipient is a patient and therefore this regulation has to be completed with the stipulations of law 41/2002, which governs patient autonomy and their rights and obligations regarding information and clinical documentation. It must be understood that the minors referred to in article 6 of the law are under the age of 16 years. The age of maturity required to be an organ donor and recipient will be covered in the section corresponding to consent.
Royal Decree 1723/2012, of 28 December, which governs the activities of obtaining and clinically using human organs, and the territorial coordination of the human organs destined for transplantation, establishing quality and safety requisites.This regulation replaces Royal Decree 2070/1999, of 30 December, which had been in force since it repealed the Decree of 22 February 1980, which had been developed for the first time by Law 30/79. Throughout its existence the law has undergone these three regulatory developments, of which the modification that occurred in 1999 was especially relevant in the field of cadaveric organ donation. The regulatory reforms have not altered the living donor donation regime, although this does not mean that new forms of donation, such as altruistic donation, are excluded from the scope of coverage of the current regulation.
The Royal Decree that is currently in force incorporates in Spanish legal code Directive 2010/53/EU of the European Parliament and Council, of 7 July 2010, on quality and safety standards for human organs destined for transplant. The Directive determines the minimum requisites that have to be applied when donating, evaluating, characterising, obtaining, preserving, transporting and transplanting human organs, with the purpose of guaranteeing high levels of quality and safety for the said organs. It also sets requisites for traceability and the development of a system for the notification and management of events and severe adverse reactions, establishing the minimum data that have to be recorded for the evaluation of donors and organs.
Its outstanding ethical foundations include those which refer to the voluntary and free nature of donation, together with consent, the protection of living donors and personal data protection. For the first time it also introduces a list of administrative infractions due to failure to comply with the regulations governing the harvesting and transplantation of organs.
Observing the requisites and principles established in the Directive, RD 1723/2012 sets quality and safety requisites for activities connected with obtaining and clinically using human organs, with the aim of guaranteeing a high level of human health protection and reducing the loss of available organs as far as possible.
Law 30/79 states that the purpose of the said activities has to be therapeutic. That is, they should be guided by the sole purpose of favouring the health or conditions of life of the recipient, without prejudice to any research that may also be taking place. The exercise of these activities also has to respect the fundamental rights of persons and the ethical postulates applied to clinical practice and biomedical research.
The principles of the regulation contained in the Royal Decree are based on Law 30/79, which develops Organic Law 3/2018, of 5 December, on Personal Data Protection and the guarantee of digital rights. They are also based on Directive 2010/53/EU, which transposes them, and they are listed below:
- Confidentiality: this principle prohibits the communication of data that would make it possible to identify the donor and recipient. The exception to this rule are those cases in which an individual publicly, freely and voluntarily identifies herself as a donor or recipient. Even when this occurs, the principle should be respected that neither donors nor their family members may know the identity of the recipient or their family members, and vice versa.
In general, any diffusion of information that may directly connect obtaining an organ and its subsequent transplantation is to be avoided. However, this restriction is not applicable to the parties directly involved in the transplantation of organs from a living donor between individuals who are genetically related or connected by kinship or close friendship. Outside such cases, the principle of confidentiality should be respected even in the case of living donor organ transplantation.
Respecting this principle does not prevent taking preventive measures when the existence of an individual or collective risk to health is suspected.
- Personal data protection: information on human organ donors and recipients is to be recorded, processed and stored in the strictest confidentiality, according to the stipulations of data protection laws, in the General Health Law and in Law 41/2002, of 14 November, which governs patient autonomy and rights and obligations in the field of information and clinical documentation.
The individual in charge of the therapy will have to adopt the technical and organizational measures necessary to guarantee the security of the data in this special category, preventing their alteration, loss, processing or unauthorized access. The individual in charge as well as the person in charge of processing are obliged to keep professional secrecy, even after their professional relationship has ceased. Infractions in this area may be penalized by the Spanish Data Protection Agency, without prejudice to any criminal responsibility that may be incurred (articles 197 and 198 of the Penal Code).
According to article 9 of Organic Law 3/2018 on Personal Data Protection and the guarantee of digital rights, by reference to article 9.2 of Regulation (EU) 2016/679, data processing in the field of health may be supported when this is required by the management of healthcare, social, public and private systems, or the execution of an insurance contract which covers the affected party.
- Limitation of Promotion and Publicity: the promotion of organ donation activity should take place in general terms, underlining its altruistic, voluntary and selfless nature. It will not be possible to advertise the donation of organs to benefit specific individuals or certain hospitals, institutions, foundations or companies.
Deceitful advertising which leads to erroneous opinions on obtaining human organs and using them clinically is expressly prohibited.
Without prejudice to the foregoing point, the competent authorities will promote the information and education of the population regarding donation and transplantation, the benefits these offer to those who need them, as well as the conditions, requisites and guarantees which apply to them.
- Free of charge: it is not possible for the donor or any other physical person or legal entity to receive any reward or economic compensation whatsoever for donation. Nor will the recipient be subjected to any demand for any payment whatsoever for the transplanted organ, and nor is it possible to offer or deliver any economic benefits or benefits of any other type in connection with the assignation of one or several organs for transplantation, and nor is it possible to request them or accept such benefits. All advertising regarding the need for an organ or the availability of one is also prohibited, as is offering or seeking any type of reward or remuneration.
The Royal Decree reproduces the exception for the compensation of economic losses when it indicates that the performance of the medical procedures associated with obtaining an organ shall not, in any case, be at the expense of the living donor. Living donors are not to be prevented from receiving repayment of their expenses and compensation for any reduction in income directly associated with the donation. However, when the said repayment is applicable, it will necessarily have to be made using the mechanisms which may be supplied for this purpose by the competent authorities.
- -
Fairness: the selection of and access to possible recipients should be subject to criteria of fairness, guaranteeing equality of opportunities for the therapy.
- -
Adoption of the quality and safety measures which are necessary to reduce the loss of organs, minimise possible risks and attempt to ensure the maximum possibilities of success for transplantation, improving the efficiency of the process of obtaining and transplanting organs.
Respecting the specific regulation of donation of this type, it is governed by article 8 in a form that is consistent with the regulation contained in law 30/79, but more completely developed. Living donation is subject to the following requisites:
- 1.
The harvesting should be of an organ or part of the same whose absence is compatible with life and whose function can be compensated by the donor's organism appropriately and sufficiently safely.
- 2.
It will not be possible to harvest organs from minors, even with the consent of their parents or tutors.
- 3.
Nor will it be possible to harvest - or, if applicable, to use - organs from a living donor when:
- -
For any reason it may be considered that consent was obtained due to an economic conditioning factor or one of any other type, social or psychological.
- -
There are suspicions that the free consent of the donor has been altered.
- -
Sufficient possibilities of transplant success are not expected.
- 4.
The right to information should include the planned procedure for the possible case in which after harvesting of the organ it cannot be transplanted in the recipient for whom it was intended. All information should be supplied to the donor in appropriate formats, so that it is accessible and comprehensible for persons with disability.
- 5.
It will be mandatory for the Ethics Committee of the transplanting hospital to issue a report.
Accreditation of the state of health and mental faculties of the donor is a requisite demanded by Law 30/79. The Royal Decree stipulates that this accreditation should be by a doctor other than the one who will perform the harvesting, after being supplied with the corresponding information. This information will necessarily include the following aspects: a) inherent risks of the intervention, b) foreseeable physical and or psychological consequences, c) possible repercussions in their personal, family or professional life, and d) expected benefits of the transplantation and the potential risks for the recipient. The living donor should also be informed of the need for them to communicate their medical history, and they will be informed that they will be given medical care for their recovery and will be clinically followed-up in connection with the harvesting of the organ.
The above points will be accredited in a medical certificate which describes the donor's state of health, the information that was supplied and the donor's freely expressed response and motivations and, if applicable, any sign of external pressure on the same. This certificate will include the list of the names of the other clinicians who have helped the certifying doctor in the tasks involved.
Authorized Hospitals. Competences of the Autonomous Regions.Article 10 of the Royal Decree lists the general requisites which living donor organ removal hospitals have to fulfil, following authorization by the competent authority of the Autonomous Community:
- 1)
It should be authorized as a deceased-donor organ removal hospital and as an organ transplantation hospital for which living-donor organ removal authorization is requested. Article 11.2 RD 1723/2012.2.
To be authorized, deceased-donor organ removal hospitals must fulfil at least the following requisites:
- a)
They must have an organization and working regime which makes it possible to ensure that organ removal is performed in a satisfactory manner;
- b)
They must have a hospital transplantation coordination unit with the appropriate personnel and material resources;
- c)
They must guarantee the availability of qualified medical personnel and the technical means of verifying death;
- d)
They must guarantee the availability of duly qualified medical and nursing personnel, as well as sufficient medical services and technical resources for the correct selection, evaluation, description and care of the donor;
- e)
They must guarantee the availability of appropriate medical services, including laboratories and imaging techniques, to carry out the determinations that are considered necessary at any time and which permit appropriate clinical assessment of the donor. These services will be staffed by qualified personnel and will have appropriate facilities and equipment;
- f)
They must guarantee the availability of the facilities and materials which are necessary for the correct performance of organ removal, according to the accepted standards in this field and the best medical practices;
- g)
They must have framework programmes which guarantee the quality and safety of the whole process;
- h)
They must have a confidential registry with restricted access, with its corresponding alphanumerical codes, to record the necessary data that makes it possible to ensure traceability, as well as to link the traceability of the tissues and cells obtained from donors;
- i)
They must keep a donor serum deposit during a period of at least ten years, for the purpose of performing biological controls, if necessary;
- j)
They must guarantee the availability of the appropriate personnel, facilities and services for the restoration of the body of the deceased person, after removal;
- k)
They must comply with the requisites established for confidentiality and the protection of personal data, together with the promotion, publicity and free nature of donations.
- 1)
They must have sufficient medical and nursing personnel with qualifications and accredited experience for correct donor assessment and selection and the performance of the removal.
- 2)
They must have sufficient facilities and materials necessary for the correct performance of organ removal, according to the accepted standards within this field and the best medical practices.
- 3)
They must have the medical services, including laboratories and imaging techniques, which are necessary to ensure the proper preoperative study of donors and to treat any complications which may arise in them. These medical services are to be staffed by qualified personnel and they are to have appropriate facilities and equipment.
- 4)
They must have protocols which ensure appropriate donor assessment and selection, communicating relevant information about the donor and recipient when harvesting and transplant are not performed in the same hospital, as well as protocols for the process of organ removal and the immediate postoperative and long-term follow-up, together with other protocols or framework programmes with the aim of ensuring the quality and safety of the whole process.
- 5)
They must have a confidential registry with restricted access and its corresponding alphanumerical codes, to record the necessary data that makes it possible to ensure traceability.
- 6)
They must guarantee recording of information about living donors and their clinical follow-up, according to the stipulations of article 31, without prejudice to the regulations on the protection of personal data and statistical confidentiality. Article 31, on information systems, establishes:
- i.
Without infringing the agreements that may be established with relevant professional and scientific associations or the systems that may be implemented by the autonomous communities for such purposes, and in cooperation with the same, the National Transplant Organization will develop and Maintain the state information systems that will record and store the data relating to: a) Donors and organs and their description; b) Organ traceability from their donation to transplantation or rejection and vice versa; c) The characteristics and movements of the patients included in the waiting list for transplantation; d) The characteristics and follow-up data of transplanted patients; e) The characteristics and follow-up data of living donors; f) Notification and measures used to manage events and severe adverse reactions.
- ii.
For each one of the above sections, the National Transplant Organization will define, in cooperation with the autonomous communities, the minimum data that will have to be supplied to the state system for each donor, organ, patient in a waiting list or recipient.
- 7)
They must comply with the established requisites regarding confidentiality and personal data protection, promotion and advertising and the free nature of donations.
The procedure for obtaining medical authorization from the corresponding autonomous community, as well as renewing or cancelling the same, without prejudice to the specific regulation in each one of the said communities, will follow the instructions of article 11 of RD 1723/2012 on authorising hospitals to remove the organs of deceased donors. This authorization must contain at least the following: a) the activity for which the hospital is authorized; b) The name(s) of the person(s) in charge of the removal process; c) The duration of the authorization, depending on the period during which it will be in force as determined by the competent authority.
When the authorization expires it may be renewed after checking that the conditions which made it possible continue. In no case may it be understood to have been renewed automatically.
Any form of substantial modification which occurs in the conditions, structure, persons in charge or working of the hospital should be reported to the competent authority, and it may give rise to review of the medical authorization or even to the cancellation of the same, even before the end of the period during which it would be in force.
The authorization will specify the person who, as well as being in charge of the medical unit in which the transplant will take place, has to approve each intervention, and this may be revoked or suspended as a result of inspection and checks by the competent authorities.
Respecting organ transplantation hospitals, article 19 of the Royal Decree stipulates, as well as the general requisites established for the hospitals where organs are removed, that the minimum specific requisites shown in appendix II will apply. To carry out any transplantation of organs from a living donor it will be indispensable that the hospital be authorized for the transplantation of the corresponding organ from a deceased donor, proving accredited experience in this intervention. Section 1 of article 18 of RD 1723/2012 states that human organ transplantation may only be performed in those hospitals which hold the specific authorization of the competent authority in their corresponding autonomous community. In section 2 it states that to be authorized, a human organ transplantation hospital must fulfil at least the following general requisites:
- a)
It must be authorized as a hospital for obtaining the organs of deceased donors and accredited with sufficient activity to guarantee the viability and quality of the transplantation scheme.
- b)
It must have a medical organization and working regime that are appropriate for performing the requested intervention.
- c)
It must have the corresponding medical and surgical unit with sufficient medical personnel and have proven experience in the type of transplantation in question.
- d)
It must guarantee the availability of specialist doctors with proven experience in the diagnosis and treatment of complications arising from the transplantation that is to be performed.
- e)
It must have a hospital transplantation coordination unit.
- f)
It must have the facilities and materials which are necessary for the appropriate performance of the transplantation process, in the preoperative stage, during the intervention and in the postoperative stage, according to the accepted standards in this field and the best medical practices.
- g)
It must have the medical departments, including laboratories and imaging techniques, which are necessary to guarantee the performance of the transplantation, appropriate clinical follow-up of the recipient and the correct treatment of the possible complications which practising the type of transplantation in question requires. These medical services will have qualified staff and the appropriate equipment and facilities.
- h)
It must have a pathological anatomy department with the necessary human and technical resources for the study of complications associated with transplantation and carrying out possible post-mortem studies.
- i)
It must have a microbiology laboratory which is able to undertake checks of the infectious complications patients may suffer.
- j)
It must guarantee the availability of an immunology laboratory and a histocompatibility unit with the technical and human resources which are necessary to guarantee the correct performance of the immunological studies which are necessary for pre- and post-transplantation monitoring.
- k)
It must have a Transplantation Commission and the protocols to ensure the appropriate selection of recipients, the transplantation process and immediate and long-term postoperative follow-up, and to guarantee the quality and safety of the whole therapeutic process, as well as other quality and safety protocols.
- l)
It must have a confidential registry with its corresponding alphanumerical codes, with records of the transplantations performed and the data that are necessary to guarantee traceability.
- m)
It must guarantee registration of the information that makes it possible to evaluate the transplantation activity undertaken in the hospital, as well as the results obtained, according to the stipulations of article 31 and without prejudice to the regulations governing the protection of personal data and statistical confidentiality.
- n)
At all times it should ensure that the actions and resources of the medical units involved in different types of transplantation are appropriate for the state of the art in science, using up-to-date diagnostic and therapeutic protocols.
- o)
It must comply with the established requisites in the field of confidentiality and personal data protection, promotion and advertising and the free nature of donations.*
The procedure to obtain authorization by the corresponding autonomous community will commence with an application that should contain at least the following data: a) The type of transplantation to be performed. b) The list of doctors who are in charge of the transplantation team, as well as the documentation which accredits their qualifications, and c) A report with the detailed description of the human and material resources and the protocols which the hospital has, according to the requisites for undertaking the corresponding activity.
Without prejudice to the specific regulations of each autonomous community, the authorization should contain at least the following: a) the type of transplantation for which the hospital is authorized. b) The name(s) of the person(s) in charge of the transplantation team, and c) the duration of the authorization, according to the period it will be in force as determined by the competent authority.
It will be the responsibility of each autonomous community to inspect or supervise transplantation coordination units, organ removal hospitals and transplantation hospitals at regular intervals. For this purpose, units and hospitals should supply all of the information in the format and way which may be requested, in connection with the activity for which they have been authorized.
Informed consent. Exceptional donors: “competent” minors and “incompetent” adults.Informed consent by the donor.Law 30/79 as well as Royal Decree 1723/2012 both demand, if donor consent is to be valid, that two requisites are fulfilled: biological and psychological.
For the first requisite the law states that the donor must be of age, and this condition is confirmed by the Royal Decree when it prohibits the harvesting of organs from minors, even with the consent of their parents or tutors. A doubt may arise concerning the limit which has to be applied to determine whether an individual is of age or a minor, as Law 41/2002 in article 9.4 excepts individuals over the age of 16 years from consent by representation when their capacity has not been legally modified, except when they are not able intellectually or emotionally to comprehend the scope of the proposed intervention. The so-called medical majority - being of age to consent to any action in the field of healthcare - has been set at 16 years.
Nevertheless, this is not the age of majority referred to by the regulation for the harvesting and transplantation of organs when it sets the conditions for the validity of the consent offered by a living donor. This age of majority coincides with what is denominated the civil age of majority, which the Constitution sets, in article 12, at 18 years. In the same way, the Civil Code states in article 315 that the age of majority commences on the 18th birthday, counting the whole day of birth for this purpose, and in article 322 it states that those over the age of majority are capable of all acts in civil life “except for the exceptions established in special cases by this Code.
The general medical age of majority -16 years- is not considered to be sufficient by legislators to make decisions such as that of assigning previous instructions, as stipulated by article 11 of Law 41/2002, which also demands the age of majority to issue such instructions for the future. Nor may a decision which affects the health and physical integrity of a healthy person be made if it is not proven that they have reached a biological age at which sufficient maturity may be presumed to make relevant decisions. Article 156 of the Penal Code is unequivocal on this point, when it confers on the consent of the donor that has been given in a manner that is valid, free, conscious and expressly issued, the power to free the doctors who harvest an organ according to the stipulations of the law from penal responsibility. Nevertheless, possible cases are excepted when consent has been obtained in an irregular manner, or by means of price or reward, or when the signer is a minor or completely lacks the aptitude necessary to give consent, in which case the consent they give or that given by their legal representatives will not be valid. That is, the Penal Code only understands the harvesting of organs from a living donor to be free of penal consequences when the said donor, being of age, has consented to the harvesting in a valid, free, conscious and express manner. Otherwise, and even when consent has been given for the harvesting, the doctors will incur in the crime defined in article 149 of the Penal Code, which punishes - with a prison sentence of from six to twelve years - those who cause another, by any means or procedure, to lose or lose the use of a principal organ.
The psychological requisite is covered by Law 30/79, which demands that the donor has full mental faculties, which must be accredited by the corresponding medical certificate. It also prohibits persons with a psychological deficiency or mental disease from donating, or those who for whatsoever other cause are unable to validly give their consent.
This regulation should not be understood to be altered by the legal modification introduced in Law 30/79 by Law 26/2011, of 1 August, on adapting the regulations to the International Convention on the rights of Persons with Disability. The said modification permits individuals with disability to become donors, but always on condition that they fulfil the requisites of being of age and in full possession of their mental faculties. In such cases the information should be supplied to the donor in appropriate formats, so that it is accessible and comprehensible for them, given the type of their disability.
Consent is always given after the information which a donor must receive, following the general rule governing all informed consent. Nevertheless, the information which has to be supplied to a living donor is far more extensive than that which is required for any other medical act, precisely because they are a healthy person who is not going to receive an intervention for their own benefit. The information on risks of all types, and the information on the expected benefits for the recipient, must be exhaustive. In this respect RD 1723/2012 states that the information should cover the following points: a) risks that are inherent in the intervention, b) foreseeable physical or psychological consequences, c) repercussions that may arise in their personal, family or professional life, and d) the expected benefits of the transplantation and potential risks for the recipient. A living donor should also be informed of the importance of communicating their personal medical history, and they will be informed that they will be given medical assistance for their recovery and will be clinically followed-up in connection with the harvesting of the organ. The medical certificate accrediting the state of health of the donor should also refer to the information they were supplied with and their freely expressed response to this and their motivations and, if applicable, the presence of any sign of external pressure applied to them.
The consent given by the donor in a medical context should be ratified by them before the legal authority. We will cover this point under the corresponding heading.
Informed consent of the recipientArticle six of Law 30/79 covers the consent given by the recipient, establishing requisites that do not differ from those corresponding to any other informed consent within a medical context. It only specifies the type of information which has to be supplied, depending on the nature of the intended intervention.
The regulation of the informed consent of the recipient by the law governing the harvesting and transplantation of organs must be understood to have been modified by the law governing patient autonomy. This is expressly stated in article 17 of RD 1723/2012 when it affirms that the transplantation of human organs may only be carried out by hospitals which have been authorized to do so, with the previous written consent of the recipient or their legal representatives, as stipulated by article 9 of Law 41/2002, of 14 November. They must be informed beforehand of the risks and benefits which the intervention involves, as well as studies which are technically appropriate for the type of transplantation in question in each case.
Exceptional donors: “competent” minors and “incompetent” adultsThe terms “competence” or “incompetence” in reference to the capacity of persons to consent to a medical act are not in themselves legal concepts. We will examine which legal concepts refer to the capacity of persons.
Legal capacity or capacity in law involves the aptitude of a subject for the full holding and exercise of rights. It is a personality attribute and in the abstract it is the same for everyone, so that the existence of a person is sufficient for its existence to be asserted (Article 30 of Civil Law: personality is acquired at the moment of live birth, once entirely free of the maternal womb).
The so-called capacity to exercise or the capacity to act is the aptitude of a subject for the exercise of their rights. Given its nature it is contingent and variable: it does not exist in all persons, and nor does it occur in all of them to the same degree, as it requires intelligence and will. Due to this the law sometimes refuses it and sometimes restricts it.
The definition of “competence” within a medical context implies the recognition of psychological aptitudes to make certain decisions. This concept must be completed with that of the “capacity of discernment”, which expresses the psychological aptitude that is necessary to reach a responsible decision “here and now”.
The special requisites and demands which the law imposes on the consent by living donors completely prevent a “competent” minor under the age of 18 from donating, and they also prevent any “incompetent” individual above the age of majority from doing so when due to any reason they are unable to give their valid consent.
Penalties for buying and selling organs. Detecting and reporting cases of illegality committed outside Spain.Penalties for buying and selling organs.Catalogue of administrative infractions.Although buying or selling organs is a serious crime, the classification of this behaviour as a crime is not incompatible with the existence of a punitive administrative law. Penal law acts in this field as a form of extra protection of the legal goods which administrative law also protects.
Article 33 of the Royal Decree includes a list of infractions, giving the regulation the indispensable element of constraint as a mechanism which sets out the consequences for the case of incompliance with its stipulations. The infractions are defined by reference to the regulations, not only those in the Royal Decree, but also the circumstances described in General Health Law 14/1986, of 25 April, and General Public Health Law 33/2011, of 4 October, together with the circumstances described in the law on personal data protection (Organic Law 3/2018 on the Protection of Personal Data and guarantee of digital rights).
The Royal Decree classifies infractions according to their severity, distinguishing between those that are very serious, serious and slight.
The following are very serious infractions:
- 1)
The performance of any activity governed by the Royal Decree without respecting the principle of confidentiality, on condition that this can be demanded.
- 2)
The performance of any activity governed by the Royal Decree without respecting the principles of a voluntary nature, altruism, the absence of desire for profit or being free of charge.
- 3)
Advertising the need for or availability of an organ, offering or requesting some type of reward or remuneration.
- 4)
Obtaining organs from a living donor in the absence of compliance with any of the previous requisites established in the Royal Decree, in particular those which refer to being of age, mental faculties, state of health and consent.
- 5)
Obtaining organs from a deceased donor in the absence of any of the previous requisites established in the Royal Decree, in particular those which refer to the study of the will of the deceased respecting the donation of organs and the diagnosis and certification of death.
- 6)
Obtaining or transplanting organs in a hospital that does not hold the necessary authorization from the competent authority.
- 7)
Failure to comply with the traceability requirements.
- 8)
The transportation into or from Spain of organs without the necessary authorization, according to the stipulations of article 15.
- 9)
Obstructing or hindering the work of inspection.
As may be seen, any activity involving the purchase or sale of living donor organs will fall under the definitions of the forms of behaviour described in numbers 2, 4 and 5, without prejudice to its penal classification. Article 34 of the Royal Decree states respecting this that in the events in which infractions may be crimes, the evidence for guilt will be passed to the competent judicial authority and the punitive procedure will not be followed until the judicial authority pronounces a firm sentence which terminates the procedure. If no crime is found to exist, the punitive process will continue on the basis of the facts which the Courts have considered to be proven.
Penalties and the punitive procedureA financial penalty is established for all types of infractions. This is stipulated in article 33 of the Royal Decree which refers, to determine the size of the amount, to the conditions set by article 58 of Law 33/2011, of 4 October, and article 36 of Law 14/1986, of 25 April. Article 58 of Law 33/2011 states:
- 1)
The commission of infractions in the field of public health will give rise to the imposition of the following penalties, without prejudice to those which may be established by the autonomous communities and local entities within the scope of their competences: a) In the case of a very serious infraction: a fine of from 60,001 euros to 600,000 euros, although this sum may be surpassed until it amounts to five times the market value of the products or services involved in the infraction). In the case of serious infractions: a fine of from 3,001 euros to 60,000 euros) In the case of slight infractions: a fine of up to 3,000 euros. These amounts may be updated by the Government in accordance with regulations.
- 2)
Without prejudice to the economic penalty that may apply, in the cases of very serious infractions the competent authority will be able to decide to temporarily close the establishments or departments involved for a maximum period of five years.
The punitive procedure will be the one established in article 60 of Law 33/2011, of 4 October, which refers to the stipulations of Law 39/2015, of 1 October, on the Common Administrative Procedure of the Public Administrative Bodies. The initiation, processing and resolution of punitive processes will correspond to the Administration that is competent due to the territory and material, without prejudice to penal or civil responsibilities or those any other type that may apply.
I) Classification of buying and selling organs. Article 156 A of the Penal Code.
Article 156 A of the Penal Code (introduced by Organic Law 5/2010, and reformed by Organic Law 1/2019, of 20 February, which modified Organic Law 10/1995, of 23 November, of the Penal Code, to transpose European Union Directives in the fields of finance and terrorism, and on how to proceed in international questions) punishes those who in any way promote, favour, facilitate, advertise or carry out human organ trafficking, setting different penalties depending on whether the organ is from a living or cadaveric donor, as penalties are greater in the former case.
This classification has the purpose of responding to the increasingly widespread phenomenon of buying and selling human organs, and the call by several international forums to make it punishable. In 2004 the World Health Organization declared that the sale of organs was contrary to the Universal Declaration of Human Rights, and it appealed to doctors not to perform transplants if they suspected that the organ involved had been the object of a transaction. In the International Summit Meeting on transplantation tourism and organ trafficking held in May 2008, the representatives of 78 countries agreed on the “Istanbul Declaration”, which states that the said practices violate the principles of equality, justice and respect for human dignity, and that they should be eradicated. Although our Penal Code already covered these crimes under the heading of injuries–article 156, interpreted “sensu contrario”-, it was considered necessary to differentiate the treatment of the said activities, expressly classifying them.
The legislator includes an authentic interpretation of what trafficking in third-party human organs should be understood to mean, including the illicit harvesting or removal of the same in the definition. This is defined as follows:
- 1)
When organs have been removed without the free, informed and express consent of the living donor, in the way and with the requisites demanded by law.
- 2)
When in exchange for the harvesting or procurement, the donor or a third party requests or receives a gift or reward of any nature, or accepts an offer or promise, themselves or by an intermediary, for their own benefit or that of a third party. The reimbursement of expenses or compensation for loss of income due to the donation will not be understood to be a gift or reward.
- 3)
Likewise penal sentences will be applied to those who, for their own benefit or that of another: a) request or receive, themselves or through an intermediary, a gift or reward of any nature, or who accept an offer or promise for proposing or recruiting a donor or a recipient of organs; b) offer or deliver, themselves or through an intermediary, a gift or reward of any nature to medical personnel, civil servants or private individuals due to the exercise of their profession or position in public or private clinics, establishments or surgeries, with the aim of undertaking or facilitating the harvesting or illicit procurement or engraftation of illicitly collected organs. Likewise, so-called manifest intentions to provoke, conspire or commit this crime will be punished with a penalty reduced by one to two degrees
The definition of criminal behaviours covers the recipient of the organ who consented to the transplantation while aware of its illicit origin. The penalty for the recipient is hard to imagine theoretically, because very probably they will allege that a state of need exonerates them because the transplant was necessary for them to remain alive. This has to be understood without prejudice to the fact that their behaviour may be considered to be necessary cooperation for illegal trafficking in third-party human organs, under the possible supposition that the necessary requisites for this were present.
In any case, as the judge is able to impose a lesser sentence due to the reduced reprehensibility of the behaviour of the recipient due to their circumstances, this seems to exclude the cause of exoneration which may be alleged.
The outlines of the legal good protected by this criminal definition were drawn by the sentence of the Second Court of the High Court on 27 October 2017. The High Court stated that the crime of organ trafficking not only has the purpose of protecting the health or integrity of persons, but rather than the object of protection goes beyond this; although it of course has the purpose of protecting physical integrity, it also seeks to protect the dignity of persons, preventing them from being treated like objects, like a container of organs which, because of their bilateral or secondary nature may be objects of trafficking. It also adds that “the national transplantation system establishes an altruistic and supportive system for obtaining and distributing organs for transplantation into patients who need them”.
There is therefore no justification whatsoever for accessing human organs by means other than those included in the national transplantation system, as such devious means always lead to types of organ trafficking that offend against the dignity of vulnerable individuals. That there may be offers of supposedly legitimate means for obtaining organs outside the national transplantation system does not exonerate those who use such means, in the knowledge that if they are unable to access an organ in Spain, this is due to the permanent imbalance between the offer and demand for organs, or to medical criteria which advise against transplantation if an organ is available.
II) Detecting and reporting illegal acts committed outside Spain.
The credibility of the national transplantation system as a part of the national health system is not reduced by the fact that someone has procured an organ illicitly and more especially in a foreign country. A person who acts in this manner does not do so because they believe the Spanish system to be ineffective, but rather because they prefer to use mechanisms that allow them faster access to an organ, even though the procedure to do so is illegal, affects the dignity and health of vulnerable people and is less safe for the recipient.
Trust in the national health system is maintained in these cases, and this leads to a demand for treatment in our country after engraftation. In such situations it is first possible to ask whether somebody who has broken the law to obtain an organ outside the procedures established for this purpose should be given medical care. Secondly, it may be asked whether the doctor should report this to a medical authority or the legal or fiscal authority, due to the suspicion of human organ trafficking.
Respecting the first question, all doctors are obliged to duly treat a patient in the said situation. This obligation will depend on whether the required medical care is included in the portfolio of services offered by their hospital, and whether the appropriate resources for such care are available. If the resources were lacking, it would always be possible to prioritise care for those who had received an organ transplant by legal means. This is because the survival prognosis for the graft will be better. From a penal viewpoint, such a refusal to offer medical care (article 196 of the Penal Code) will only constitute a crime when this refusal leads to a serious risk for the health of persons.
With regards to the obligation to report, this is imposed by article 262 of the Criminal Justice Law which, in spite of the evident anachronism of its language, is still in force. According to this precept, “those who because of their position, profession or trade learn of a public crime” are obliged to report this to the Attorney General's office or competent court. The anachronism is clear after reading the second paragraph, which states: “if the person who omitted to report were Qualified in Medicine, Surgery or Pharmacy and it occurred in connection with the exercise of their professional activities, the fine may be no less than 125 pesetas nor higher than 250”. It adds “if the individual who commits the omission were a public employee, their immediate superior will also be informed for the relevant administrative purposes”.
Although there is no doubt regarding the validity of the obligation to report, if this obligation is not obeyed then the doctor involved would not have committed the crime defined by article 408 of the Penal Code. This precept punishes the person in authority or civil servant who, disobeying the obligation arising from their position, intentionally fails to prosecute the crimes they detect or those responsible for them. However, the subjective scope of the crime cannot extend to cover medical personnel, because they are not civil servants with the obligation to prosecute crimes. The crime does not in fact consist of not reporting to a legal body that a crime has supposedly been committed, but rather in not prosecuting it; omitting the necessary investigation.
The obligation to report a well-founded suspicion to the medical administration that an organ trafficking crime has been committed is not countermanded by a supposed exercise of the right to professional secrecy. Thus professional secrecy is not affected by informing the authority of a well-founded suspicion that a crime has been committed. Knowledge of the supposed crime arises from simple observation or the physical examination of the patient, and it is not information about a pathological process that would be subject to the duty of confidentiality.
The most appropriate procedure for reporting would be to inform the competent medical authority that medical assistance has been given to a person with a graft that had not been engrafted using legally approved channels, i.e., the national transplantation system. The medical authority will then, directly or by means of its legal departments, prepare the corresponding report for the attorney general or the examining magistrate.
Another difficulty should be taken into account, apart the one which arises in the case of the recipient of an organ that had been trafficked alleging exoneration due to a state of need (which as we pointed out will not usually apply). This additional difficulty involves prosecuting someone in Spain for a crime committed abroad, when organ trafficking is not defined as a crime in the country where it occurred. The general principle of attributing jurisdiction to Spanish courts is territoriality (Article 23.1 of the Organic Law of Judicial Power), although some suppositions exist as exceptions, in which Spanish courts extend their jurisdiction to cover crimes committed abroad, by Spanish citizens or foreigners. These exceptions (principles of personality and the protection of interests) generally require, unless an international treaty creates an exemption from this requisite that the act committed should be punishable at the place where it occurred. Thus in the absence of a treaty, if the behaviour considered to be criminal in Spain occurred in a country where organ trafficking is not defined as a crime, then Spanish courts will not be able to prosecute it. There would be nothing to prosecute in the country where it occurred because the behaviour in question is not considered to be criminal.
Documentation and custody of the sameArticle 32 of the Royal Decree governs the information systems that have to be implemented for the registration and custody of certain data. These are data on: a) The donors and the organs, and their description. b) The traceability of organs from donation until transplantation or rejection and vice versa. c) The characteristics and movements of the patients included in the transplantation waiting list. d) The characteristics and follow-up data of transplanted patients. e) The characteristics and follow-up data of living donors, and f) Notification and measures taken in the case of severe events and adverse reactions.
The National Transplant Organization has the function of developing and maintaining the state information systems in which the said data are registered and stored, in cooperation with the autonomous communities and without infringing on the systems the latter may develop and the agreements which may be entered into with the relevant professional and scientific associations.
In cooperation with the autonomous communities, the National Transplant Organization will establish the minimum data which will have to be supplied to the state system for each donor, organ, waiting list patient or recipient. The state information systems, which will have to permit regular statistical analyses, will receive data from the hospitals where organs are obtained or transplanted, as appropriate, either directly or through the available autonomous community systems. The National Transplant Organization will establish, in cooperation with the autonomous communities, the procedures that will make it possible for the state to compile the information.
With the data included in the systems, and without prejudice to other reports that may be prepared, the National Transplant Organization will prepare annual reports on the activity undertaken in hospitals where organs are obtained and transplanted throughout national territory, including aggregated data on living and deceased donors, the number and type of organs obtained and those which were transplanted or rejected. These reports will never contain the personal data of donors or recipients, and they will be supplied to the transplantation coordination network and the transplantation teams, and they will be publically accessible.
As well as permitting general use of the data in question, state information systems may also be used by hospital or autonomous community information systems for their own data, when the corresponding autonomous community or hospital requires this.
Access to any of the data contained in the information systems will be restricted to authorized persons in hospitals, autonomous community coordination units or in the National Transplant Organization, and all of the systems at hospital, autonomous community or state level will comply with the current regulations on personal data protection, confidentiality and statistical secrecy.
Paired transplantation and altruistic donation. Confidentiality in indirect donations.Paired donationThe imbalance between the need for organs and their availability for transplantation explains the initiatives of the medical administration to try to increase the number of potential donors, deceased as well as living. Living donation between unrelated individuals with no emotional ties is one of these initiatives. However, it raises the following questions: is it voluntary, altruistic and selfless, and if this is so, is it covered or not by our legal system?
World Health Organization Resolution WHA 63.22, of 21 May 2010, established the main guidelines for organ transplantation. Guiding principle 3 stipulates that in the donation of organs from living donors, the donors must be related to the recipients genetically, legally or emotionally. In our country, article 5 of RD 1723/2012 refers, to exclude donor and recipient from the general rule of confidentiality, to persons who are genetically related or are related by kinship or close friendship. The latter concept is equivalent to the emotional tie referred to by the World Health Organization, and it does not exclude donation to recipients united by emotional ties other than close friendship. Nor can donation between persons who have no ties be ruled out, because the said tie is solely mentioned by the Royal Decree for the purpose of introducing an exception to the obligation of confidentiality respecting donor and recipient identities.
As we have warned, donation to unknown recipients may arouse suspicions about the existence of some type of reward for the donor, who will lose one of their organs for the sole purpose of substantially improving the life expectancy or conditions of someone completely unknown to them. The definition of altruism must be kept in mind, as it consists of diligently ensuring good for another even when this reduces your own welfare, as this is exactly the attitude that guides living KT donors. To confirm the altruistic nature of the donation and eliminate all suspicion of spurious ends, investigation takes place to confirm the will of the donor to improve the health or conditions of the recipient. This is achieved by the corresponding psychosocial reports on the donor, which will accredit their true motivations and supply data on their professional, economic and family circumstances.
However, paired donation takes place precisely to help someone with whom the donor has some form of emotional tie. This is why they donate, even if the recipient of their organ will be somebody they do not know. They agree to donate an organ because they know that someone else is doing the same for their close friend, and the second donor also knows that their action will achieve their initial purpose. Both donors know that the person they wish to help by donating will receive an organ, if they donate for someone they do not know.
It should be made clear that this is not a determining factor for the donation - which would be expressly prohibited by law, as we know - but rather a legally valid condition which can be expressed as follows: “I donate because you donate” (a condition), not “I donate so that you donate” (a determining factor).
The objective here in terms of medical policy is also governed by the law, as the aim is to overcome the constant imbalance between the number of patients in the waiting list for a kidney and the number of donors.
The legal validity of this donation makes it necessary to verify, as well as the other requisites for living donation (age, capacity and information, etc.), that the first donor who wishes to donate in favour of a close friend, still wishes to do so in favour of a close friend of the second donor, and that the latter who wishes to donate in favour of someone close to them still wishes to do so to benefit the person close to the first donor. Both donors are unable to donate their organs to aid the person they know because the recipients are incompatible with them, although this is not the case for the recipients they do not know.
Paired renal donation is configured as simultaneous, based on a previous desire to donate to improve the health and life conditions of a known specific recipient. Given this, and that the donor and known recipient are incompatible, the donor wishes to donate in favour of an unknown person so that another donor does so for the individual they wish to help. The whole process has the structure of an exchange of previous accredited desires to donate, favouring those who were unable to receive an organ from the person they knew even though they wanted to donate and had decided to do so.
As we pointed out above, this form of donation is fully covered by our laws, and this legal protection also covers the case in which although the donor and the recipient they are close to are not incompatible, the aim is to gain a real benefit from the paired KT process (such as an age gain). The therapeutic purpose is to achieve a better result of transplantation, and this would justify the choice of a paired KT as the correct therapy in this situation.
Purely altruistic donationSomebody who donates to someone who is totally unknown to them with the sole purpose of improving their health or life conditions, fulfils more completely than anyone else the legal demand that donation be voluntary, altruistic and selfless.
The problem which may arise in donations of this type is the same as the one covered in the section on paired donation. It consists of the suspicion that there is a hidden non-altruistic motivation–usually economic in nature - that negates the validity of the consent that has been given. Although transactions involving your own organs is not included within the definition contained in article 156 A of the Penal Code (which understands organ trafficking to involve harvesting or illegally obtaining human organs from others), the recipient's actions may be understood to be included if a price is paid. It is true that organs donated in this way could never be accepted by the national transplantation system.
The procedures that may be established to guarantee the altruistic motivation of the donor–psychosocial reports, structured questionnaires, investigation of their professional, economic, personal and family circumstances, etc. - will be subject to their consent as part of the donation process, and the results should be presented to the examining magistrate, so that they can decide whether the consent given by the donor to the said authority is free of any economic, social, psychological or other type of determining factor. This does not change the situation regarding any other living organ donation, without prejudice to the fact that in this case legal examination of the consent may require a broader foundation of proof.
The legal possibility of accepting donations by a living donor to a waiting list of recipients should be examined, even though this considerably reduces (although it does not eliminate) the risk of motivation by payment masked by the will to donate. Obviously the beneficiary of such a donation would be unknown to the donor, preventing any type of economic transaction between them. A greater problem would arise in those cases when the selection of a recipient is reduced to a specific group with certain characteristics in terms of their age or clinical situation, etc., to improve the probability of a successful transplantation. In such cases it seems that there could be an indication of who the possible recipient or a small group of the same could be, so that precautions should be maximized to ensure that any donor is purely altruistic and selfless. If this were the case, we would reach the same conclusion as that corresponding to paired renal donation.
Confidentiality in non-directed donationsArticle 5.2 of the Royal Decree establishes the general rule of confidentiality that neither the donors nor their family members may know the identity of the recipient or their family members and vice versa, preventing the diffusion of any information that may connect obtaining and then transplanting an organ. Nevertheless, for reasons which derive from the living donor organ transplantation technique itself, this general rule excepts “those who are directly involved in the transplantation of living donor organs between individuals who are related genetically, by kinship or by close friendship”. Non-directed donation is therefore an exception to the exception, so that it is therefore subject to the general rule of confidentiality.
With the aim of preserving the confidentiality of the pairs in this process, the National Renal Donation Protocol created a registry of donor-recipient pairs in the National Paired Renal Donation Programme. This registry complies with the requirements of the personal data protection law, and its functions include giving each member of the pair a unique identification number. This number is used to preserve the anonymity of the pair when comparing before an examining magistrate. In this way, no information which permits identification of the recipient will be shown in the document with which the donor appears before the judge. Nor will it be possible for third parties to identify the members of the other pair who will take part in the exchange, only their identification number in the paired renal donation registry. The transplantation coordinator of the hospital where the operation will take place in each case will give the judge an informative sheet supplied by the National Transplant Organization, accrediting the identities of the two donor-recipient pairs and their correspondence with the identification number.
Appearance in courtLaw 30/79 refers to the development of regulations governing the granting of donor consent, as well as determining the authority before which consent must be granted. In Royal Decree 426/1980, of 22 February, and until RD 1723/2012 came into force, the regulations of the sector stipulated that consent had to be granted before a judge, and more specifically, the judge in charge of the Civil Registry. The second additional Regulation of Law 20/2011, of 21 July, on the Civil Registry, when it refers to the legal regime of the person In Charge of the Central Civil Registry Office and the General Civil Registry Offices, states that the posts of being in charge of Civil Registries were to be filled by career civil servants in Subgroup A1 who held a degree in Law or the university qualification which had replaced this, and the legal secretaries (now justice administration lawyers). Thus the legislator ordered the Civil Registry to be more independent of the judicial system, turning it into an administrative organ which was no longer under the control of a judge. In connection with the said legal project, RD 1723/2012 tried to keep to the same line as the previous regulations, stating that the authority before which donors had to give their consent was still a judicial authority. Nevertheless, given the legal plan to make civil registries independent of the judiciary, the Royal Decree attributed the competence to the examining magistrate. This also agrees with the project for the Law of Voluntary Jurisdiction, which was being processed at the time and is now in force (Law 15/2015, of 2 July, on Voluntary Jurisdiction).
The report issued by the General Council of Judicial Power on the project for Royal Decree 1723/2012 found in favour of the maintenance of judicial intervention in case of living donation, as it proved that the introduced reform is not “less important, or a trivial question, as the legal modification which made the Civil Registry independent of the judiciary involves, respecting the purposes of this report, the disappearance of the jurisdictional guarantee of the rights, in this case, of the freedom of a living donor to give consent, and the awareness of the same of the importance and repercussions of their decision (definitively, that they have the information that is necessary to freely give their consent), which had been conferred by the Judge in charge of the Civil Registry, and which cannot be substituted by the civil servant who is in charge of the Civil Registry, according to the model established in Law 20/2011”.
It is true that the civil registries have not become independent of the judiciary. Royal Decree-law 16/2020, of 28 April, on the organizational and procedural measures to combat COVID-19 within the context of the Legal Administration, modifies the final tenth regulation of Law 20/2011, of 21 July, on the Civil Registry, and stipulates that the same will come into force on 30 April 2021.
In any case the authority for giving consent is now definitively held by the examining magistrate of the court of first instance which exists in each judicial administrative area, with jurisdiction over the entire territory of the same and with its seat in its capital, who is the authority on recognition in civil law procedures, including procedures of voluntary jurisdiction.
The procedure which must be followed to obtain living donor organs requires the presentation, before the court of first instance of the locality where the harvesting or transplantation is to take place, as selected by the promotor of the proceedings, of a request by the donor or a communication by the director of the medical centre where this will be carried out, or the delegated individual, describing the personal and familial circumstances of the donor, the purpose of the donation, the medical centre where harvesting is to take place and the identity of the doctor in charge of the transplant, all this accompanied by the medical certificate referring to the mental and physical health of the donor.
The donor must give their express consent before the judge during the appearance that takes place in the voluntary jurisdiction proceedings (which require the intervention of a legal body for the tutelage of rights and interests, without any controversy or conflict which would have to take the form of litigation) and which commences, following the explanations of the doctor who is to carry out the harvesting and in the presence of the doctor who accredits the physical and mental health of the donor, the doctor in charge of the transplantation and the person who has to approve the intervention, according to the document which authorises the harvesting of organs in question.
The document transferring the organ where the donor expresses their agreement will be issued by the Judge and signed by the donor, the doctor who is to execute the harvesting and the others who are present. If any of the former doubt that consent was given expressly, freely, consciously and selflessly, they may effectively oppose the donation. A copy of this transference document will be given to the donor. In no case may organs be obtained without this document being signed beforehand.
To confirm that the consent of the donor is firm, the legislators set a period of at least twenty four hours from the moment the organ transfer document is signed to the moment of harvesting, during which the donor is able to revoke their consent at any time prior to the intervention without the need for any formality whatsoever. The said revocation may not give rise to compensation of any type.
In any case and prior to the presentation of the request to appear before the court of first instance, the potential donor will have signed an informed consent document in which they declare that they were sufficiently informed duly in advance of the proceedings to which they would be subjected, as well as the risks, possible complications, expected benefits and alternatives, with the possibility of asking all of the questions they considered to be appropriate and stating that all of their questions had been answered.
Voluntary Jurisdiction Law 15/2015, of 2 July, considers the giving of consent for the donation of organs by a living donor to be one of the acts in which a judge has to take part, for the effective tutelage of their rights and interests under civil law. The publication of this regulation, apart from establishing the legal procedure to follow to formalise consent for donation, does not modify the legal regime created by Royal Decree 1723/2012.
Articles 78, 79 and 80 (Chapter X under the heading “On the harvesting of organs from living donors”) of this Law centre on regulating this area, and they always consider that the judicial intervention has the sole aim of verifying that the consent of the donor is given freely, voluntarily, selflessly and that is complies with the other requisites of Law 30/79 and the resulting regulations.
The law stipulates that the request must be presented before the judicial body by the donor or through communication with the director of the medical centre, describing the personal and familial circumstances of the donor, the purpose of the donation, the medical centre where harvesting is to take place and the identity of the doctor in charge of the transplantation or harvesting, or the person who is delegated for this purpose. The said request or communication must be accompanied by a medical certificate on the mental and physical health of the donor. The appearance before the examining magistrate must be attended by the donor, the doctor who is to carry out the harvesting, the doctor who signed the health certificate and the doctor in charge of the transplantation or their delegate. The person who has to authorise the intervention must also be present. The examining magistrate is to authorise the transfer document if they consider that consent was expressly given freely, consciously and selflessly, and that the other requisites established by the law governing the harvesting and transplantation of organs have also been fulfilled. As demanded by the said legislation, the Voluntary Jurisdiction Law orders that the transfer document describes the possibility of revoking consent at any time prior to the intervention.
Both texts are completely consistent, as they have to be. One of them is for the medical sector and orders how it is to proceed, while the other is for the judge, informing them of the legal procedure to be followed. Giving consent before the legal authority confers a double guarantee on living donor organ donation. On the one hand, consent has to be given before an authority which is both external to and independent of the public health system. On the other hand, the fact that the organ transfer document is issued by a judge adds to security, as this validates the checks on consent undertaken in the medical sector.
3Ethical aspects of living donor donation and KTLDKT is considered to be a highly effective procedure for the treatment of CRD. Nevertheless, due to its nature it is necessary to comply with legal and ethical norms which guarantee donor suitability and protection, while also governing the actions of those who intervene in the procedure 59.
Organ donation ethics is a dynamic discipline, as every scientific advance creates a new ethical dilemma that requires new and better definitions 60. Ethics starts by suggesting criteria or guidelines for action which subsequently, in some cases, are ordered and raised to the level of Law, as not all ethical approaches are expressed in law. Law is therefore coercive, while the ethics which governs living donation would be classified within the imperfect obligations, as it will never be possible to demand that somebody donates, as this decision is strictly personal and nobody may ever be placed under pressure in favour of or against donation 61.
Several conflicts exist regarding living organ donation. The key ethical dilemma lies in the fact that living donation inevitably involves physical harm, and it causes a potential health risk for a healthy individual 62. Individual autonomy is therefore especially important in their decision to donate.
Altruism has formed the basis of organ donation from the start, and it is justified as a selfless gift without any expectation of remuneration. Altruism in donation may have the aim of benefiting family members, friends or even unknown individuals, and it underlines the philosophy of voluntary donation without payment through solidarity between the donor and recipient 63.
Dignity is associated with the Kantian concept of intrinsic dignity or the special status of the human body, where dignity and price are mutually exclusive. To keep their dignity human beings must remain above any negotiable price. Thus any form of payment for parts of the body would violate human dignity even if the donor herself does not feel degraded. The Oviedo Convention under the auspices of the Council of Europe 64 sought to guarantee dignity and identity without any type of discrimination, together with rights and freedoms that are fundamental in biological and medical interventions.
The clinicians who take part in LDKT should therefore apply high ethical standards, so that their decisions will harmonise with good clinical practice, offering complete transparency to medical workers and society 65.
Living donation is considered to be included within the basic level of the ethical principle of beneficence, and the ethical requisites which have to be examined include those associated with autonomy and non-maleficence. Beneficence therefore describes actions that facilitate the well-being of third parties. Maximising benefits while minimising risks is considered to be ethical. Autonomy is the right of individuals to reach decisions independently. A decision is autonomous when it is made freely and without coercion. Finally, non-maleficence restricts interventions that could cause prejudice or harm, while attempting to ensure that the risk-benefit ratio is correct.
The World Health Organization has established eleven guiding principles for the transplantation of human cells, tissues and organs, with the aim of providing an ordered, ethical and acceptable framework for donation and transplantation for therapeutic purposes. The majority of these principles are applicable to live donors 66.
The most important ethical considerations which may be raised when studying potential living KT donors are:
Analysis of donor autonomy- •
The safety and well-being of the candidate donor should prevail over the potential benefit for the recipient, so that all of the clinicians who intervene in the study have the ethical duty to protect the best interests of the living donor (Quality of evidence: NG).
- •
We recommend that the requisites for acceptance be specified with the donor, together with the information that they would wish to share with the recipient and the procedure in case of unexpected results, whether or not they are relevant for the donation (B).
- •
We suggest that the studies of the donor and recipient should be carried out separately, although the teams involved have to be properly coordinated to guarantee good communication, without compromising donor and recipient independence (C).
- •
We suggest that professional support and confidentiality be offered when a potential donor decides not to donate and expresses difficulties in communicating this decision to the recipient (NG).
The decision to donate while alive is a voluntary act that potential donors will evaluate finally after they have received complete information. Nevertheless, in the first meeting with the donor, the clinician who commences the study is to accredit their capacity and the reason which has led them to decide to donate one of their kidneys. Although qualified professionals will subsequently report on and accredit their mental state and capacity, the donor should be alone in this interview, in the absence of the recipient and even other family members. The chief benefit which donation will confer on the donor is psychological, based on the personal satisfaction which arises from improving the health of a sick person by means of an altruistic donation. The absence of pressures which mask other motivations should be evaluated at first by means of open questions such as: “Could you let me know your main motivation for becoming a donor?”; “Do you think that if you do not donate, the patient may die due to a lack of alternatives?”; “Do you think that your personal, financial or social situation may have influenced your decision to become a donor?”; “Have you ever felt pressured by anyone to become a donor?”.
In the first meeting the potential donor will be informed of the alternative therapies which are available to the recipient, as well as their survival estimations and waiting times. They will be assured that commencing the study for becoming a donor does not commit them to donating, as they are able to stop the process at any moment without any consequences at all.
In situations when LDKT is considered to be urgent (for example, a highly sensitized patient without vascular access) and study of the donor has to be completed within a short time, the potential donor may be under pressure to a certain degree to donate, thereby invalidating their consent. Due to this, emergency living donation should only be considered in exceptional circumstances, seeking other alternatives using a deceased donor 67.
Sometimes, if risks to the donor's health are increased by the donation, this may be accepted on condition that the consequences for their health are not unacceptable, and if complete and verified information is expressly shown in the consent document. Nevertheless, a conflict may arise between the autonomy of the donor who wishes to donate and the absence of malice of the medical team, which wishes to protect and exclude them. This dilemma may be resolved by reference to the ranking of the principles, in which justice and the absence of malice would be level 1 (the public sphere), while autonomy and beneficence would be level 2 (the private sphere) 61. Public sphere ethical principles affect everybody in the same way, and they take preference over private principles, which everyone manages according to their own ideals and life plan.
Finally, all living donors will be informed at the start of the study that if they find any difficulty in communicating their decision not to donate, they will receive assistance from the transplantation team to do so.
Decisions in donor selection based on sex- •
We suggest that the clinicians who are in charge of selecting donors minimise sex-based imbalances, ruling out the possible coercion of women to donate (NG).
The professionals who take part in the decisions to select the best of several possible donors should consider the ethical need to minimise any possible sex-based imbalance. Although many studies report that the majority of living renal donors are women, the reasons for this disparity in the donation rates of men and women are still controversial 68. According to the ONT 69 and the Amsterdam Forum 8, 65% of living renal donors are women, while 65% of the recipients were men 8. These differences make it necessary to consider the sex of donors from an ethical standpoint before selecting one. In some circumstances and due to cultural or social pressure, it would seem that women have no other choice but to be selected to donate. If this bias is suspected, the medical professionals and psychologists who evaluate the donor should rule out the existence of possible coercion due to sex.
Analysis of altruism- •
We suggest that precautions be maximized under certain circumstances or when suspicions exist that there may be hidden economic agreements or other spurious benefits, above all in donors who are unrelated (NG).
This evaluation is more complicated in the case of donors who are not genetically or legally related. According to World Health Organization estimations, 8% of the more than 100,000 transplantations performed every year in the world use organs that have been illegally trafficked 70. The Istanbul Declaration defines commercialising organ transplantation as “a practice in which an organ is treated as a basic product, even buying it, selling it or using it to obtain another benefit71. With the exception of Iran, where payment is made and controlled by the state for living donation to unrelated recipients, this practice is universally rejected. Over and above its highly controversial ethical aspects 72, this practice is said to reduce the growth in donations from deceased donors 73. Such agreements are clandestine and are denied in other countries, although subsequent disagreements or failures to fulfil conditions bring to light acts of this type, in which intermediaries exploit the neediest individuals for profit. They also corrupt professionals and clinics in countries with limited resources to investigate and prosecute these illegal practices. Professionals who commence studying a living renal donor have to maximise precautions against certain circumstances which may mask economic agreements or other specious benefits. In the case of donors who are unrelated, unemployed, immigrants or prisoners, the first requirement is to accredit what forms the basis of the relationship of the donor and recipient. The next step is for a multidisciplinary team to help evaluate what motivates the donor, with the aim of reaching a decision. Such vulnerable donors should be considered to be victims, as their decision to donate may be due to coercion, regardless of any promised economic benefit or payment of any other type.
It is a crime to offer or accept an economic reward for donating an organ, and this should be reported if it is suspected. It is also unacceptable in ethical terms when the reward is intangible, as this undermines the principles of dignity, justice and equality. Such practices enable the richest patients to gain solutions for their health problems which are unattainable for ordinary people. Internet has made it easier to buy and sell kidneys from living donors. Although they are banned, it is not hard to find offers or requests for donation in exchange for economic rewards, and the authorities should prosecute these practices. Internet has also enabled the creation of web pages that have the purpose of finding unrelated living donors for direct transplantation. This is ethically inacceptable, unless such donation is anonymous and controlled by medical professionals, as is the case in “Good Samaritan” paired donation programmes with altruistic donors 74. Otherwise we would be turning these websites into what have been called beauty contests, where recipients are evaluated on the basis of their personal appearance and biography, which are irrelevant variables for organ assignation 75.
Accreditation of donor identity and their relationship with the recipient- •
In the case of genetically unrelated donors we suggest that their relationship of kinship or the circumstances which accredit the donor-recipient relationship be documented, to ensure that the donation complies with the law and ethical standards (NG).
Exhaustive knowledge of the identity of the donor and their genetic or legal relationship with the recipient should be the priority in the initial phases of the suitability study. The professionals who take part in the study of the donor (nephrologists, urologists, psychologists/psychiatrists, nurses, social workers and transplantation coordinators, etc.) should investigate the identity of each donor and their relationship with the recipient in depth. To this end they will request all of the documentation which permits accreditation of kinship relationships and, in the case of unrelated donors, the circumstances which verify the intensity and duration of their relationship and its nature, to guarantee that altruism is the main motivation for the donation. The Convention on Human Rights and Biomedicine of the Council of Europe protocol on the Transplantation of Human Organs and Tissues urges protection of the dignity and identity of donors and recipients, guaranteeing individual rights and freedoms without discrimination to attain the maximum professional standards while minimising risks 76.
Anonymity- •
It is obligatory in Spain to maintain anonymity in living donor transplantations involving paired donation and non-directed altruistic donors (NG).
This regulation is not applicable to directed LDKT between individuals related genetically, by legal kinship or close friendship. Anonymity in Spain is obligatory for paired donation LDKT and in non-directed altruistic (Good Samaritan) donations which permit chain transplantations. Nevertheless, not all of the European countries with paired and altruistic donation programmes have legally regulated anonymity 51.
In a study of 414 donors and recipients who took place in non-directed and paired transplantation in Germany and Sweden, where such programmes demand anonymity, 22% of the donors and 31% of the recipients expressed the desire to meet after the transplantation 77. Other similar studies agree that more recipients than donors wish to meet the other party. Although donors and recipients are generally satisfied with anonymity, they consider a strict policy of anonymity to be unnecessary.
Experts in bioethics are divided on this point. Breaking with anonymity may lead to psychological or economic pressures on the beneficiary of the donation, terminating in conflict. Although such situations would be rare, they would cause stress for donors and recipients. As well as being legally obligatory in Spain, keeping anonymity is an ethically acceptable practice, because it encourages altruism and prevents forms of behaviour that could damage social perceptions of donation by living as well as deceased donors.
Occasionally, some donors request to be studied without the recipient knowing. It is not good clinical practice to assume that all recipients will accept related LDKT, although many health professionals or family members believe this. The ethical principle of autonomy for the LDKT recipient may be relevant in specific cases, in which their refusal to receive a certain kidney from a living donor would prevail.
Procedure in the case of unforeseen results- •
We recommend specifying the requisites for acceptance with donors; the information they wish to share with the recipient, as well as the procedure in cases of unexpected results, whether or not they are relevant for the donation (B).
Potential donors will be informed of the procedure to be followed in the case of unexpected results that identify a highly improbable genetic relationship. HLA analysis during donor assessment may reveal, for example, cases of misattributed paternity (0.25% - 0.50% of all living donors) and also, although to a lesser degree, cases of siblings and children born to adolescent mothers who grew up believing that another relation in their family was their mother 78. The way to handle such information is controversial, and there is no standard answer, so that each case has to be dealt with according to its own singular characteristics.
In the same way, potential donors should be informed of the procedure to be followed in the case of unforeseen analytical findings (cases of HIV or HCV) the positive nature of which may have implications for the health of third persons, who will have to learn of this circumstance so that they can be referred to specialists. Respecting the recipient, the donor will have to be informed of any previously unknown analytical results (viral serology or specific antibodies) which may alter the survival of the transplanted organ.
Risks analysis and information for the donor- •
The donation of one kidney for transplantation involves accepting a degree of risk for the donor's health that must be minimum, known and accepted by the donor before harvesting (NG).
- •
We suggest that the information for the potential donor on the risks and benefits of LDKT be given in at least two phases, and in oral and written format (D).
- •
All donors are recommended to undergo a lifelong medical follow-up (B).
From an ethical viewpoint the professionals who assess donors must not forget that they have to work in favour of the donors’ interests. Nephrectomy of a kidney for transplantation and the consequences of this for the donor will never be free of possible complications, and some may consider this risk to be ethically unacceptable. However, in LDKT it is accepted that to attain the benefit of the transplant it is possible to accept a minimum or reasonable level of risk for the donor. Due to this, all living renal donors must receive precise information as many times as is necessary about the risks of major surgery and the restrictions or precautions that are involved in living with a single kidney 79. We suggest that this information be offered in at least two phases, in oral and written format 80. The general risks shared by all living renal donors will be described in the first phase, and in the second phase, after the results of the tests on the donor's state of health have been received, the specific risks for the donor must be described (CRD, hypertension, diabetes mellitus, preeclampsia, etc.). These risks are covered in other chapters of this Guide. Tools such as the ESRD Risk Tool for Kidney Donor Candidates” may aid objective work when evaluating each one of these risks 81.
Additionally, donors must accept undergoing regular check-ups after nephrectomy to permit the early detection of cardiovascular and renal risk factors, among others, which can be controlled by changes in lifestyle, dietary recommendations, pharmacological therapies or surgical procedures. The data on living renal donors lost to follow-up in Spain (2010-2017) are 18% and 13% in the fourth and sixth years, respectively 69; some studies in other countries detect far higher percentages 82.
Post-donation follow-up for living renal donors is also an ethical obligation to protect their health. It is an indispensable tool which uses the best scientific methodology to verify the risks of donation at different times, with the smallest possible distortions 83. Living renal donor follow-up registries are included in a directive of the European Parliament 84 as well as in our regulations.
Evaluation of non-resident living donors- •
Donors with a genetic or emotional tie who do not live in the same country as the recipient are especially vulnerable, so that extraordinary vigilance is needed to ensure their protection and care after donation (NG).
- •
We suggest that donation by non-resident donors should not be accepted when no long-term medical follow-up can be guaranteed for them (NG).
The Committee of Ministers of the Council of Europe is aware that many countries accept donations from non-resident living donors with or without a genetic, legal or emotional tie with the recipient and who arrive in the country legally (with a visa or other authorization) and who wish to donate. It therefore adopted a resolution in 2017 for the selection, assessment, donation and follow-up of non-resident living donors, as it recognized that such individuals are especially vulnerable, so that they require extraordinary vigilance to ensure their protection and care after donating 85.
It is important to maintain close vigilance to ensure the early detection of possible cases of human trafficking for the purpose of organ harvesting for transplantation. This question may be particularly difficult to detect when assessing and accepting a non-resident living donor.
- Recipient and living donor who live outside Spain. Before they travel to Spain the hospital where the transplantation is to take place should receive a detailed report which accredits the recipient and possible donor as suitable for transplantation and donation, respectively, according to Amsterdam Forum criteria for living donation. The recipient is to supply clear proof of genetic or legal kinship. In exceptional cases it will be possible to accept another type of emotional tie if this is accredited with absolute certainty within the legal framework in force in Spain. If a visa is required for entry into the country this must have a duration of at least six months. The medical reports on suitability, informed consent, approval by the Ethics Committee and the legal appearance are to be prepared according to the legal requisites, and a translation is to be supplied when applicable. The recipient will have no access to deceased donor KT in Spain. The expenses deriving from the procedure are payable by the recipient or secondarily by their country of origin.
- Recipient resident in Spain and a non-resident living donor. All of the above safeguards will be applied regarding the medical report on the donor, which must be prepared in the donor's own country. In the specific case of living donor transplantation, the transplant will be linked to the care associated with the donation process, as well as any possible complications of the same 86. This will therefore be applied to donors without social security or recipients who are covered by other regimes such as MUFACE.
Centres which are authorized for LDKT must have a specific protocol for acceptance of the recipient of an organ from a non-resident living donor. They should previously inform the ONT through the autonomous community coordination system of the dates on which the surgical operations are to take place, and comply with the protocol according to the document “Living donor KT in non-resident recipients” 85,87.
Signing the informed consent (IC) document- •
We recommend that the IC document should include the risks which are common to all donors, the particular risks for the donor in question and that it be signed by the latter without the presence of the recipient, family members or persons who could influence their decision (B).
- •
Open questions are to be used in the signing of the IC document to confirm that the donor has understood the information on nephrectomy and its risks, and that their decision to donate is firm and without duress (NG).
- •
Donors are to be informed of their right to leave the study at any time, as well as the procedure to be followed if after the removal of a kidney it were not possible to engraft it in the recipient (NG).
The instructions in this step must be followed exactly. The wording of the IC document varies widely from one hospital to another. Now may be the time to standardise a specific national informed consent document that includes the peculiarities associated with nephrectomy for LDKT. A copy of this must be given to the donor and recipient. This initiative agrees with the opinions of professionals involved in transplantation in transplanting hospitals in Spain and other European countries 47.
The act of signing the informed consent document (IC) is considered optimum when it takes place in the presence of professionals who have taken part in the study of the donor and a surgeon who is a member of the donation or transplantation team. In this session all of the analytical and radiological tests will be discussed, together with reports by other specialities and any other result which may affect the donor's safety or the success of the transplantation. Now will be the time to offer all of the additional explanations that the donor may require. Before signing, the medical team will use open questions to confirm that the donor has comprehended the information, is aware of the nephrectomy process and its intrinsic risks, and that their decision is firm and was made without any duress whatsoever. The donor will also be informed that they are free at any time to revoke their consent, and it is therefore advisable for the recipient to be absent during this step.
The IC document for the donor is to include at least the following:
- -
The risks of the surgical procedure (type of surgery, anaesthesia, transfusions, infections, further surgery, mortality).
- -
The risks deriving from the nephrectomy such as the possibility of CRD, hypertension or cardiac pathology.
- -
The individualized risks for the donor studied, due to borderline analytical results, pre-existing disease or family history. In young women with the potential to become pregnant, risks of gestational hypertension or preeclampsia will be reported.
- -
Actions in case the collected kidney cannot be engrafted in the recipient. The donor must decide at the moment of signing the IC if the kidney should be transplanted to another person or re-engrafted in the donor.
- -
The right to withdraw their IC at any time prior to surgery.
- -
Quantification of the risk or possibility that the kidney will not function in the recipient or that it will cease working prematurely.
- -
Commitment to medical follow-up after donation.
The IC document for the recipient is to include at least the following:
- -
The risks of the surgical procedure (anaesthesia, transfusions, infections, further surgery, mortality).
- -
The risks that the transplanted kidney will not function (thrombosis, rejection) or will cease to work prematurely.
- -
The adverse effects of immunosuppressant medication.
- -
The risks of catching diseases that were not detected in the donor due to the window period or the impossibility of diagnosis.
- -
The recurrence of the original renal disease.
- •
The function of the Healthcare Ethics Committee is to protect the rights of donors and, in the case of conflict, to help decision-making (NG).
- •
We suggest that the medical reports arising from the study of the donor and recipient be sent to the Healthcare Ethics Committee, as well as all of the information which is considered to be relevant for risks and motivation analysis (C).
RD 1723/2012 covers intervention by the Healthcare Ethics Committee in the evaluation of a living donor: “In any case, before proceeding to obtain the organ, it will be necessary to have a report by the corresponding Ethics Committee”. To this end the professionals who have studied the donor and recipient will send a request to the Healthcare Ethics Committee for its report, accompanied by the clinical reports on the donor and recipient which contain a detailed clinical history and the tests used to assess their state of health. These will be accompanied by the donor's mental health report and the informed consent documents of the donor and recipient. The Healthcare Ethics Committee has the function of protecting the rights of donors and, in case of conflict, to help decision-making. Its mission is not to evaluate the suitability of the procedure, but rather to ensure that the donor is properly informed of the probabilities of success and the risks which they accept. In some cases they may request an interview with the donor to confirm the degree to which they are informed and the absence of coercion.
The Healthcare Ethics Committee will issue a document on the suitability of the living donation, or it may request an additional report or consideration in connection with the guarantees for the donor. This report will never substitute the responsibility of those who control the process of living donation and transplantation.
In North American hospitals LDKT programmes have been introduced recently which include the so-called “independent living donor advocate”. This individual is independent of the transplantation team and is not involved in the study of the donor and recipient. Their mission is to ensure that the donor and recipient have all of the relevant information about the donation process and that they have understood it properly, ruling out the possibility of coercion or commercialization as far as possible and defending the donor's rights 88. One hospital in our country which is very active in LDKT has an independent professional who is unconnected with the transplantation team, who accredits the correctness of the whole process prior to the LDKT procedure. Nevertheless, for the majority of hospitals the authors consider that the Healthcare Ethics Committee included in Spanish law perfectly fulfil the role of this independent advocate and that therefore there would be no need to involve other professionals for the same purpose.
4Information in living renal donationThe chief aim of the information given to potential living renal donors is to obtain their consent for donation. Obtaining this consent is more of a process than it is an event. The KT scheme is responsible for establishing that potential donors are able to understand the relevant information, that they are suitably informed about the risks and benefits of the donation and the alternative therapies available to the recipient, and that they understand this information and act voluntarily.
In the Spanish guidelines published in 2010 1, this section about information gave general recommendations and reviewed the literature available to date while centring on when to supply information and the nature of the information to be supplied 89. This section now contains recommendations to ensure that the process is suitable. Sections 2 (legal aspects) and 3 (ethical aspects), are also relevant here, together with sections 5 to 8 and 17, on the physical and psychosocial risks for the donor.
The lack of randomized studies or systematic reviews justifies the fact that all of the recommendations under this heading are “ungraded”.
Information about types of renal replacement therapy- •
Selection of the type of renal replacement therapy is a shared decision-making process by the patient and the medical team (Quality of evidence: NG)
- •
All patients who are considered candidates for receiving a KT should be offered the possibility of receiving a kidney from a living donor (NG).
- •
All patients who are considered candidates for receiving a KT should be informed that the best option is transplantation before they need treatment by dialysis (NG).
The decision-making process for the type of renal replacement therapy (RRT) to be used is completely covered by the concept of shared decisions, with at least two parties: the patient and the medical team of clinicians, the nephrologist and a nephrology nurse. The clinicians will supply scientific evidence on the diagnosis, aetiology, prognosis and different therapeutic options, including conservative treatment without dialysis or a KT. The patient will describe their values, their social, family and economic circumstances and their worries and preferences. Both parties will eventually reach an agreement on the best options to be selected.
This selection of a RRT, within the context of an unstoppable flow of information associated with the increasingly explicit right of patients to independently decide on every aspect that affects their health, implies the complete abandonment of the classical paternalistic model. In this new deliberative model the patient decides, while medical professionals pay respectful sensitive attention to their individual preferences, needs and values, before agreeing to the decision of the true protagonist in this process. When choosing a RRT this deliberative model is far more suitable than the purely technical and informative alternative, in which the clinician simply functions as an advisor. In the deliberative model which this Guide proposes, the patient enters a trusting relationship with the clinician and nurses that is based on this active two-way exchange of information, to reach the best shared decision. The clinician guarantees that the information is appropriate, comprehensible and easy to access, especially the information about therapies and their advantages and risks.
The best shared decisions are those which have a sufficiently scientific basis for their efficacy and safety, while being as suitable as possible for the values, preferences and aims of the patient. The tools which can aid this decision-making are instruments that help the patient to obtain information and answers to their key questions about what they should do or what they have to know to reach a decision. These tools can come in a wide range of formats: pamphlets, information sheets, videos, on-line applications or interactive websites, etc. The information supplied should be expressed appropriately and effectively, high quality and in an easy-to-understand clear format 90,91.
When a patient reaches stage 4 CRD, with an estimated glomerular filtration rate (eGFR) below 30ml/min, the first step should be their assessment as a possible candidate for LDKT 92. Parallel to this option, it is also necessary to select one form of dialysis or the other (peritoneal or haemodialysis), in case it is impossible to carry out transplantation before dialysis is required. The ideal context for this process of information and selection of RRT type is during consultation for ESRD, where apart from this process a series of increasingly standardized activities are also implemented, in a way that could be formally accredited (Table 1).
Patient route map in the end-stage renal disease unit of the Hospital del Mar, Barcelona.
| General assessment and evaluation by nursing.Decision-making process on renal replacement therapy (living or deceased donor kidney transplantation, haemodialysis or peritoneal dialysis).Referral to the appropriate specific department (haemodialysis or peritoneal dialysis); if the patient is a transplantation candidate, referral to the specific living donor surgery or inclusion in the deceased donor transplantation list, if applicable.Patients who are not candidates for renal replacement therapy will be evaluated immediately to increase their comfort and treat any complications, referring them to their primary care centre with a report for palliative care, if applicable.An analytical study, a comorbidity study, screening for tumours and viral markers (HIV, B-C virus), and regular follow-up procedures.Vaccination against virus B and others.Surveys on quality of life and degree of dependency.Signing of their specific consent for the selected technique. |
The webpage set up by the Departament de Salut of the Generalitat de Catalunya contains a detailed set of tools for use in a joint decision for the treatment of ESRD 91 (Table 2). Whether online or personally, the different options have to be compared, and it would be advisable to detect patients’ preferences by means of a test. This space would allow patients to reflect and use the results in conversation with their clinician.
Shared decision-making tool and material for selecting a renal replacement therapy [Modified from Ref 89].
| What are the treatment options? | |||
|---|---|---|---|
| RENAL TRANSPLANTATION | PERITONEAL DIALYSIS | HAEMODIALYSIS | |
| What does it consist of? | A kidney from a deceased or living donor is implanted | Blood is filtered and cleaned through a natural membrane in the abdomen | Blood is filtered and cleaned through a an external circuit with a machine |
| What is needed? | A good general state of health, with no medical or surgical contraindications | A thin flexible tube inside the abdomen | Access in a vein or a created fistula (minor surgery) or a catheter |
| Is surgery required? | Yes | Yes, to position the catheter | Yes, to make the fistula |
| Does it hurt? | Discomfort in the wound after surgery | No | Injections in the fistula in each session |
| Where is it performed? | In hospital, but only while admitted, afterwards you are seen as an outpatient | At home or anywhere clean and suitable | In hospital or a haemodialysis centre |
| Who does it? | An expert surgeon | You yourself | A medical professional; sometimes yourself at home |
| How often? | Once if there is no acute or chronic rejection. Follow-up visits are needed for life | Every 3-4hours during the day or during 8-10hours at night | Three times a week during 4hours in a care centre; flexible at home |
| Does it involve restrictions in liquid intake and diet? | Less than the other treatments | Few | Yes |
| Does it have side effects? | Surgical complications and the therapies to prevent rejection: infections and tumours | Risk of infection of the catheter orifice or in the abdomen (peritonitis) | Tiredness, risk of low arterial pressure after the session, infections |
| What are the long-term results? | Better than the other therapies | Good; better than haemodialysis over the short term, but the same over the long-term | Good |
| How long may it work? | Half work from 10-12 years, some more, some less. A new transplantation is possible | Several years | Several years |
Shared decision-making ensures that patients know more and are better informed, with greater awareness of their own values and better able to perceive risks and advantages: they are able to decide without negatively affecting their health 93.
There is currently debate about the relevance of using quantitative methods to select the type of RRT to be used, to calculate the risk of mortality or the probability of survival. Calculators of this type are widely used in the Anglo-Saxon world, while they are used less often in Spain. One of the best is iChoose Kidney, which was developed in the Emory (Atlanta, U.S.A.) transplantation programme 94. This calculator was found to be useful in a randomized trial 95, and it was validated in Ontario, Canada 96. It includes age, sex, race, time in dialysis and the most important comorbidities. The Canadian validation of this calculator, which included 0,520 patients in dialysis and 4,505 transplanted patients, was able to predict mortality at 3 years. An example shown in the publication 96, is that a 70 year-old women of African American, with more than one year in dialysis, hypertensive and diabetic, has a risk of mortality at 3 years of 27% in dialysis and 11% if she receives a transplant. If the kidney comes from a living donor the risk is 6%, and if the donor is deceased it stands at 12%.
Apart from the different strategies developed by each hospital or ESRD unit to properly process the shared decision on the type of RRT to be used, other specific strategies have been developed to help the specific decision involved in LDKT (Table 3) 97. The materials which aid the decision (booklets, videos, online material, text messages and calculators, etc.) centre on general education about the process involved in renal disease and transplantation. They are more or less adapted to patients in general or culturally sensitive aspects, and they are aimed at potential donors as well as potential recipients. The strategy in which each unit prepares its own materials is more widespread in Spain, essentially pamphlets or small explanatory books which in a series of chapters explain different aspects associated with the process of donation, describing advantages and drawbacks, risks for the donor and recipient, and the results that can be expected. The questions which any material for potential renal donors should answer are shown in Table 4. Booklets can never completely replace a conversation which lasts for as long as a potential donor may require, in which they are able to raise any questions 98.
Summary and short description of the tools that are available to aid decision-making in living donor kidney transplantation (LDKT).
| Decision Aid | Type Of Tool | Short Description |
|---|---|---|
| Comprehensive educational decision-making aids | ||
| PREPARED: Patients’ readiness to make decisions about kidney disease | Booklet, video, website | Knowledge. Economic aspects. Video testimonies by patients, family members and professionals, describing concerns |
| La gran pregunta, el gran don (“The big question, the great gift”) | Printed material | Material for renal patients and their families, as well as potential donors, centring on LDKT and their assessment process |
| Specific or culturally sensitive instruments | ||
| Living ACTS: Opciones en trasplante (“Transplant options”) | DVD and book | This has the special purpose of overcoming communication barriers about LDKT in the Afro-American community |
| Infórmate (about living donor renal donation for Latin Americans) | Website | Culturally adapted information in English and Spanish for patients and families |
| Decision aids for potential recipients | ||
| Tu ruta al trasplante (“Your route to transplant”) | Messages adapted and personalised by computer | A programme that generate personalized education based on the analysis of a specific recipient, advantages and disadvantages of LDKT |
| Explora el trasplanteCasa(“Explore transplantation home”) | Text messages | Educational material about the advantages of LDKT |
| Mi entrenador de trasplante(“My transplantation trainer”) | iOS application | This offers predictions for a specific patient and their survival after different therapeutic options. It promotes consideration of LDKT. |
| iChoose Kidney | Smartphone app | Transplantation vs dialysis |
| Decision aids for donor education | ||
| Live Donor Champion | In person | Training for a potential representative or “advocate” of potential living renal donors |
| DONOR | A platform for renal patients which allows them to share their experiences. By answering a series of questions, a narrated history is created that is easy to share | |
| Calculadora de riesgo de enfermedad renal avanzada para candidatos a donante renal (“End-stage renal disease risk calculator for renal donor candidates”) | Website | This shows the risk of reaching the stage of needing renal replacement therapy 15 years after donating a kidney |
| Decision aids for professionals and providers | ||
| Living donor KDPI | Website | To determine the recipient risk in case of a range of living donors, according to their characteristics, and comparing this with a potential deceased donor |
Questions which any material supplied to a potential renal donor should answer.
| What is end-stage chronic renal disease? |
| What is living donation? |
| Who can be a living donor? |
| Who cannot be a living donor? |
| What can the potential recipient do? |
| What risks are involved in living donation for the donor? |
| What should we expect of the transplantation? |
| What type of assessment is used for the donor-recipient pair? |
| What are the risks for the donor? |
| What are the risks for the recipient? |
All CRD patients should be considered and assessed for KT, except for those with an absolute contraindication, because KT offers a higher quality and longer life than dialysis 3,4. Patients who receive an early KT from a living or deceased donor have better personal and graft survival than is the case for those who had already started dialysis 99. Thus an early KT should be offered to all of the patients who have the possibility of a living renal donor. Patients and their family will be informed of this option in the decision-making process in cases of ESRD, and they will be given informative booklets and referred for a first pre-transplantation consultation.
The European guides recommend that patients with gradual deterioration of renal function and an eGFR <15ml/min/1.73 m2 should be assessed for inclusion in the waiting list for a preventive or early KT 3,4. The patients who are considered to be potential candidates for KT will be referred to start assessment when their GFR is <20ml/min, once the possibility of a living donor KT has been examined.
The basic information required for potential renal donors to give their consent- •
The information given to the donor will be standardized and will include details about the assessment process and acceptance; the risks, benefits and evolution that can be expected in the donor's health; alternative therapies for the recipient; how personal health data are used and donor support personnel (NG).
KT programmes should develop the process by which candidate donors are informed 100. The use of standardized checklists has been suggested, to ensure that all of the relevant information is supplied 101. In the United States, the Organ Procurement and Transplantation Network has developed a list to ensure the suitability of the process 102, as well as a document for potential donors in language that is easy to understand 103.
This information should be communicated in each KT scheme in the most favourable environment in each case; the most frequent and appropriate circumstances for this are consultation in the case of ESRD, consultation before transplantation or transplantation coordination, where patients are referred after a general visit to nephrology or dialysis units. It often happens that the possibility of living donation is raised in the context of the selection of a RRT in a nephrology consultation. This leads to a specific visit and analysis of this type of transplant with specialized nephrologists. It is therefore essential that the nephrologists who are in charge of CRD patients always keep in mind the possibility of treating them with the best available therapy, which is living donor KT, if possible prior to the need for dialysis. The best time to present this possibility and check whether potential donors are available is when the potential recipient is in stage 4 CRD, with an eGFR of from 16ml/min to 30ml/min.
A range of printed material may be used to offer this information systematically, facilitating the presentation and discussion of the basic concepts with the patient or potential donors. From the first, potential donors should receive clear information on the details of the assessment process. The language used has to aid communication and the mutual understanding of a potential candidate and the clinicians 104,105. The climate in which this exchange takes place should permit questions of all types, adapting to the particularities of the candidate 105,106.
The information which has to be supplied is included in many guides and declarations 1,2,104,107,108. Some regulations stipulate a minimum content that should be present in the informed consent process 107. Table 5 shows the information that should be supplied to the potential donor.
Information that a potential renal donor must be given in the process of obtaining their consent. [Modified from Ref. 91].
| Type of information | Information that must be given to a potential donor |
|---|---|
| Personal health data of the potential donor | Personal information about the health of the candidate donor is confidentialThe transplantation scheme will supply the recipient with confidential information about the donor with the permission of the latter: immunological compatibility and relevant medical characteristics for the future of the graft. |
| Risks of positive findings in the assessment | Possible diseases that require study and treatmentPossible transmissible diseases that have to be reported to the authoritiesPossible ruling out of family relationships that were previously assumed, or findings of new family relationships that were previously unknown |
| Risks of donation | Physical, medical, surgical, psychological and social risks of renal donation, over the short- and long-term |
| Alternative therapies for the potential recipient | Information about dialysis techniques and deceased donor kidney transplantation, and the comparative results respecting living donor kidney transplantation. |
| Personal health data of the potential recipient | The personal information about the health of the candidate to receive the kidney transplantation is confidentialThe transplantation scheme will give confidential information about the potential recipient to the donor if the donor gives permission for this, on condition that these findings are relevant in the donation decision-making process. |
| Processes involved in the evaluation of a potential donor, donation and follow-up | It is possible that some procedures will require specific informed consentIt is necessary to insist that the donation is completely free of rewardThe possibility of deciding not to donate at any moment in the process should be describedAfter the assessment process there are two final possibilities which are subject to the decision of the transplantation team and communicated to the candidate: that they are suitable for donation or they are not. In the latter case the reasons why they were rejected should be explained to the candidate who was ruled out.The whole process of hospitalization, nephrectomy, postoperative recuperation, discharge from hospital and subsequent short- and long term follow-up should be explainedThe social, medial and economic advantages arising from the donation should be described, including priority for deceased donor transplantation if the donor develops chronic end-stage renal disease |
The most common worry expressed by renal donors prior to donating is the possibility of suffering renal failure themselves in the future, together with the recovery process, its duration and possible complications 100,109,110.
Potential renal donors have to know that they will only be able to donate once in their life, and they should also be aware of the alternative renal replacement therapies available to any potential recipient. When incompatibility due to blood group or HLA sensitization is detected, the donor should be informed of the range of options that may be available, such as ABO-incompatible KT, paired KT 111–113 or HLA desensitization, which in general usually require a specific informed consent.
The assessment process for a potential renal donor involves the risk of discovering previously hidden pathologies and diseases, and these may even require diagnosis and therapy. All findings and the decision about what to do should be shared with the individual who has now ceased to be a renal donor candidate and is now a patient. One such finding may be that a previously accepted family relationship (such as paternity) is ruled out, or that a new previously unknown relationship is discovered 78. There is no agreement on the appropriate behaviour in these cases. At an early stage it is advisable to warn that such a discovery may occur. The KT scheme should establish an information protocol for such cases, regarding when and how to inform the interested parties 78,114–117.
Renal donor candidates should receive information on the surgical, medical and psychosocial risks involved (sections 5-7 and 17), including the necessary individualization and the inevitable degree of uncertainty and lack of evidence in the majority of cases. For example, the risk of mortality 3 months after donating is estimated to stand at 0.03%, and no profile has been established of donors who are at increased risk 118–120.
The surgeon who carries out nephrectomy on a renal donor has a particular responsibility to ensure that they understand the potential short and long-term risks. The surgeon is usually a urologist in Spain, and they will have their own process for informing the donor, centring on the nephrectomy itself, which is usually performed laparoscopically. The urologist will explain the purpose of the surgery and describe the technique they will use and the risks associated with it, obtaining the donor's informed consent. On the other hand, potential donors should also receive information on the circumstances that may arise while they live with a single kidney. These will include exceptional risks such as severe injuries or medical problems involving lithiasis or infections that may compromise the working of their remaining kidney. It can be confirmed that over the long-term there is no significant tendency for GFR to gradually decrease in any age group 121, and that the incidence of hypertension does not increase over time 122.
A question that young women who are potential donors ask is about the possibility of gestation. An increase in gestational hypertension and preeclampsia has been described in renal donors in comparison with their general non-donor health control group. Nevertheless, these findings are not associated with any extra complications for the mother or foetus 123. Special attention should be paid to checks of arterial pressure, weight gain and proteinuria, although these are generally the same as those recommended for all pregnant women 124.
Although it may be advisable to use transparent quantitative tools in the process of informing potential donors about the long-terms risks involved 125, calculations of this type are highly limited and we have no exact means of estimating the risk that could be attributed to renal donation. Even though no official regulation exists on this point, potential donors should be told that in the unlikely event of their developing ESRD, they would be given maximum priority to receive a kidney transplantation 126,127.
With the same degree of emphasis that is used to inform them of the risks involved, the transplant scheme should inform renal donation candidates of the potential benefits of their donation. The difficulty of measuring this benefit is not very different from the above-mentioned difficulty of measuring the risk 128.
The donor should be informed about the estimated survival of the kidney as well as the recipient at 1, 5 and 10 years, after LDKT as well as a deceased donor KT or treatment using different dialysis techniques. This information should preferentially be based on the results of the renal donation scheme in question. Although in the U.S.A. potential donors should be told about the survival of the kidney and recipient one year after KT 107, there is no such obligation in Spain.
Donor candidates should know that the potential recipient will not be informed of any aspect of their health without their express consent 129,130. Equally, the donor will not be informed of anything about the recipient without the consent of the latter 2,107. The potential donor should also know the economic and social implications of their donation, the impact that it may have on their professional performance and the length of time they will be temporarily off work, under normal circumstances as well as in case of complications. On the other hand, the donor should also be told that it is illegal to obtain any material benefit (economic or any other type) as a reward for the donation.
The informing clinician should tell the renal donor that they are able to decide not to donate at any time during the process, and that regardless of the reason for this, they will continue to have the full support of the transplantation scheme. On the other hand, the majority of programmes reserve the right to accept or refuse a potential renal donor, so that the latter should be informed of this in detail, together with the reason for the decision 131. In general, it is assumed that a transplantation scheme which rejects a donor due to unacceptable risk for their or the recipient's health should seek a second opinion from another scheme if the potential donor requests this.
The renal donor should be informed of the risks and benefits which the recipient of the kidney will experience, including among others: 1) that the therapy is not definitive, and that gradual loss of renal function over the years often occurs, due above all to the development of chronic forms of rejection, 2) that there is a possibility of around 5% that the graft will be lost at an early stage because of acute rejection, primary failure of the graft, thrombosis or infectious complications. This aspect is covered in greater detail in Table 689.
Potential risks accepted by the recipient of a kidney transplantation that the potential renal donor should be aware of in the process of obtaining their consent [Modified from Ref. 89].
| The kidney | Surgery | Drugs | Unmet expectations |
|---|---|---|---|
| Never functioning (5%) | Complications associated with anaesthesia (<1%) | Need for drugs every day for life | Not leaving hospital: follow-up for life |
| Functioning much less than expected (10%) | Complications with haemorrhaging (3%-5%) | They cause side effects such as diabetes or hypertension | A transplant will often not function lifelong, there is chronic rejection and a return to dialysis (50%) |
| Dialysis sessions are necessary until it functions (25%) | A urinary fistula, lymph or blood appear around the kidney or infection of the wound (5%-8%) | Chronic administration is associated with infections and malign tumours | The original disease may “reproduce” in the renal transplant |
| Rejection and other situations require a biopsy (15%) | A blood transfusion may be necessary (20%) | Doubts about whether it would not have been better to remain in dialysis |
The essential point of the process is to hold a suitable interview in a completely trusting and frank atmosphere, without the potential recipient or any of their family being present. A persistent doubt or ambivalence will normally lead to a refusal to donate, and in this case as well as in that of a clear negation, the scheme should respectfully and confidentially support the decision and the individual who had been a potential donor and finally decided not to proceed 132. Although it often used to be employed in the past, it is unadvisable to use a “medical alibi”, or justification of the decision not to donate for the potential recipient by giving a false medical reason to justify the choice. It would seem to be better to state that “this donor is unsuitable” in general, without giving a false reason 133. The transplantation scheme should help the donor to communicate this if they wish. On the other hand, in some cases the potential recipient will not accept a voluntary renal donation, and in this context conflict reduction procedures should also be in place, permitting a refusal without any other consequences.
Preliminary approach to the viability of donation- •
The process of studying a potential renal donor permits a very wide range of approaches which depend on the donor's characteristics, the urgency of the study and local facilities and waiting times for examinations (NG).
- •
The first step consists of the screening of a potential renal donor to rule out unviable cases with a minimum of inconvenience for the donor and resource consumption. This screening should therefore include: a review of the pre-transplantation study of the recipient, donor-recipient compatibility (ABO group, crossmatch test) and evaluation of the potential donor, with basic analysis and estimation of their GFR rate (NG).
The process of studying a potential renal donor permits a very wide range of approaches which depend on the donor's characteristics, the urgency of the study and local facilities and waiting times for examinations. Studies may take place simultaneously from the first in very clear cases, above all if the aim is to reach a swift conclusion. The order in which examinations take place should also be varied depending on the case; for example, in donors with specific pathologies that require approval by other specialists, no in-depth studies should be undertaken until their approval has been obtained.
The first step has to consist of the screening of a potential renal donor within the context of the initial consideration of the donor-recipient pair, using hardly invasive and immediate basic examination techniques that will make it possible to decide whether the donation is possible. The aim here is to rule out unviable cases with minimum inconvenience for the donor and resource consumption. This also gives the donor time to confirm their decision. No written informed consent is required for this first step, simply the verbal acceptance of the potential donor. Written consent will be required later, when invasive examinations take place, as well as the final consent to nephrectomy.
This first screening should include three fundamental aspects:
- 1)
Review of the pre-transplantation study of the recipient: confirm the indication for transplantation, that the pre-transplantation study is appropriate and complete, and assessment of the risk and prognosis.
- 2)
Donor-recipient compatibility: determination or repetition of the ABO group in blood bank, donor HLA typing (and of the recipient, if they are not included in the deceased donor list) and the first crossmatch test. This makes it possible to consider the possibility of directed transplant if there is ABO compatibility and a negative crossmatch test; otherwise, it makes it possible to offer the option of a paired KT or a desensitization procedure.
- 3)
Assessment of the potential donor, with anamnesis and physical examination, basic analysis with serum creatinine and eGFR, glycaemia, liver function, haemogram, haemostasis, HBV, HCV and HIV serologies and microalbuminuria/creatinine and sporadic sediment in urine.
- •
Informing society about living donor transplantation should stimulate promotion of the same (NG).
- •
Attending to the media and proactive information should be priorities (NG).
The scientific evidence in favour of LDKT, especially when it takes place before the need for dialysis, should stimulate its promotion in educational spaces for all types of healthcare professionals, including those who work in primary care 134. On the other hand, dialysis therapy providers should be fully aware of the advantages of transplantation, especially from a living donor, so that they will be able to educate the patients they treat regarding this.
Agreements should be reached at all levels of society, the media and even religious organizations to promote and drive LDKT. Technological advances should therefore be used to spread a wide range of educational and training tools throughout society. This educational work should be even more intense where there is a low educational level in different communities and regions, to overcome economic and social concerns.
Attending to the media has always been a priority for the ONT, as it is for regional and hospital transplantation coordinators. They are all aware that information given to the media will directly affect the whole of society. All medical professionals involved in the donation and transplantation of organs should cooperate all of the time with the media, always being available to answer their requests and questions, while attempting to quell the possible negative effects of a poorly expressed news item as quickly as possible. Medical professionals should maintain what is known in communication theory as “proactive communication”. Journalists welcome this attitude, and it leads to personalized daily attention. The continuous publication of positive news has helped to create the good image enjoyed by transplantation in our country, where it is considered to be not only normal, but also and above all a basic service of our healthcare system. In the social imagination transplantation is associated by Spanish citizens with life, and this perception surely aids the donation of organs in general, and donation by living individuals in particular. Basic communication principles such as the need to use clear, concise and well-argued messages, coherent arguments, properly explained complexities, the tone of messages and of course the most suitable techniques to express them are of key importance for the system to work.
As the ex-director of the ONT, Rafael Matesanz, writes: “You should always attend to journalists when they call, in the best possible way and regardless of where they are calling from. And you have to do so as quickly as possible, because journalists usually don’t have the luxury of being able to wait. That way we will be able to prevent lots of misunderstandings that may end up as malicious rumours, while on the other hand we’ll have the opportunity of spreading a positive image of the process.” 135
Time off work, economic costs, life insurance policies and applying for loans- •
Extracting a kidney for donation should be included as a specific cause which justifies the declaration of temporary incapacity to work, with the resulting financial support from the social security system (NG).
- •
The direct accredited costs incurred by a donor due to donation should be repayable, and their medical care should be guaranteed for life (NG).
- •
The change in the health circumstances of an individual because they have donated cannot give rise to penalizations in contracting or renewing insurance policies or loans of any type (NG).
Kidney donation leads to problems at work in a certain number of cases. Donors have to miss a lot of workdays due to medical tests and the postoperative period. It is possible that they may even lose their job due to the consideration that interventions of this type are “voluntary”. Restrictive interpretation of the General Social Security Law (art. 128) prevents recognition of temporary incapacity to work with the right to economic support, as the donor is considered to be neither sick nor to have suffered an industrial accident. Many donors therefore take a holiday or unpaid leave during the donation process.
This lack of work-related protection for living donors has to be resolved, and the harvesting of an organ for donation should be included as a specific cause which justifies the declaration of temporary incapacity to work and the resulting economic support from the social security system during the harvesting and subsequent recovery of the donor, including the impossibility of dismissing the individual during the donation process. Although the current regulation governing temporary incapacity already permits this form of interpretation, it would be advisable for Spanish law to include this explicitly.
A more ambitious and encouraging initiative could take the form of social and work-related protection similar to the current system for pregnancy and birth, with a similar amount of leave to that granted to parents. This measure would affect about 400 people per year, a very low figure. Resolving this problem would encourage people to donate kidneys while alive. The direct and accredited costs incurred by donors due to their donation should be repaid, and they should be guaranteed life-long healthcare. Nevertheless, no economic reward can be set, as this is prohibited by current law.
The change in their health circumstances which someone may undergo due to having donated a kidney should not lead to penalties when they take out or renew an insurance policy or any type of loan. 136.
5Assessment, clinical history and specific tests for living renal donorsThe study of a renal donor is a complex process in which many specialists take part. This study should ensure that the physical and psychological health of the donor is suitable, that their donation is conscious, voluntary and selfless, that the risks for the donor over the short, medium and long-term are very low, and that the possibilities of success in the recipient are high. The transmission of diseases from the donor to the recipient should also be prevented, especially infectious and/or neoplastic ones. It is very important to confirm that a potential donor and the recipient are free of hereditary diseases. It is also necessary to ensure that the donor and recipient have a good level of immunological compatibility. This compatibility and the metabolic study of the donor are described in other chapters. This chapter describes how the study of the donor should proceed, and the main findings which may contraindicate donation.
General analysis and RadiologyAlthough it is not the same in all hospitals, the majority recommend a study protocol similar to the one shown below 9,101,137,138:
Haematological analysis- -
Haemogram, coagulation study, iron metabolism.
- -
Biochemistry (glucose, HbA1c, urea, creatinine, CRD-EPI, sodium, potassium, calcium, phosphorus, uric acid, venous gasometry, hepatic biochemistry, proteinogram, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, reactive C protein).
- -
Serological studies:
- -
Human immunodeficiency virus (HIV). PCR-HIV before surgery.
- -
Hepatitis B: HBV-surface antigen (HBsAg), HBVcAc (HBcAc IgM/IgG), HBV-surface antibody (HBsAc), PCR-HBV in plasma if HBcAc positive.
- -
Hepatitis C (ELISA). Evaluate RNA-HCV in some cases.
- -
Cytomegalovirus (CMV IgG/IgM).
- -
Epstein-Barr virus (EBV IgG/IgM)
- -
Toxoplasma.
- -
Syphilis: RPR (Rapid Plasma Reagin)-FTA.
- -
Coronavirus. Screening for SARS-CoV-2.
- -
Human T- lymphotropic virus-HTLV I-II.
- -
PSA (men >40 years).
- -
Pregnancy test, if applicable.
- -
Oral glucose overload test, if applicable.
- -
Elemental, twice.
- -
Albumin/creatinine ratio, proteins/creatinine ratio
- -
Creatinine clearance, calciuria and proteinuria in a 24 hr. sample
- -
Urine culture.
To be considered depending on risk assessment based on endemic geographical areas:
- -
Trypanosoma cruzi
- -
Strongyloides
- -
Rapid detection test for malaria (RDT) using antigen detectors and antibody determination using indirect immunofluorescence (IIF) against the same
- -
Schistosomiasis
- -
Coccidiomycosis, histoplasmosis
- -
Ebola
- -
Zika
- -
Western Nile Virus
- -
Thoracic radiography (posteroanterior and lateral)
- -
Abdominal CT-angiography. This will inform us of any possible alterations in individual organs, detecting vascular problems, lithiasis and tumours, offering a description of both kidneys that will contribute definitively to the selection of the one to be nephrectomized. It will also permit the characterization of the renal excretory system. Anomalies detected by CT-angiography are usually the first cause of rejection.
- •
We suggest that donor studies should include stress tests such as the myocardial SPECT test or MR imaging with dobutamine in the case of an abnormal ECG or echocardiogram, individuals older than 60 years or multiple risk factors (C).
The cardiovascular study has the aim of detecting significant cardiac pathologies which would constitute a contraindication due to increased risk for the donor: ischemic heart disease, heart failure, cardiac valve pathologies, left ventricular hypertrophy or significant arrhythmia. After finishing this study and having been selected, donors do not seem to suffer any increase in cardiovascular risk after donation 139.
Together with the detailed anamnesis, physical examination, thoracic radiography and the ECG, age or other potential donor risk factors such as hypertension, heart murmurs, stress dyspnoea or end stage age lead us to use echocardiography, Holter (to rule out arrhythmia) and stress tests such as the myocardial SPECT or MR imaging with dobutamine in the case of an abnormal ECG or echocardiogram, age over 60 years or multiple risk factors: age >45 years in men/>55 years in women, smoking, dyslipidaemia, hypertension or a family history) 137. If the case so requires, the cardiologist will request other donor assessment tests.
Respiratory function tests would be indicated in the case of chronic lung disease or heavy smoking. There is an increased risk for donors in the case of forced expiratory volume in 1 second (FEV1) or forced vital capacity (FVC) <70%, or a FEV1/FVC ratio of <65% 138.
Evaluation of donor renal function- •
We recommend that the initial study should include the estimation of GFR using validated formulas (Quality of evidence: B).
- •
We recommend that the measurement of glomerular filtration should be confirmed by means of more precise exogenous markers, such as 125- iothalamate, 51 Cr-EDTA, iohexol or Tc-DTPA, especially in patients with borderline renal function (B).
- •
Study using 99Tc DTPA is suggested when there is a size difference between both kidneys greater than 10% (C).
- •
The donor GFR measured before donation should be sufficient so that afterwards the foreseeable GFR will remain within values that are acceptable for their age and sex during the rest of their life (B).
- •
We suggest that the decision to accept a candidate with renal function below the required threshold or who has additional risk factors for progression to chronic renal failure should be made on an individual basis (D).
- •
We suggest that the donor be informed and that the informed consent document should contain a statement of the slight increase in the risk of suffering chronic renal failure during their lifetime (D).
- •
We recommend that all donors should be followed up for life, with especial attention to renal function, proteinuria and arterial blood pressure (B).
Donor renal function must not be evaluated using only plasmatic creatinine. This is of limited value when it seems to be normal, although if it is known previously and it is equal to 1.5mg/dl or higher, this may help to rule out donation. In general standardized GFR estimation equations for body surface and creatinine clearance in urine over 24hours are recommended. It is necessary to collect urine in the absence of fever, menstruation, urinary infections and previous heavy physical exercise. It is also important for the intake of proteins in the diet to be at least 1g/kg weight, as a low protein diet may reduce creatinine clearance by up to 10ml/min 140. The possibility of incomplete or excessive urine collection should always be taken into account. For this we base ourselves on the excretion of creatinine (which should be within the range from 15mg/kg - 25mg/kg). This should be determined on two occasions to minimise errors.
Estimating the GFR using formulas validated by international standards is accepted in the initial study and should be repeated later (9,137). The most widely used standards are Cockroft-Gault, MDRD and CRD-EPI. These formulas have not been validated for this specific population of renal donors. Nevertheless, it has been communicated that average Cr clearance and MDRD give a good approximation to GFR measured by 125-iothamalate 141. It is currently thought that CRD-EPI may offer a better fit with measured GFR than other formulas in patients with normal function 142.
The KDIGO and British guides recommend subsequent checks and the evaluation of renal function using more “exact” methods, such as measuring GFR using exogenic markers such as 125iothalamate, 51Cr-EDTA, iohexol or 99Tc-DTPA (9,137). These tests are even more important, if this is possible, in the presence of borderline renal function as measured by other formulas.
If it is not feasible to measure GFR it is recommended that at least Cystatin C be determined, using eGFR in these cases as determined by formulas based on creatinine or Cystatin C. However, these techniques are only necessary in cases where creatinine clearance is close to the limit. Study using 99Tc DTPA is recommended when the difference in size between both kidneys is greater than 10%. This has the additional advantage of estimating the functioning of both kidneys separately 137. If vascular or urological anomalies are detected that do not contradict donation, the best kidney must be kept for the donor.
To support these guidelines the Organ Procurement and Transplantation Network (OPTN) developed an online GFR calculator, to calculate GFR and compare it with the measured GF 143. This tool was validated by a study of candidate donors 144, which suggests that calculated GFR may be sufficiently accurate for decision-making, without the need to measure it directly. Nevertheless, the use of eGFR alone is not recognized by the policy of the OPTN, although a strategy of detection with eGFR followed by confirmation by 24hour creatinine clearance or measured GFR may be sufficient and comply with the established norms.
The donor's GFR measured prior to donation should be sufficient so that, after donation, their foreseeable GFR will remain within acceptable values for their age and sex during the rest of their life 137. British guides offer a table as guidance on this point (Table 7).
Minimum glomerular filtration rate for the acceptance of a living renal donor.
| Age (years) | Minimum glomerular filtration rate (ml/min/1.73 m2) | |
|---|---|---|
| Men | Women | |
| 20-29 | 90 | 90 |
| 30-50 | 80 | 80 |
| 55 | 80 | 75 |
| 60 | 76 | 70 |
| 65 | 71 | 64 |
| 70 | 67 | 59 |
| 75 | 63 | 54 |
| 80 | 58 | 49 |
In general, rates of GF>90ml/min/1.73m2 are considered to be optimum for donation, with the recommendation for individual evaluation when rates are from 60-90ml/min/1.73 m2. If they are below 60, donation is generally considered to be contraindicated. The decision to accept a candidate whose renal functioning is below the required threshold or who has other additional risk factors for progression to chronic renal failure should be individualized and based on the probability that they will develop chronic renal failure during their expected lifespan 137.
The necessary renal functioning for the future recipient should also be taken into account. This has to be compared with the donor's calculated renal functioning for the purpose of deciding whether or not it will be sufficient. This decision is highly important in directed donation as well as when undirected donation is possible.
The donor will have a risk of terminal renal failure after donation which is no higher than that of the general population 33. Nevertheless, there will be a slightly increased risk of this occurring during their lifetime 35,37. A potential donor should be aware of this aspect and they should have consented to it 9.
Finally, it should be added that the donor should be followed up during their whole life, paying especial attention to their renal function, proteinuria and arterial blood pressure 8.
Proteinuria- •
We recommend that the excretion of protein in urine should be quantified for all potential living donors (B).
- •
We recommend that donors with less than 30mg/day excretion of albumin in urine or with an albumin/creatinine ratio lower than 30mg/g be accepted as candidates. Individualise cases from 30mg/day - 300mg/day (30mg/g - 300mg/g) and contraindicate those with higher levels of albuminuria (A).
- •
We suggest that the risk of CRD and cardiovascular risk run parallel to an increase in albuminuria or proteinuria (C).
The excretion of protein in urine should be measured in all potential living donors 137. Although it is preferable to determine donor albuminuria rather than total proteinuria, the majority of hospitals measure both. We recommend testing the albumin/creatinine ratio in a urine sample, and confirming this with total albuminuria in 24hour urine or an albumin/creatinine in 24hour urine coefficient. Excretion of less than 30mg/day albumin in urine or an albumin/creatinine ratio lower than 30mg/g is considered to be acceptable. It is advisable to individualise cases from 30mg/day - 300mg/day (30-300mg/g) and to contraindicate the ones with higher albuminuria 9. An acceptable alternative is the protein/creatinine ratio in a urine sample. In this case it is recommended that it be below 50mg/mmol. The values that would clearly contraindicate donation would be albumin >300mg/day, an albumin/creatinine ratio >300mg/g (>30mg/mmol), a protein/creatinine ratio>500mg/g (50mg/mmol) or protein >500mg/day 9,137,143.
The significance of moderate albuminuria or proteinuria (albumin/creatinine 3-30mg/mmol, protein/creatinine 15-50mg/mmol, albumin 30-300mg/day or protein from 150mg/day - 500mg/day) in living renal donors has yet to be determined. We do know that the risk of CRD and cardiovascular risk run parallel to the increase in albumin or protein, so that these may be relative contraindications against donation 101,137. However, each hospital may have its own different acceptance threshold. In the United States, a survey on the protein threshold set by different hospitals for the acceptance or rejection of a living renal donor found that 37% defined this as protein<150mg/day, and 44% set it lower than 300mg/day or even higher 145.
Microhaematuria- •
We recommend that erythrocytes in urine be determined at least twice in all donors (B).
- •
We recommend that further studies should take place of donors with persistent microhaematuria on two or more occasions (A).
- •
We recommend that a renal biopsy should be taken if microhaematuria probably has a glomerular origin, to rule out the existence of glomerular pathologies (B).
- •
We recommend that a genetic specialist should be consulted in case of the suspicion of hereditary renal disease (B).
- •
We recommend that cystoscopy should be performed patients over the age of 40 years with microhaematuria that has an unknown cause (B).
All donors should be subjected to at least two determinations of erythrocytes in urine during the study 137. If a donor has persistent microhaematuria, (>3 erythrocytes/field or 5 erythrocytes x 106/l) on two or more occasions, this should be studied to determine the cause (dysmorphic erythrocytes, urine culture, urine cytology, cystoscopy, a renal biopsy, study of lithiasis) 137. If the probable cause is glomerular (dysmorphic erythrocytes) a renal biopsy will be considered to rule out the existence of glomerular pathologies (IgA/IgM nephropathy, Alport's syndrome, thin basement membrane), medullary sponge kidney and significant glomerulosclerosis 146. If a hereditary renal disease is suspected, a geneticist could examine the possible alternative of creating a panel of hereditary diseases that could lead us to a diagnosis without the need for a renal biopsy 147. Glomerular pathology would contraindicate donation in general, except for thin basement membrane disease 101,137. In any case, the absolute absence of risk for developing ESRD in donors with basement membrane disease cannot be guaranteed, so that it is suggested that such donors be more than 40 years old at the moment of donation. If no cause is found, in patients who are more than 40 years old it would be recommendable to perform a cystoscopy to rule out bladder pathology. It is possible that renal donors with microhaematuria are at greater risk of gradual renal function deterioration 148.
The decision whether to accept a potential donor will depend on the cause, reversibility and/or risk of progression in their case.
Leukocyturia- •
We suggest that donation should be contraindicated in case of persistent leukocyturia in the absence of urine infection or prostatitis (C).
Donation will be considered to be contraindicated when leukocyturia is persistent in the absence of urine infection or prostatitis. If it is not explained by an infection, a renal biopsy may be required to rule out interstitial nephritis or chronic pyelonephritis (which exclude donation).3
Urinary tuberculosis which contraindicates donation must always be ruled out by at least three mycobacteria cultures.
Renal lithiasis- •
We suggest that specialists should be consulted if lithiasis or associated metabolic alterations are present, informing the donor and recipient of the risks and appropriate follow-up (C).
- •
We suggest that in donors with unilateral lithiasis who are accepted the affected kidney should be nephrectomized, so that the donor keeps the kidney that is free of lithiasis (C).
The existence of renal lithiasis should be ruled out in all potential renal donors. The clinical history (chronology and composition of the calculi) and the CT-angiography performed during the study are important for this 9. If there is a clinical history then the cause of the lithiasis should be determined and a metabolic study of 24hour urine should be undertaken. Acceptance of the donor will depend on the probability of the lithiasis recurring in their remaining kidney, and the donor should perfectly understand the risks which donation involves in this case. In the U.S.A. the majority of hospitals will accept a donor with a history of lithiasis if at the current time they have no lithiasis and their metabolic urine study is normal 145. Independently of whether or not they donate, a potential donor with a history of lithiasis should follow the usual criteria to prevent the relapse of lithiasis.
Renal lithiasis may be considered to be an absolute contraindication in certain situations with a high risk of relapse, or a renal lesion such as nephrocalcinosis, if it is bilateral, recurring or with a probability of doing so with associated disorders (hypercalciuria, hyperphosphatemia, hypocitraturia, hyperuricaemia, hyperuricosuria, cystinuria, hyperoxaluria, distal tubular acidosis, recurring urine infections, sarcoidosis or inflammatory intestinal disease) 138,149. The metabolic abnormalities of a donor should always be discussed with an expert in lithiasis, especially if no clear metabolic cause for lithiasis has been found 137. It is generally accepted that, in the absence of a major metabolic anomaly, potential donors with a limited history of renal lithiasis or slight current lithiasis may be considered for donation. It is necessary to fully communicate the risks to the donor and recipient, together with an appropriate follow-up of the donor over time 137.
If there is unilateral lithiasis and donation is accepted, the kidney affected by the lithiasis should be subjected to nephrectomy (while attempting to extract the calculus during the operation), so that the donor is left with a lithiasis-free kidney 137.
Detection of tumours in the donor- •
A carefully prepared clinical history is recommended, together with a detailed physical examination, to find any undetected neoplasia prior to renal donation, especially when the donor is more than 50 years old (B).
- •
We recommend that potential donors with a minimum risk of low stage tumour transmission (<1%) be accepted after careful assessment and discussion (B).
- •
We suggest that kidneys from donors with a T1a renal tumour may be accepted after performing tumorectomy during the harvesting (D).
- •
We suggest that donation should be rejected in the case ofbilateral angiomyolipoma. In cases that are unilateral andlarger than 4cm the affected kidney can be collected fortumour exeresis ex vivo. If they are are unilateral and less than 4cm in size they do not contraindicate donation, and if they are less than 1cm in size, the kidney with angiomyolipoma may be left in the donor or given to the recipient (C).
The possibility that a potential donor may be a carrier of tumours that could be transmitted to the recipient must be ruled out. The study should take place according to the guides used in each country. A painstaking clinical history and detailed physical examination are essential, especially for donors above the age of 50 years 137. They should be complemented by general analysis, thoracic X-ray, CT-angiography, digital rectal examination and PSA in men aged over 50 years, blood in stool, colonoscopy or cystoscopy if applicable and a gynaecological study (examination, cytology, gynaecological ultrasound imaging, mammography). Specific studies may also be used depending on the findings of the preliminary study or personal or family background; for example: a dermatological examination if there is a family history of melanoma or a high number of nevi 138.
In general, if an active malign process is detected during the study of a donor, or if they have a history of this, it usually rule out donation 150. Donation is rejected if there is a previous diagnosis of haematological, gastrointestinal, testicular, melanoma, lung, breast or urinary cancer with certain exceptions, coriocarcinoma or monoclonal gammopathy 9. Only potential donors with a minimum risk of transmission (<1%) may be considered if they have low stage tumours and after careful evaluation and discussion 9,137,138), including basocellular skin tumours, in situ colon or cervix carcinoma, kidneys which carry a Bosniak II-III cyst, or even a T1a renal tumour after tumorectomy 151,152.
Radiological study by CT with contrast, ultrasound scan or MR imaging usually make it possible to distinguish between benign lesions such as angiomyolipoma and malign ones such as clear cell renal carcinoma. The help of an experienced radiologist is required for this 137.
Bilateral angiomyolipomas contraindicate donation. In unilateral cases that are larger than 4cm some groups consider what is known as therapeutic donation, performing exeresis of the angiomyolipoma during the nephrectomy ex vivo. Unilateral cases that are smaller than 4cm do not usually contraindicate donation. If cases are unilateral and smaller than 1cm, it is considered that the kidney with the angiomyolipoma may either remain in the donor or be given to the recipient 137.
Hereditary renal diseases or those with a familial component- •
We recommend that a detailed clinical history should be prepared for all potential KT recipients, with especially detailed content on any family history of renal disease. It is indispensable to exclude hereditary renal disease from potential donors (A).
All potential KT recipients should have a detailed history of renal disease in their family and, when possible, the diagnoses of the same. This will help to detect familial risks in potential living donors. At the same time, all donors should be questioned about their personal or family history of hereditary diseases. If the disease of the recipient is due to a hereditary renal disease, it is indispensable to rule this out in the donor, using radiological examinations, renal biopsy or genetic studies if they are necessary 9,137,138. Autosomal dominant polycystic renal disease stands out among the most important diseases. Classically certain criteria were used to diagnose this and differentiate it from simple isolated cysts 153. In case of doubt a genetic diagnosis will help us to be sure that the donor will not suffer the disease in the future 154. Other hereditary diseases or ones with a familial component that should be ruled out in donors are Alport's syndrome, thin basement membrane disease, glomerular pathologies with increased familial involvement (IgA, membranous, focal and segmentary, membranoproliferative), LES and atypical haemolytic - uremic syndrome. Potential donors who are carriers of the same disease as the recipient must not be accepted as donors.
The risk of transmitting infectious diseases 155- •
Tests to detect transmissible infections should be applied to all potential living donors (B).
- •
We recommend that if a potentially transmissible infectious disease is found, the medical team and recipient should accept or reject the suitability of the donation after receiving detailed information about the risks that would be involved (B).
- •
Positivity for HIV or HTLV (humanT lymphotropic virus) is considered to be an absolute contraindication against living donation (B).
- •
We recommend that the donor and recipient be informed of the risk of post-transplant primo infection in the case of a CMV+ donor and CMV- recipient, and the risk of lymphoproliferative disease in the case of an EBV+ donor and EBV- recipient (B).
The tests to detect transmissible infections which should be applied to all living donors were described above 156,157. We recommend that these tests should be applied shortly before the transplant for the highest risk diseases, so that window periods can be ruled out. Especially for HIV, HBV and HCV it is preferable that the relevant tests be applied in the month before donation 9. Some active or latent infections will not be a contraindication if it is possible to treat the donor for them effectively. It is important to undertake a correct anamnesis of the risk factors for latent diseases in the donor, with special emphasis on factors involving their social behaviour, addictions, professional risks, geographical risks or exposure to animals. The criteria for accepting transmissible diseases in the donor vary from country to country 26,27 and they have changed rapidly over recent years. When the donor has a potentially transmissible disease, the fully informed medical team and recipient will reach a decision on whether it is possible to accept the risks of transmission 9,137.
Hepatitis B virus (HBV)Positivity for HbsAg or the presence of viral DNA in the blood contraindicates donation to HBsAg negative patients. Positivity for HBcAc-IgM, which indicates recent infection, and isolated positivity for HbcAc make it necessary to rule out active replication by DNA or viral mutations. Positivity for HBcAc-IgG with/without positive HbsAc and negative viral DNA implies a very slight (although not zero) risk of transmission. Although this makes donation acceptable, the recipient should be naturally immunied or immunized by effective vaccination 9,138 (NG).
Hepatitis C virus (HCV)The presence of HCV antibodies has classically been a contraindication against renal donation, due to the risk of transmission to the recipient as well as the risk for the donor of glomerulopathy. From 2013 the recommendation has been to determine RNA-HCV in the study of renal donors 158. The appearance of new direct antiviral treatments against HCV and their high degree of efficacy have changed the scenario. For example, kidneys from positive deceased donors are now being transplanted into negative recipients who will receive intensive treatment for their viral load, or direct antiretroviral treatment, if they give their consent 159,160. This situation may extend to LDKT if the donor receives treatment beforehand (NG).
Human immunodeficiency virus (HIV)The detection of HIV or HTLV (humanT lymphotropic virus) is considered to be an absolute contraindication against living donation 137. Nevertheless, in South Africa there is experience of transplantation from HIV+ donors to HIV+ recipients after informed consent, with good results 161.
Cytomegalovirus (CMV)The donor and recipient should both be tested serologically before transplantation to discover their degree of immunity against CMV. In living donor programmes serological states against CMV do not contraindicate donation, and nor are they a normal donor selection criterion. However, this status should be determined to discover whether it is necessary to apply prophylaxis if the donor is positive and the recipient is negative, or in other situations with excess immunosuppression 162. Some guides consider the need to inform the donor and recipient of the post-transplantation risk in the case of a positive donor and negative recipient 137.
Epstein-Barr virus (EBV)The serological status of the donor and recipient against EBV should be determined prior to transplantation. The presence of antibodies against EBV means that there was a previous infection which is possibly in a latent phase and may be transmitted to the recipient. Although this does not contraindicate donation, when the recipient is negative they will be at greater risk of developing a disease caused by EBV, which may run from a trivial viremia, the symptoms of infectious mononucleosis or a post-transplantation lymphoproliferative disease 163. The latter situation is seen more often in the case of recipients of paediatric age. The British guides recommend informing about this risk when the donor is EBV+ and the recipient is EBV- 137.
Other herpes virusesThe need to screen for herpesvirus 6 and 7 (HHV 6 and 7) (which is almost universal) and herpesvirus 8 (HHV 8) (with a highly variable prevalence depending on geographical area) has not been clearly established. HHV8 may be transmitted by transplantation, and it has been associated with increased risk of Kaposi's sarcoma 164,165.
West Nile VirusThis virus has spread to certain regions where it was not autochthonous. It should be screened for in donors from regions at risk by PCR, as serological study is complex 166.
TuberculosisIt is calculated that 30% of world population have been infected by Mycobacterium Tuberculosis. It is very important to show the country of origin in the clinical history of the donor, as well as any journeys they have made to countries where the risk is present 137. The majority of transplanting hospitals include at least the PPD test in the study of the transplant donor and recipient. Others only include it if there is a certain degree of prevalence of tuberculosis in the local population. If the PPD test is positive, active TBC should be ruled out (as this would be a contraindication) by clinical examination and imaging tests (thoracic X-ray or CT, IV urography) and microbiological tests (mycobacteria culture in sputum and urine, or an interferon-gamma release assay, IGRA) 138,166. Latent TBC is not a contraindication, and the recommendation is to treat it before donation (9 months isoniazid or 3 months rifampicin). Post-transplantation prophylaxis should be considered in the recipient (NG).
SyphilisAll donors are initially given a RPR test. Positivity for RPR should be completed with Treponema tests. Although the presence of latent syphilis does not contraindicate donation, the donor should receive appropriate treatment (3 weekly IM doses of 2.4 million benzacine-benzylpenicillin). If there is considered to be risk of transmission to the recipient, it is preferable to administer prophylactic treatment (2.5 MU of a single IM dose of benzacine-benzylpenicillin or 100mg/day oral doxycycline during 14 days or 1g azithromycin in a single oral dose) 167.
Emerging infectious diseases. ParasitesDonors from zones that are endemic for other emerging diseases or which have endemic parasites and donors who have lived in them should be investigated for them. We recommend that the infectious diseases department provide support during this consultation. The main such diseases are malaria, Chagas, toxoplasmosis, Schistosomiasis and Strongyloidosis. After study of the donor, donation may be considered following effective treatment of the disease 137,138,168–170. When in the previous 28 days the donor has visited a zone where the Ebola virus or Zika virus is endemic, donation should be deferred for a prudent time to ensure that the donor has not been infected 9 (NG).
Urinary tract infectionsAll potential donors should be tested with a urine culture. It is relatively common to find asymptomatic bacteriuria in women. If the donor is a man with bacteriuria or if there is a family history of urinary tract infections, then it is recommendable to perform a radiological study (CT, urography, voiding cystourethrography) to rule out renal cortical lesions or other urinary tract anomalies, while such studies sometimes include a cystoscopy or urodynamic study 137,138. Lower urinary tract infections in women, even recurring ones, do not contraindicate donation, although repeated pyelonephritis does. Donor urine must be sterile at the moment of donation.
Prion diseasesDonation is contraindicated when there is a personal or family history of Creutzfeldt-Jakob disease. There is currently no effective diagnostic test. Transmission due to a transplanted organ is exceptional. Genetic advice may be required regarding the familial component. The risk factors are blood transfusions after 1980, dura mater grafts or treatments with pituitary hormone or gonadotrophin 137.
SARS-CoV-2The 2020 pandemic makes screening for this indispensable for the donor and recipient. The guidelines for this are constantly evolving, so that it is advisable to consult the recommendations of the ONT at any specific moment. The transmission of this disease from a deceased donor is exceptional.
Haematological diseases of the donor- •
An exhaustive study of the donor is recommended if they have anaemia (A).
Many hospitals already systematically perform hypercoagulability studies of all donors. In spite of this, such studies are considered to be obligatory only if there is a history of thrombosis. A previous incident of pulmonary thromboembolism or deep vein thrombosis may constitute a contraindication against donation (NG). In women who are carriers of V-Leiden factor the preoperative suspension of contraceptives or hormone replacement therapy would be indicated.
In the case of anaemia an exhaustive study prior to donation is indispensable 137. This is also the case when a haemoglobinopathy is detected.
6Psychological evaluation of living renal donorsLiving renal donation is a complex process which includes ethical, psychological, social and physical aspects. It takes place with the expectation that the risk for the donor will be compensated by the psychosocial benefits (quality of life, social and psychological well-being, body image and self-perception) and the improvement in health for the recipient 171.
Additionally, the physical and mental health of the donor must be accredited and certified by a qualified doctor. This is a legal requirement in Spain for donation and transplantation between people with an emotional relationship and also between people who do not know each other, on condition that donation is altruistic and selfless 172. The ONT is decidedly in favour of encouraging living donation, for which the protocols governing the study of living donors have to be updated, including those on psychological evaluation 58,172.
A unified protocol is suggested for the psychological evaluation of a living renal donor. This protocol combines a personal interview and a psychometric evaluation, thereby facilitating their informed consent and ensuring that donors’ psychological health is appropriate 173,174.
For altruistic renal donation the Spanish National protocol indicates that the assessment which takes place will make it possible to: “a) express the whole complexity and depth involved in the experience of altruistic donation, b) evaluate their mental health, quality of life and the psychosocial situation in which they live, and c) understand the underlying motivation for the donation” 58,172. In the case of the altruistic or Good Samaritan donor, the fact that motivation plays an important role here should be taken into account, so that this will require a more exhaustive evaluation.
Professionals with knowledge and experience of transplantation should take part in the assessment process and the evaluation and follow-up of donors and recipients. It is therefore appropriate to include a psychologist in multidisciplinary transplantation teams, who will focus on care for transplanted patients, assessing and following up donors, as well as providing emotional support for team members.
The psychological and social assessment of living donors should take into account all of the variables which may influence their perception of the donation process.
The members of the psychological assessment team for living renal donors are listed in Table 8, as follows:
Items in the psychological assessment interview for a living renal donor.
| Exploring the donor |
| Information they have about donation and transplantation |
| Relationship with the recipient |
| Comprehension of possible risks and complications associated with the procedure |
| Verify that they are competent and free to take the decision voluntarily |
| Explore possible risk behaviours (tattoos, sexual promiscuity, addictions...) |
| Motivation that leads them to take the decision |
| Expectation for after the intervention. |
| Sociodemographic interview |
| Social and family support |
| Status regarding partner and children. |
| Educational level |
| Work and economic situation |
| Clinical history (therapeutic interventions, diseases and psychological disorders) |
| Religious beliefs |
| Healthy habits |
| Consumption of tobacco, alcohol, drugs or other substances. |
| Cognitive assessment |
| Verification of cognitive capacities to confirm maturity and voluntary capacity |
| Emotional assessment |
| Evaluate possible emotional involvement that may influence the process:- Emotional disorders (anxiety, depression, etc.)- Impulsiveness- Habitual stress triggers- Explore possible suicidal ideation (previous or current)- Existence or otherwise of sexual abuse (previous or maintained). |
| Evaluation of coping strategies |
| Verify donor coping strategies, whether or not they are adaptive and may help them after the intervention in the case of possible complications. |
- •
We suggest that psychological assessment should be based on a clinical interview and psychometric evaluation (Quality of evidence: C).
- •
We suggest that psychological assessment should be undertaken by psychologists or psychiatrists with clinical experience and who do not take part in care of the recipient (NG).
- •
We suggest that the contents of the psychological assessment protocol should be uniform, regardless of donor type (NG).
- •
We suggest that psychologists should be included in the multidisciplinary transplantation teams (NG).
Psychological assessment should include mental health evaluation of the donor, as well as their comprehension of the risks and circumstances of donation. The relationship between the potential donor and patient should be analysed, together with the motivation to donate and coping strategies for surgery and possible complications, among other factors 174–178. A wide range of methods and criteria exist for psychological assessment; in some cases formal criteria are used, while others use semi-structured clinical interviews 173,179,180. We therefore suggest that an interview and psychometric evaluation should be used, so that sufficient data are available for the generation of a final report. Psychosocial assessment should be performed by psychologists or psychiatrists with clinical experience and suitable qualifications, as well as mental health expertise. We also recommend that they should not take part in care of the recipient, to reduce conflicts of interest in their assessment of the donor candidate. Psychosocial assessment should take place in the absence of the recipient, family members or any other individuals who may influence the donor's decision, regardless of their type of relationship with the recipient.
Living donor selection and acceptance criteria- •
We recommend not to proceed with the living donation process in case of severe clinical or personality disorders, as well as acute phase disorders and situations of stress, vulnerability, personal defencelessness or serious addictive disorders (B).
- •
We recommend contraindicating donation in altruistic or Good Samaritan candidate donors if they have a notable or exaggerated predisposition to arouse the admiration of others or be the centre of attention (B).
In general, the main psychosocial contraindications for living donation would include severe psychiatric problems or substance abuse, the presence of major financial stresses that may have a coercive effect on the decision to donate, or which may worsen significantly as a result of donation, and any medical complication, evidence that the potential donor has experienced pressure or the undue pressure of other individuals to donate, restricted understanding or capacity to understand the risks and benefits for the renal donor or transplant candidate, or ambivalence about whether to proceed with the donation 172,181.
The following contraindications should also be taken into account for good Samaritan donors candidates: a) An unrealistic opinion that transplantation involves no risk of rejection or failures; b) Donor confusion about who is responsible in case of transplantation failure; c) Seeking monetary reward; d) Desire for media attention; e) Use of donation as a palliative for a psychological problem or another underlying disease; f) Donor willingness to decide on the type of recipient (regarding gender, age, race or ethnic group, and g) Desire to meet the recipient and become involved in their life 182.
The psychological interview- •
We suggest that the psychological interview should elicit motivation and post-donation expectations, sociodemographic aspects and background. It should include cognitive evaluation, emotional evaluation and the evaluation of coping strategies, resilience and available emotional support (NG).
- •
We recommend that altruistic or Good Samaritan donors should be subjected to a more exhaustive evaluation, with a careful assessment of their motivation as the most important criterion (B).
In the psychological interview with a potential donor all of the areas that could compromise the intervention in some way should be taken into account. These include not only the medical area, but also social, economic and professional, emotional, cognitive and familial factors. It is essential to ensure that the donor is aware of possible negative effects that may arise due to the donation 174,175. At the start of the interview it is advisable to check the knowledge of the donor about donation, in order to give comprehensive additional information about the procedure, possible risks, benefits, results and post-donation follow-up, etc. During the assessment some additional aspects should be assessed, such as that the donation is selfless and seeks no economic interest, publicity or any type of personal promotion in all cases, and most especially for Good Samaritan donors. This latter type of donation is anonymous in Spain by national law 172. The interview should cover a list of subjects including mental health history, drug abuse, that the donor is able to understand the risks, social support, motivation and voluntary nature and the donor recipient relationship, etc. 183. During the initial phase of the interview factors in connection with the information held by the donor about the procedure, risks and possible complications will be explored. In connection with post-intervention expectations, it is necessary to verify whether the donor has taken into account that, although rare, they may suffer renal complications that may threaten their health to the extent that they need renal replacement therapy. All of the above points will give us information about the motivation which leads to donation, and this is especially important for Good Samaritan donations.
The interview will focus on the sociodemographic data that are necessary for all psychological assessments and especially those which are relevant for organ donation, such as healthy habits and possible substance consumption.
The cognitive assessment will evaluate the intellectual capacity and maturity of the donor to ensure that they understand the significance and possible consequences of their actions. The aim here is to maximise precautions to ensure that the process of giving consent is free, without any family, professional or economic pressures 184.
The emotional assessment will explore the possible existence of emotional disorders which affect their free decision to donate, such as anxiety, depressive or impulse control disorders, etc. This assessment will be confirmed in the psychometric evaluation. Whether the decision is a mature one and the result of proper reflection will also be taken into account, or whether on the contrary it was due to a thoughtless act that may have been the consequence of a momentary impulsive action. Lastly the normal coping strategies used by the donor will be taken into account, as these may be predictors of good post-intervention well-being. The capacity of the donor to practically resolve problems will therefore be explored, together with their capacity to control their emotions and manage stressful situations, taking into account the fact that donating an organ is intrinsically stressful. Adaptive coping strategies such as active confrontation, planning, impulse control, positive reinterpretation and the search for social support may all be considered. On the contrary, negation, avoiding concentrating on their own negative emotions and addictive forms of behaviour would all be considered strategies that fail to adapt, and they would therefore be predictors of poorer post-intervention welfare 185.
Psychometric assessment- •
We suggest that psychometric assessment based on the use of an objective test should be used, making it possible to evaluate clinical personality traits (C).
- •
We suggest that the Millon MCMI Questionnaire (which is now in its IV edition) should be used for psychometric assessment (NG).
- •
We suggest that the evaluator should be familiar with this test and have sufficient experience in applying it (NG).
Aside from the results of the interview, it is always advisable not to reach a diagnosis or take important decisions for patients without the support of objective data which complement clinical judgement or diagnostic impression 186.
We propose using the Millon MCMI Questionnaire for this purpose, because it is able to identify the presence of severe clinical syndromes such as clinical personality traits and severe personality pathologies. It supplies information and a nomenclature which better fit ICD and DSM diagnostic criteria, as medical personnel in general are more used to these ones 187,188. This questionnaire offers a dimensional vision of the normal-abnormality continuum in psychopathology, making it especially interesting for the evaluation of a collective (consisting of potential donors) who in principle are not considered to have any psychological pathologies at all 189–190, and it has been used to evaluate living renal donors 191,192.
Donor follow-up- •
We suggest that living donors should be followed up psychologically using interviews, especially those in whom pathological personality traits had been detected (NG).
- •
We suggest that recipients should be followed up psychologically to improve the survival of the organ and patient (NG).
In the same way that follow-up of living donors is recommended over the short, medium and long-term to prevent or treat risk factors that could compromise their health in general and their renal function in particular 193, follow-up of the recipient should also be standardized to cover possible psychological complications that may arise after transplantation. Respecting this, high levels of anxiety and depression have been detected in the immediate post-transplantation period 194.
According to the Altruistic Renal Donation Protocol of the ONT, it would be advisable for candidates who had been accepted for donation to receive appropriate psychological assistance from the moment they are included in the scheme. This would aid the emotional expression and management of any possible anxiety and suffering caused during the donation process, with the aim of preventing future emotional complications and the development of psychological disorders triggered after donation 172. Identifying which donors could potentially have the greatest post-transplantation psychological problems could help to limit the follow-up of such cases 192, reducing the probability of subsequent complications emerging 195.
7Living renal donors with borderline alterations: acceptance and rejection criteria.The experience accumulated in thousands of donors over recent decades makes it possible to evaluate the results and procedures of donation to date. Solid recommendations have therefore be established for the acceptance of living renal donors who will experience a favourable post-donation result. However, when we examine borderline cases of donation there are far fewer examples of experience, and the results are not homogeneous. This means that the said limits are inherently a field of uncertainty where research is needed, and here the onset of new data may move the limits in one direction or another.
In donation, as is the case in almost all biological phenomena, the risk associated with a certain variable does not follow a discontinuous pattern but is rather a continuum. This makes it necessary to arbitrarily set the limit at a certain point that will be more of a transition zone than a clear borderline.
Limits to donation based on donor GFR- •
All donors should at least fulfil the GFR thresholds set for their age and sex (Table 7). The thresholds are valid under appropriate conditions of post-donation compensation and preservation of renal function (Quality of evidence: B).
- •
We recommend that the GFR should be ≥ 90ml/min/1.73 m2 in donors<30 years old; and that the GFR is ≥ 80ml/min/1.73 m2 in donors aged from 30 to 45 years. The absence of additional risk factors should be confirmed in both cases (C).
- •
The use of the Grams et al. risk calculator [21] is not recommended for young candidates as it may seriously under-estimate the risk of end-stage CRD (ESRD) (B).
- •
In donors>45 years old with a GFR higher than the minimum rates which are required, but with other risk factors, it is recommended to estimate the risk of ESRD using the Grams et al. risk calculator tool [21] (C).
- •
We suggest that donors with a GFR<70ml/min/1.73 m2 should not be accepted, except for elderly donors and after a careful assessment (C).
The GFR is now accepted to be the best renal function indicator, in healthy individuals as well as in patients with CRD. In spite of this, there is no clear agreement on what the GFR should be in any specific individual or how the GFR should be adjusted to the changes that occur with age. Much of the literature to date accepts that the average GFR for a young Caucasian male is 125ml/min/1.73 m2196,197. Nevertheless, more recent studies based largely on populations of healthy donors show lower GFR rates for the same average individual, at around 107ml/min/1.73 m2198–200 (Figure 5). In the same way, we have evidence that the GFR falls physiologically after a certain age, although the speed of this fall and its cause are also the subjects of intense debate 201–203. The study of potential healthy donors has shown that there is a constant drop in the GFR after the age of 40-45 years, amounting to approximately 0.9ml/min/1.73 m2 per year 198,199. These data, obtained from studies of the general population as well as candidate donors, have even been used to create a new proposal for the estimation of the GFR and its normal intervals as an alternative to the staging currently used for CRD in the KDIGO guides, so that the classification of CRD and its staging based on GFR thresholds uses limits which are adjusted to the age of the specific individual 204.
Pre-donation glomerular filtration rates (mL/min/1.73m2) measured (United Kingdom, UK) 215 or estimated rates (Spain, United States, US) 200,237 per age group. The population figures in the United Kingdom correspond to the average of those published for men and women. The figures shown in the population of donors in the United States (US) correspond to representative values for the said population per age group. The figures for the Spanish population correspond to the average in donors of the Spanish Registry of Living Donor Kidney Transplantation 2010-2017.
These circumstances are important when evaluating a candidate donor and, in fact and in consonance with them, there are now two different approaches in evaluating acceptable or borderline GFR for donation. The first approach is suggested by the KDIGO, and it is the result of classifying CRD in GFR intervals independently of the individual's age. It states that a donor is suitable if their GFR ≥ 90ml/min/1.73 m2 (9). The second approach is proposed by the Guides of the British Transplantation Society, BTS, and it accepts the concept that a physiological fall occurs in the GFR with ageing. It proposes GFR thresholds that are acceptable for donation in the normalcy ranges defined for each interval of donor age (Figure 6) 2. The use of one approach or the other has important consequences in the classification of candidates as suitable or not for donation 205.
Graph and table showing the minimum estimated glomerular filtration rates (eGFR) for donation to be accepted, according to candidate age for men (-•-), women (- -), and both sexes (-•-) proposed by the latest British Transplantation Society Guides 2, comparing them with the limiting threshold per age group proposed for the definition of chronic renal disease by Delayane et al. 203.
The aim in both cases is that the GFR after donation will not compromise the prognosis for the donor during their whole life expectancy, with zero or minimum impact on the long-term health of the donor. It is therefore necessary to know the expected donor GFR following donation, and the threshold GFR below which an excess of morbimortality is detected.
Fall in the GFR after renal donationDonors lose approximately 50% of their renal mass in donation, although this loss under optimum conditions is followed by a period of compensation which leads to a GFR 12 months after donation of approximately 70% of the baseline GFR (60-75%) 206,207. A recent analysis of more than 21,000 donors in the American registry found that eGFR at two years after donation was 68.7% of the eGFR before donation, equivalent to an absolute fall of 31.2ml/min/1.73 m2208.
Consequentially, depending on the GFR accepted pre-donation, a large percentage of donors with a normal GFR pre-donation may fall to a GFR <60ml/min/1.73 m2 post-donation. Using the current classification of the KDIGO they would therefore be considered to be within the CRD category. In spite of the fact that serious differences exist regarding the importance of a low GFR in donors, several studies have found when following up donors that there is an increase in the prevalence of several complications associated with CRD, including hypertension, preeclampsia, hyperuricaemia, gout and hyperparathyroidism (122,123,209,210). Even more importantly, several recent studies showed small increases in the long-term risk of end-stage CRD (ESRD) in donors 35,37.
Regarding the expected fall in the GFR that occurs with ageing, long series of donors confirm that, under appropriate selection conditions, the loss of GFR with age is even less in donors than it is in the healthy general population.
A long period of stabilization or even positive compensation of the GFR after donation is described in the follow-up of donors, and this period may last for years 211. This compensation process seems to be limited by several risk factors such as age, male sex or obesity 212. Several studies show lower GFR compensation in elderly donors during the earliest phases, as well as over the longer term 213,214. This may be linked to a different capacity and speed in the compensation mechanisms that are associated with ageing. Similarly, although the mechanisms involved are less obvious, slower compensation and less capacity to compensate the GFR have been described in donors who are related to the recipient 211,213.
Determining pre-donation functional renal reserve has not been shown to date to offer an added value in predicting the post-donation GFR, so that this determination is restricted to the field of research 215.
The GFR attained in the recipient.The GFR prior to donation is closely linked to the GFR obtained in the recipient, and it is essential for appropriate survival of the graft. The GFR is in fact the most important characteristic in predicting renal function in the recipients of living donor KT 216. Moreover, the development of ESRD in the donor is a risk factor that is independent of the pre-donation GFR for recipient death-censored loss of the graft 217. A study of 275 kidney recipients whose donors developed ESRD showed that their censored graft loss and mortality rate increased in comparison with a control group of LDKT recipients, adjusted for all of the relevant confusion factors in the donor, recipient and transplant, and independently of the donor-recipient relationship 218. This work shows that there may be subclinical renal disease prior to donation, or that there are factors which predispose to CRD that are not detected in the routine examination of candidate donors. Detailed analyses of pre-implantation biopsies of living donor grafts may elucidate this question. A review of almost 3000 donor - recipient pairs has shown that the presence of subclinical lesions in the pre-implantation biopsy is associated, independently of the clinical variables corresponding to the donor and recipient, with patient death-censored graft survival 219. Other components of renal function in the donor, as well as GFR, have been correlated with recipient GFR 220.
The final GFR in the recipient will be the result of variables associated with the donor as well as with the donor - recipient pairing. Several attempts have been made to combine all of these variables to develop predictive models of renal functioning or living donor graft survival 221,222. Although they have limitations, these models offer an individualized score for each donor, and this is important for decision-making and even for selection among different donors.
Individualization of donor CRD riskConcerns about the long-term consequences of donation have underlined the concept of focussing donor assessment on their future risk of developing ESRD. KDIGO recommendations include individual estimation of the future risk of ESRD after donation, as the central axis of a new donor assessment framework 9. This approach differs from the previous one, in which donation was either accepted or rejected depending on whether the donor fulfilled a series of health and renal function criteria, rather than an attempt to quantify their overall risk.
The risk of ESRD should first be calculated without taking donation into account, adjusting for the demographic and clinical characteristics of the candidate, including their GFR. A calculator has been developed which makes it possible to estimate the risk of ESRD and dialysis at 15 years and throughout the life expectancy of an individual (http://www.transplantmodels.com/esrdrisk/). This calculator is based on a meta-analysis of several cohorts of individuals in the general population of the U.S.A. Almost 5 million individuals were then selected with a low risk profile for CRD according to clinical and analytical variables. Multiple variables were considered, and finally 10 were selected which had been found to be predictive of the said risk, and these were included in the calculator 125 (Table 9).
Variables associated with the future risk of ESRD and corresponding Hazard Ratios (ref. 237).
| Characteristic | Hazard ratio (95% CI) |
|---|---|
| Race (black vs Caucasian) | NA |
| Sex | NA |
| Age | NA |
| eGFR CKD-EPI< 60mL/min/1.73m260-89mL/min/1.73m290-119mL/min/1.73m2> 120mL/min/1.73m2 | 6.61 (4.87–8.96)1.63 (1.53–1.74)1.02 (0.85–1.23)0.79 (0.56–1.10) |
| Systolic arterial pressurePer increases of 20mmHg above 120mmHg | 1.42 (1.27–1.58) |
| Antihypertensive therapy (Yes vs No) | 1.35 (1.01 - 1.82) |
| Non-insulin dependent DM (Yes vs No) | 3.01 (1.91–4.74) |
| BMIPer increase of 5 points above>30 Kg/m2 | 1.16 (1.04–1.29) |
| SmokingEx-smokerActive smoker | 1.45 (1.23 - 1.71)1.76 (1.29 - 2.41) |
| Albumin coefficient/Cr in urine (mg/g)Per 10 times increase over levels of 4mg/g | 2.94 (0.99–8.75) |
Cr, creatinine; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; CI, confidence interval; BMI, body mass index; NA, not available
The second step consists of calculating the risk of ESRD that can be attributed to donation, and this risk will be added to the basal risk of the donor. This risk is not clearly defined, and estimations of it cover a very wide range (35,37,125). The KDIGO suggest calculating the final risk of ESRD after donation by multiplying the basal risk by a factor of from 3.5 to 5.3, depending on the sex and race of the donor 125. This multiplier factor is the coefficient between the expected risk of ESRD after 15 years in the general population analysed by Grams et al. stratified according to race and sex, and the observed risk after 15 years in the series of more than 96,000 donors in the American registry 35,125.
This process has several limitations. Firstly, some variables are excluded from the calculation of an individual's future risk of ESRD. The absence of the risk factor which arises from the relationship between the donor and recipient is especially important, as the genetic component may have a highly important specific weight in the overall risk of ESRD for the candidate 2,37,223,224. Secondly, it should be taken into account that the attribution of basal risk to each donor profile will be strongly distorted by the follow-up time of the general population included in the Grams et al. meta-analysis (average follow-up time 4-16 years), as this was used to build the risk calculation tool 125. Uncertainty in calculating the basal long-term risk of ESRD therefore increases considerably in young donors, or in other words, the younger a donor is (with a longer life expectancy), the lower the predictive value of a normal result at the moment of donation will be 225.
There are also major uncertainties regarding calculation of the risk fraction that can be attributed to donation. Different studies give slightly different figures, from an excess of 27 to 41 cases of ESRD at 15 years for every 10,000 donors 35,37. Moreover, there are insufficient data to know with any exactitude how the risk fraction will change depending on the demographic and clinical characteristics of the candidate. Lastly, we have to consider that the future risk of ESRD does not seem to be lineal over time, as it is likely to rise exponentially more than 10 years after donation 223.
As a result of these limitations, several publications have raised doubts about the process proposed by the KDIGO for the calculation of the individual risk of a specific donor 47,226–228. Due to all of these considerations, it is obvious that the risk calculation tool proposed should only be used on condition that its inherent limitations are clearly understood, together with the fact that it may give rise to serious under-estimations of long-term risk. This method of donor assessment should be used as just one of different tools, in a process that requires a global and multidisciplinary clinical vision, one which has to take into account multiple factors as well as the quantitative estimations of risk.
Massie et al. recently published a donor ESRD risk estimation calculation tool 229. This tool, which is also available in internet (http://www.transplantmodels.com/donesrd/), includes fewer variables than the one published by Grams et al. For example, it does not include certain highly important risk factors, such as albuminuria or high blood pressure, while it does nevertheless include a family history of CRD, which is lacking from Grams’ calculation tool. In any case, both risk calculation tools would need external validation, and this has yet to be done.
Analysis of the recommendations in the main Clinical GuidesThe KDIGO guides recommend the standard GFR for a candidate donor of ≥ 90ml/min/1.73 m2, while a GFR <60ml/min/1.73 m2 should exclude donation 9. These limits differ from the ones that were accepted previously, which set the optimum limit at a GFR ≥80ml/min/1.73 m2, and they were not set using evidence-based medicine, as they were based on studies in non-donors. Long-term studies of large series of donors, with an average age of 40-50 years and a measured GFR ≥ 80ml/min/1.73 m2, showed that there is very little increased risk of ESRD in these populations 35,37.
Although the selection of this new standard may be correct when assessing the youngest donors, its significance in older donors is unclear. This lack of clarity is due to the relationship between ageing and a falling normal GFR 201,202,204.
For a GFR of from 60 to 90ml/min/1.73 m2 the KDIGO recommend evaluating the long-term risk of ESRD based on the health and demographic characteristics of each candidate. They offer an online risk calculation tool for this purpose (http://www.transplantmodels.com/esrdrisk/). Use of this tool shows that a broad range of GFR figures may be considered valid for donation, depending on donor age and other characteristics. However, given the importance of basal GFR in determining the results and future of the donor, these Clinical Guides offer no specific advice on the borderline GFR that is acceptable in each case.
The BTS guides, which were published soon after the KDIGO, accept two principles questioned by the KDIGO as valid: 1) Using GFR data measured in potential British donors 230,231, they set a standard GFR for young adult men that is lower than the historical level of 120ml/min/1.73 m2; and 2) they take into account the ageing-related physiological decrease in GFR, leading to different ranges of normality for each donor age cohort 2. They thereby set minimum GFR thresholds for donation, so that the renal function of the donor remains within normal limits during their lifetime.
Interpreting the BTS guides requires some clarifications:
- 1)
The thresholds for each age group, differentiated according to sex, predict that the GFR will remain within normal limits while the donor ages, on condition that it runs in an “ideal” GFR decline curve. Existing data on the evolution of the GFR after donation show that the fall in the GFR is no greater than the physiological fall in the healthy population, and it may even be less. However, we have to remember that these data correspond essentially to Caucasian series of donors with an average age of around 45 years, who are not hypertensive or diabetic and with normal albuminuria 33,211,232,233.
The minimum threshold is set at 80ml/min/1.73 m2 for female donors aged from 30 to 50 years and men aged 30 to 55. The limit is set at 90ml/min/1.73 m2 for donors under the age of 30 years, and this is the same minimum figure recommended by the KDIGO. If these thresholds are accepted then it has to be taken into account that the post-donation GFR in donors aged from 30 to 45 years may even fall below the normal range. The BTS guides contain no clear justification for this decision, which implicitly assumes that the accumulated experience over recent decades, accepting donors with a GFR >80ml/min/1.73 m2, has been followed by very slight increases in the absolute risk of ESRD over the long-term 35,37.
- 2)
The BTS have selected the figure of 75% of basal renal function as the degree to which renal function is compensated by the remaining kidney, which is in the upper range of observations 206,207,231. Due to this, post-donation estimations of GFR and its long-term evolution may not extrapolate to very elderly donors or those with comorbidities.
- 3)
Like the KDIGO, the BTS recognise the possibility of donating with basal GFR figures at from 60ml/min/1.73 m2 to 70ml/min/1.73 m2, while the BTS even do so when rates are lower than 60ml/min/1.73 m2. In both cases, GFR thresholds that are so distant from standard advisable GFR and previously published experience lack supporting evidence, and they should only be accepted in exceptional cases in experienced hospitals.
- •
We suggest that donation by young people, especially those who are under 30 years old, should be restricted to candidates who have a GFR of at least the average for their age group, without any additional risk factors and a strictly normal result of evaluation of their health status (C).
- •
We recommend that advanced age alone should not contraindicate donation, although such candidates are subject to more perioperative risks (A).
- •
We suggest that hypertension, one of the risk factors for CRD, may have less impact in elderly donors, due to their reduced life expectancy (C).
- •
We recommend that donor-recipient pairs should be informed that when donors are elderly, especially when they are older than 60 years, the renal function attained in the recipient and probably the long-term survival of the graft may be reduced (B).
Increasing numbers of elderly donors have been accepted in recent decades, and more than 30% of LDKT donors are now older than 50 234. This tendency reflects the change in the perceived long-term risk of ESRD for donors, as this affects younger candidates to a greater extent.
We now recognise that all donors have an increased risk of ESRD. This risk is very small in absolute terms, and it arises over the very long-term after donation (one or several decades), and it seems to increase exponentially with time after donation 223,229,235. Analysis of the donors who have reached a stage of ESRD shows that the majority of these patients took more than 10-15 years to arrive at this point, and they donated when they were young. Donation when young is therefore a risk factor of the first order for donors to reach stages of ESRD 235–238. Only 2% of the donors in the American UNOS registry were younger than 35 years old 223.
Young patients have a very long time of exposure to the risk of new diseases such as hypertension and DM, which usually debut after the age of 45 years. These diseases are difficult to predict, but may affect their future risk of CRD 223. We also know that hypertension and/or DM cause practically 50% of the cases of ESRD in donors, a situation that occurs several decades after donation 238. All these factors reduce the predictive power of a normal evaluation result in young candidates when they donate, and it may lead to an under-estimate of the risk 225. On the other hand, there is less available evidence for how young donors will evolve than there is for intermediate-age donors 223.
All these factors are inversely applicable for elderly donors. Although the latter are exposed to higher risk during nephrectomy and the immediate post-donation period, older donors with CRD risk factors such as hypertension and DM are less likely to be exposed to them for long enough to lead to ESRD 239. In spite of this, age may be a clear limiting factor for donation. According to the detailed analyses in other sections of these recommendations, advanced age is a risk factor for surgical complications during donation, recovery and the development of hypertension after donation 240,241. Donors older than 50 should therefore be subjected to exhaustive cardiorespiratory evaluation 242.
Age is an independent risk factor in for ESRD in donors, with the exception of African Americans 37,224,229. This fact may be due to the higher risk of developing hypertension and DM after donation in the African American population, so that the longer life expectancy in young and intermediate age donors would increase the long-term risk of ESRD 224.
Minor urinary alterations and their impact on donationThe term “minor” urinary alterations does not always correlate with their importance for an individual's renal prognosis, so that the term “minor” is only applicable when it refers to lower grade urinary alterations that are close to normality.
Proteinuria and Albuminuria- •
We recommend that donor proteinuria should be evaluated by measuring the excretion of albumin in urine, not solely by measuring total proteinuria in urine. In spite of this, both determinations are necessary when evaluating a candidate (A).
- •
We recommend that the amount of albuminuria be included in the estimation of the future risk of cardiovascular disease and ESRD in donors (http://www.transplantmodels.com/esrdrisk/) (B).
- •
Albuminuria of less than 30mg/day permits unrestricted donation (B).
- •
Albuminuria levels of from 30 to 100mg/day are a relative contraindication against donation. Accepting a donor in this range of albuminuria may be considered in elderly candidates when no other risks are present (D).
- •
We suggest that albuminuria from 100 to 300mg/day may be accepted in exceptional cases, when there are no other comorbidities. (D).
Increased protein excretion in urine is a strong renal disease marker, and it sometimes appears earlier than the fall in the GFR. Albuminuria signals an increase in glomerular permeability, while the elimination of low molecular weight proteins reflects a failure in tubular reabsorption. Although both may be associated with different forms of renal disease, the determination of albuminuria is a better marker in evaluating renal disease. All of the methodological aspects in connection with the determination of proteinuria or albuminuria are described in section 5.
Independently of the GFR, in the general population albuminuria higher than standard normal levels is associated with an increased risk of CRD, cardiovascular disease and mortality 243–260. Albuminuria also predicts the progression of CRD and cardiovascular events in patients with CRD, cardiovascular disease or diabetes 0,61. Although albuminuria of 30mg/day is usually considered to be the limit of normality, ranges above 5mg/day already show an increased risk that is proportional to the rise in albuminuria 217,247. A meta-analysis of almost 5 million individuals in the U.S.A., selected for their low risk of CRD and with an average follow-up of from 4 to 16 years, found that for each 10-time rise of albuminuria, a 3-times increase in the risk of ESRD is detected. Although this finding did not attain statistical significance, the projected incidence of ESRD at 15 years showed that the increase in albuminuria was associated with a higher risk in all age groups independently of sex 125. Orthostatic proteinuria is an exception, given that it has a benign prognosis and must not be considered a contraindication for donation 247.
In spite of all the above considerations, we lack solid evidence that would allow us to set a clear limit that excludes donation. This is because there is practically no published experience of the long-term evolution of donors with different levels of proteinuria or albuminuria.
The KDIGO and BTS guides set a level of albuminuria <30mg/day, or a ratio of albumin/creatinine in urine <30mg/g as a suitable limit for accepting a donor. However, they also recognise that there are individuals within this range who have normal albuminuria, and others with a slightly raised level for their age and sex. At the other extreme, albuminuria higher than 300mg/day for the BTS guides, or higher than 100mg/day for the KDIGO, are a contraindication. Donation is relatively contraindicated between both extremes, and the decision to accept a donor should be taken on an individual basis, according to their demographic characteristics and health. According to current recommendations these “intermediate” albuminuria values (30mg/day - 300mg/day) are sufficient to establish a diagnosis of CRD, so that even admitting the weakness of the available evidence, donation is not advisable. The current KDIGO guides justify accepting these donors because the projected risk of ESRD in elderly individuals with albuminuria levels from 30mg/day to 100mg/day is very low, on condition that a reduced GFR and other comorbidities are ruled out 125.
Microhaematuria- •
We recommend that after ruling out the most common causes, a renal biopsy should be indicated if isolated persistent microhaematuria is found in the study of a living donor (B).
- •
Any pathological entity detected in a renal biopsy is a contraindication against donation, including IgA glomerulopathy. Only TBMD would require a different decision (B).
- •
Candidates with TBMD face an increased risk of ESRD. Donation may be considered in individuals older than 40 or 50 years, after clinical, histological and genetic evaluation and after they have received detailed information on the risks and uncertainties associated with donation (D).
The presence of microhaematuria or non-visible persistent haematuria is defined by the presence in microscopic evaluation of urinary sediment of more than 2-5 erythrocytes per microscope field in at least 2 separate determinations. Lower degrees of haematuria, i.e., the presence of erythrocytes in urine in any degree (1-3 erythrocytes per microscope field) are considered to be irrelevant in the general population. Nevertheless, the few available studies prove that the histological examination of such cases shows a significant presence of underlying nephropathy 146,148. Therefore, and even recognising that the indication for biopsy is weaker, the study of a donor candidate with detectable haematuria does not substantially differ from that of a candidate with persistent isolated microhaematuria. The methodological aspects of defining and determining microhaematuria and the diagnostic sequence after finding it are described in section 5.
Microhaematuria may have many causes. Firstly, microhaematuria associated with intense exercise, sexual activity, menstrual contamination, or injury should be excluded 248,249. The most frequent causes of microhaematuria include a non-malign urinary aetiology such as urinary infection, prostatic hypertrophy or urolithiasis. There are also many other less frequent causes, such as systemic diseases, infectious diseases, urothelial neoplasia or primary glomerulopathy 249–251. The majority of cases are therefore due to benign causes and are potentially reversible. Donation will be permissible once the underlying cause and associated factors have been resolved. In fewer cases microhaematuria is due to a pathology which affects the health of the donor or the safety of donation, making it a contradiction against donation. After the relevant study and once other causes have been ruled out, the study of microhaematuria for the purpose of enabling donation must conclude by indicating a renal biopsy. In such cases the presence of different histological findings may raise doubts about whether to accept the donation.
In an individual with persistent microhaematuria, any pathological entity detected in a renal biopsy will be a contraindication against donation. This includes IgA glomerulopathy, regardless of its clinical or histological expression. Only thin basement membrane disease (TBMD) will give rise to a doubt regarding the acceptance of a potential donor 173,180. This entity, which had only been defined using histological criteria, now corresponds in genetic terms with autosomal dominant Alport's syndrome (ADAS) 252. These patients only have a pathogenic variant of the COL4A3 or COL4A4 gene, and its possible clinical presentation varies widely, from isolated microhaematuria to the development of ESRD, generally over the age of 40 years 253. The BTS guides consider the possibility of donation by patients with persistent isolated microhaematuria due to TBMD 2. This is based on the fact that there are precedents for donations under these circumstances when there was awareness of donor TBMD and when there was not, and no adverse events have been reported in the follow-up of either type of donor 146,148. Nevertheless, even when this decision is based on rigorous genetic evaluation and family history, it does not exclude the risk of developing ESRD. Only individuals with a complete genetic, histological and clinical evaluation who are older than 40 to 50 years may be considered as donors, with especial emphasis on detailed information about the risks and uncertainties associated with donation. TBMD should be differentiated in Alport-carrier patients with a heredity linked to chromosome X, who have a significant risk of ESRD, at 12% before they are 40 years old and increasing to 30% and 40% at 60 and 80 years old, respectively 253, so that these candidates are not considered to be acceptable donors. Donation in women who are Alport's syndrome carriers with heredity linked to chromosome X has been followed by the development of CRD, proteinuria and hypertension in more than 50% of cases 254.
In exceptional cases, and after extensive study which includes all of the relevant complementary examinations, including renal biopsy with electron microscopy and immunofluorescence studies, donation may be accepted from individuals with isolated microhaematuria as the sole finding. It is fundamental in these cases to exclude any family history of renal disease, with a targeted genetic study in the case of the slightest doubt. Several experiences support the acceptance of these donors. One recent study which targeted the general young population (16 to 25 years old), with an average of 22 years of follow-up, found a small excess long-term risk of ESRD associated with the presence of isolated microhaematuria in individuals with less than 200mg/day proteinuria and normal range creatinine levels. The majority of the ESRD cases corresponded to the development of primary glomerular disease 255. Taking into account that these patients were not subjected to such an exhaustive or extensive evaluation process as the one proposed, it is possible that this evaluation would detected the individuals with underlying renal pathology, thereby eliminating the excess risk detected in this study. This occurred in a Dutch study including renal biopsy of a series of 49 patients with persistent isolated microhaematuria. The 20 patients with normal histology showed no worsening of renal functioning or developing proteinuria over the 11 years of follow-up 256.
Dyslipidaemia- •
We suggest that donation should not be contraindicated if dyslipidaemia is an isolated finding (C).
- •
We suggest that dyslipidaemia should be taken into account when estimating the overall cardiovascular risk of a donor (C).
The prevalence of dyslipidaemia in the general Spanish population stands at 30%–51%, making it one of the most prevalent cardiovascular risk factors, surpassed only by hypertension in some studies 258. Additionally, within dyslipidaemia, hypertriglyceridemia and low levels of HDL cholesterol form part of the diagnosis of metabolic syndrome 259. Few studies have analysed the possible association between pre-donation lipid levels and the results after nephrectomy. One study in México found that metabolic syndrome had a negative impact, leading to a lower GFR at 5 years, although none of the components of metabolic syndrome that were analysed (abdominal perimeter, fasting glycaemia, hypertriglyceridemia, HDL cholesterol levels or hypertension) had any impact alone on post-donation renal functioning. In an Israeli study donors were found to be at higher risk of metabolic syndrome than the control group 261. However, no studies analyse the influence of dyslipidaemia as an isolated prognostic factor for renal donors. A meta-analysis in the general population found no association between LDL cholesterol levels and the development of ESRD 125. All of the guides consulted (2,4,9,262,263) recommend the determination of the lipid profile in the study of live donors, although none of them sets a limit for non-acceptance.
Hyperuricaemia and gout- •
We suggest that hyperuricaemia or a history of gout in a donor are not a contraindication against donation (C).
- •
We suggest that candidates with a history of hyperuricaemia and/or gout should be informed that donation is associated with increased levels of uric acid and perhaps a higher risk of gout (C).
A fall in the GFR is accompanied by a reduction in the excretion of uric acid and a subsequent increase of uric acid in the blood, while the risk of gout also rises 264. Within the context of the fall in the GFR which occurs after donor nephrectomy, an 8% increase has been observed in uric acid levels 6 months after donation in donors compared to healthy controls, and this increase persists 36 months after nephrectomy 210. If we compare donors pre- and post-donation, uric acid was found to have increased by 20% 7 years after nephrectomy and more episodes of gout were detected, although at a low absolute frequency 209. African American, male and older donors are the most likely to suffer gout after renal donation 265. A recent Japanese retrospective study found that donors with higher uric acid levels before donation were at greater risk of developing suboptimum compensatory renal hypertrophy 266.
As well as the risk of gout, high levels of uric acid have been associated with increased cardiovascular risk and the risk of developing CRD in the general population 209,267. These data have not been confirmed in cohorts of renal donors.
Of the recently published guides, only the 2017 KDIGO make recommendation about renal donor hyperuricaemia 204. As the development of hyperuricaemia and gout after donation seems to be strongly associated with sociodemographic factors 265, donors should be assessed on an individual basis, asking about possible episodes of gout and informing them about the potential risk of hyperuricaemia and/or gout after donation.
Smoking- •
We suggest that the impact of smoking on whether candidate donors are accepted should be individualized, depending on the overall risk of the candidate (C).
- •
Smoking cessation is recommended before donation as well as during donor follow-up (C).
- •
Potential donors should be properly informed of the risks associated with smoking and having smoked (NG).
Tobacco consumption is a major but modifiable cardiovascular risk factor, with a prevalence in Spain of 39% among adults aged from 35-64 years 268. As well as its widely known cardiovascular and carcinogenic risks, smoking may also cause direct renal damage by promoting atherosclerosis and microvascular damage in tissues, and it has been associated with the appearance of CRD 269. The aforesaid meta-analysis on risk factors for developing ESRD in the general population after 15 years showed that active smokers had a 76% increase in risk, while ex-smokers too had a 45% increased risk 125.
There is little literature on the risk for smoking donors after nephrectomy. An American study found a risk of mortality at 12 years to be 5 times higher in donors who were smokers than it was in non-smokers 120.
No guide recommends contraindicating donation for smokers 2,4,9,262,263. They do recommend undertaking respiratory function tests to rule out structural pulmonary pathologies 2 and, depending on the accumulated packs per year index, it may also be necessary to use low radiation thoracic scan imaging to rule out lung neoplasia 263.
Alcohol consumption and addiction to other drugs- •
The overall and psychosocial assessment of donor candidates should specifically examine their consumption of alcohol and other drugs (NG).
- •
We suggest that all donors should be encouraged to reduce their alcohol consumption to the recommended levels for the general population (D).
There is no evidence or recommendation whatsoever regarding the evaluation of alcohol consumption and/or addiction to other drugs in potential renal donors 2,4,9,262,263. The advice to reduce consumption or to consume alcohol in “moderation” seems to be insufficient to correctly assess donor risk, as alcohol consumption may be associated with the development of hypertension or gout, for example 204. Moreover, addiction in itself should be included within the psychological assessment of donors.
The European guides examined “alcohol consumption” in depth 4. The authors concluded that a reduction in alcohol consumption should not only be recommended but also encouraged and sought. The target was to reduce consumption to below 60 grams per day in men and 40 grams per day in women, according to World Health Organization criteria 270. Although extrapolation from renal recipients to donors should not be the norm, it would seem recommendable to at least evaluate donor alcohol consumption, over and above a simple question such as: “Do you drink?” Alcohol consumption prevalence in the previous 30 days stands at 60% in adults in Spain, and up to 25% of men aged from 55-64 years consume it daily 264. It is therefore necessary to assess alcohol consumption more precisely in our renal donor candidates, so that we can offer suitable information and detect possible alcohol dependency disorders. Excessive consumption can also be identified using indirect signs, such as altered liver function in analysis or ultrasound scan imaging.
On the other hand, there is no evidence or any recommendation on assessing donors for sporadic drug consumption. In fact, the majority of guides contextualise drug consumption within their donor assessment in terms of infectious diseases. Within this scenario, psychological assessment is the most important means of ruling out problems with addiction, so that the decision whether to accept a donor is based on their complete psychological and medical evaluation.
8Metabolic evaluation and healthy lifestyles before and after living renal donationThe prevalence of ESRD is increasing worldwide as the result of a higher prevalence of comorbidities such as hypertension, DM and obesity. These comorbidities are generally contraindications against accepting living donors because of the long-term risk of developing ESRD and increasing post-transplantation morbimortality, particularly in elderly donors. Likewise, metabolic syndrome, which affects 30% of the general population, is a prelude to these comorbidities and may be a potential limitation. Nevertheless, the majority of potential donors are in older age groups, and there is limited scientific evidence on long-term donor health. Decisions about donation by these populations are therefore controversial. The majority of studies suggest that living renal donors have a minimum life-long risk of developing ESRD. However, the long-term complications and psychological and physiological after-effects of donation are still unclear, due to the lack of optimum donor follow-up after KT.
Optimising the selection of these donors should guarantee the safety and efficacy of this replacement therapy. The detection of risk factors for the development of ESRD is of crucial importance in this assessment. The increase in these risk factors in potential donors has led to the broadening of the selection criteria for them, modifying absolute and relative contraindications, so that individualized assessment is necessary. The identification of these risk factors makes it possible to set donor selection and exclusion criteria, as well as recommending specific changes in their lifestyle.
Obesity- •
We suggest that the body mass index (BMI) should be used for the clinical evaluation of overweight and obese subjects (Quality of evidence: C).
- •
We suggest that individuals with a BMI>35kg/m2 should be advised against renal donation (C).
- •
We recommend that living donor renal donation should be contraindicated in obese patients with a second risk factor (B).
- •
We recommend that obesity (BMI>30kg/m2) should be considered a relative contraindication against donation, and that this should be evaluated on an individual basis to detect the presence of other cardiovascular risk factors such as glucose intolerance, hypertension and proteinuria (B).
- •
We recommend that obese donors should be advised on the increased risk of perioperative morbidity and the lack of data on long-term safety (B).
- •
Donors with a BMI >27kg/m2 should be informed that they may be at higher long-term risk of developing CRD and that therefore, they should lose weight prior to donating and keep to a healthy weight afterwards (C).
Being overweight or obese is a public health problem due to its high rate of prevalence in the general population, as a result of a sedentary lifestyle and overeating. National Health Institutes define obesity as a BMI≥30kg/m2. Although historically living donors were free of pathologies, the selection criteria for marginal donors have been gradually broadened 271. Currently more than 25% of living donors are obese at the moment of donation, as opposed to 8% in the 1970s, and up to 43% are overweight.
Obesity is an independent risk factor for ESRD in the general population. It may cause functional and structural changes at renal level, such as glomerular hyperfiltration, glomerulomegalia and focal segmental glomerulosclerosis lesions. The risk of ESRD increases in patients with a solitary kidney, who develop compensatory glomerular hyperfiltration. Patients with a BMI >30kg/m2 at the moment of nephrectomy are therefore at higher risk of developing proteinuria and renal failure 10 years afterwards, due to reasons other than renal donation 272.
Obesity is associated with a higher risk of hypertension, DM, CRD, metabolic syndrome and cardiovascular disease in the general population, and this is also the case in living donors. Although BMI is a useful tool in the identification of overweight and obese patients, there is no established agreement on what constitutes a safe BMI for donors, and there are several different recommendations 2–4. The long-term impact of obesity on donors is therefore uncertain, and it is hard to find the limit that would predict a higher post-donation risk, so that candidates could be excluded during the pre-donation assessment, or lifestyle strategies could be implemented to modify this factor.
Obesity in living renal donors does not seem to affect their long-term health, except for a more complicated wound and a longer time in surgery. There are no differences in the GFR in comparison with non-obese donors in the years following donation 213,273. However, the long-term impact may be negative. In a national study in the U.S.A., obesity alone (without hypertension or DM) was associated with a higher rate of CRD and mortality in comparison with non-obese donors 271. They also developed DM and hypertension more often and earlier than donors who were not obese after donating 274,275, although this risk was not aggravated by the nephrectomy 276.
The above findings have important implications for the selection of living donors in terms of the BMI cut-off points set previously as contraindications for donation. It therefore varies from a BMI of 30kg/m2 to 35kg/m2, depending on the hospital. Being overweight may lead to a high risk of ESRD in the long-term in comparison with a normal BMI, so that potential donors who are obese or overweight should be advised to lose weight before donation. Moreover, a lower BMI is associated with an improvement in other cardiovascular risk factors over the short-term. Donors with normal BMI at the time of donation underwent significant weight loss prior to donation and kept a healthy bodyweight afterwards; donors with a BMI ≥25kg/m2 at the moment of donation underwent a significant weight gain during 1 year after the donation, suggesting the need to improve weight control efforts among obese renal donors and those with excess weight before and after donation to reduce their risk of advanced CRD 277.
To summarise, obese potential donors should be evaluated on an individual basis to detect other risk factors associated with CRD, such as glucose intolerance, hypertension and persistent proteinuria. Potential donors with metabolic syndrome should be evaluated individually, given the reversible nature of the alterations. Post-donation evaluation and follow-up to control weight, as well as to underline the importance of daily physical exercise and coordination with nutritionists for individualized healthy diet programmes should be an objective in living donor KT programmes, to minimise cardiovascular risks and to improve long-term renal survival.
Arterial hypertension- •
We suggest that arterial blood pressure should be measured at least twice, separated by an interval (C).
- •
We suggest that arterial blood pressure should be measured in the following cases: donors with hypertension under treatment, when measurement in the surgery shows raised blood pressure >140/90mmHg or normal-raised>130/85mmHg (C).
- •
Eligible donors with controlled hypertension<140/90mmHg with one or two antihypertensive drugs, without evidence of organic harm that could be due to hypertension. Consider excluding donors with controlled hypertension but also with other associated risk factors, such as obesity, smoking or advanced age (C).
- •
We suggest that potential donors with a blood pressure>140/90mmHg who are under antihypertensive treatment or signs of organic lesion should be excluded (C).
- •
We suggest that blood pressure and other modifiable risk factors should be monitored annually after donation (C).
In the general population, the prevalence of hypertension in adults is estimated to stand at 30%-45%, reaching 60% in individuals over the age of 60 278. Hypertension is a relative contraindication for living renal donation, and individual assessment is necessary to determine whether there are any other associated comorbidities. The most recent clinical guides set an arterial blood pressure of >140/90mmHg with one or two antihypertensive drugs or evidence of organic damage induced by hypertension as contraindications for living renal donation. Nevertheless, the definition of hypertension may undergo slight variations over time 279.
A meta-analysis that included 48 studies in 28 countries with 5,145 living renal donors found an average weighted increase in systolic blood pressure of 6mm Hg, while the diastolic increase was 4mm Hg, which is higher than what would be foreseen in normal ageing 122. Donor may be at greater short - to long-term risk of developing hypertension 280–284. The associated risk factors are age, male sex, African-American ethnicity, a family history of hypertension, BMI and higher systolic and diastolic blood pressure. Nevertheless, given that these risk factors are similar to those in the general population of the Framingham cohort, it may be inferred that the effect of nephrectomy neither modifies nor increases the risk 283.
Donors with pre-existing hypertension maintain a higher blood pressure beforehand and two months after donation than their controls. They are similar at 5 years, and no differences in renal function or proteinuria have been found 285.
Although the majority of hospitals exclude patients with hypertension as renal donor candidates, they usually base this on blood pressure measured in a clinical examination, and this does not reflect their real blood pressure. In a study of 178 donors, 63 of them had hypertension when measured in the surgery, but two thirds of these were found to correspond to “white coat hypertension”, as when ambulatory blood pressure monitoring (ABPM) was used, these patients were found to be eligible for donation. On the contrary, of the 115 individuals found to be normotensive in clinical measurement, 17% were found to have masked hypertension in the ABPM, and they were excluded from donation. The individuals with masked hypertension were older, male and had a somewhat higher clinical blood pressure than the individuals with sustained normotension 286. ABPM better reflected the real blood pressure than measurements in the surgery. Several studies have pointed to the value of ABPM in renal donors when clinical measurement of blood pressure is in the hypertensive range. 30%-60% of potential donors who had hypertension during clinical assessment were found to be normotensive when studied using ABPM 287.
Eligible hypertensive donors should therefore be appropriately monitored using by ABPM and have no evidence of hypertension-induced organic harm. In the same way as for the general population, it is probable that a rise in blood pressure in living renal donors will increase their cardiovascular risk and the risk of CRD. The first therapeutic step would be to administer renin angiotensin system blockers. The donors without pre-existing hypertension should be informed that donation may accelerate the increase in blood pressure respecting what would be expected with normal ageing, and that it is necessary to monitor blood pressure regularly.
Diabetes mellitus and metabolic syndrome- •
We recommend that basal glycaemia and glycated haemoglobin (HbA1c) should be determined in all donors (B).
- •
We recommend that an oral overload glucose test should be performed if there is a history of gestational diabetes, a first-degree family history of type 2 diabetes, impaired fasting glycaemia, overweight, hypertension or dyslipidaemia (B).
- •
Donors with type 1 DM should be excluded (D).
- •
Donors with type 2 DM should generally be excluded, although they may be considered under special circumstances (C).
- •
In cases with pre-diabetes or metabolic syndrome the decision should be taken on an individual basis (D).
- •
Recommendations for a healthy lifestyle (C).
- •
Follow-up after donation to monitor CV risk factors (C).
There has been a major increase in the prevalence of DM over recent decades 288,289. Potential renal donors should have a determination of basal glycaemia and glycated haemoglobin (HbA1c), In those ones with a first-degree family history of type 2 diabetes, a history of gestational diabetes, impaired fasting glycaemia (IFG), obesity, hypertension or dyslipidaemia, an Oral Glucose Tolerance Test (OGTT) should be performed.
Maintained hyperglycaemia triggers a series of haemodynamic and structural changes which, when they coincide with a genetic basis and converge with other factors such as obesity or hypertension, may lead to the appearance of diabetic nephropathy 290,291. If this is combined with hyperfiltration due to reduction in the nephron mass after nephrectomy, the risk of developing diabetic nephropathy in renal donors with diabetes may be considerable. The presence of type 1 DM has therefore classically been considered to be an exclusion criterion for renal donation. Nevertheless, in type 2 DM and under certain circumstances, especially if there are no other factors such as hypertension or overweight, donation may be considered 292. In a series of 225 donors, the 14 diabetics at the moment of donation were compared with the 211 non-diabetics over an average follow-up time of about 4 years. There were no differences in renal function or albuminuria between both groups 293. Another series found no differences in survival over the long-term, and none of the diabetic donors had advanced CRD 294.
Up to 70% of prediabetic individuals (basal glucose from 100mg/dl to 125mg/dl, or an altered OGTT) will develop type 2 DM 295,296. The proportion of donors with glucose intolerance has increased from 7% to 22% over the past two decades 297, and studies carried out with donors of this type show good outcomes, without differences in the GFR or albuminuria after 10 years of follow-up in comparison with non-prediabetics 298. A study of 93 donors with HbA1c and OGTT distributed them in three groups: high risk of DM (HbA1c and OGTT in the prediabetic range), low risk (one of both parameters altered) and the control group (with both parameters normal). After an average follow-up time of 76 months, a high percentage of the high risk group developed DM, in comparison with the low risk group and the control group (31.3% vs 6.5% vs 0%). They showed no higher risk of hypertension or microalbuminuria, and the prediabetic donors had a similar GFR to the control group. The conclusion was that although HbA1c identifies prediabetic donors at risk of progressing to DM, with careful selection these donors would not be at higher risk of renal function deterioration 299.
Notwithstanding all of the above, these results should be interpreted with caution since there is no long-term follow-up. A study of 2954 living renal donors found that after an average follow-up time of almost 18 years, 154 had developed type 2 DM. During the first decade, they had no increased risk of CRD after developing diabetes, although this was not the case for increased albuminuria 300. In a later publication this same group presented the outcomes of these diabetic donors, and over the longer term they showed faster deterioration of the GFR than the non-diabetic control group if they also had hypertension and proteinuria 301.
Several risk indexes have been prepared for the general population to estimate the probability of developing type 2 DM, but their predictive capacity varies widely as they exclude major risk factors. In practice, basal fasting glucose and the OGTT are the standard recommended tests for the diagnosis of type 2 DM, in renal patients too. Figure 7 shows a flow diagram that may help to detect type 2 DM before renal donation.
Possible mechanisms which lead to hyperfiltration, progressive renal disease and proteinuria in potential living renal donors who have metabolic syndrome prior to donation. GFR: Glomerular Filtration Rate. Adapted from Hernández D 305.
Metabolic syndrome arises when at least three of the following circumstances coincide: abdominal perimeter (waist circumference) >102cm in men and >88cm in women, fasting serum triglycerides ≥150mg/dl, HDL-cholesterol <40mg/dl in men and <50mg/dl in women, systolic blood pressure ≥130mmHg and/or diastolic≥85mmHg or taking antihypertensive drugs and fasting glucose ≥100mg/dl or taking hypoglycaemiant drugs. This syndrome has a prevalence in Spain of 25%, and it is associated with CV morbimortality, diabetes and renal damage 302–304. The high prevalence of metabolic syndrome, its comorbidities and its negative consequences, make it necessary to undertake an exhaustive search for this entity in potential renal donors 305. Metabolic syndrome and each one of its components are independently associated with a higher risk of CRD 306. However, it is less clear whether the presence of metabolic syndrome in a donor is associated with worse renal function after donation. In this respect, an interesting study analyses the association between the syndrome, renal function and histological damage in living renal donors. Of a total of 410 donors, 12% had metabolic syndrome at the time of donation; the pre-implantation renal biopsies of the donors with metabolic syndrome showed more histological signs of chronicity, and this finding was associated with slower recovery of the GFR after donation 307. One group of renal donors with metabolic syndrome reached a GFR <70ml/min in a shorter period of time (an average of 5 years vs 12 years) 260. Theoretically, in individuals with metabolic syndrome who are subjected to nephrectomy for living donation, insulin resistance and the fall in renal mass may predispose their remaining kidney to hyperfiltration, with proteinuria and progressive renal disease (Figure 8).
Flow diagram for the detection of diabetes mellitus before living donation. OGTT: Oral Glucose Tolerance Test; AOGTT: Altered Oral Glucose Tolerance Test; AFG: Altered Fasting Glucose. DM: Diabetes Mellitus. Adapted from Hernández D et al. 305.
In potential donors with type 2 DM, prediabetes or metabolic syndrome, changes should be recommended in lifestyle, diet, weight loss and physical exercise. The aim would be to prevent progression to diabetes and reduce CV risk and the risk of progression to CRD 308.
The benefits of weight reduction to slow the development of diabetes have been clearly proven, at least in the short-term 309–311. However, this benefit is not usually maintained over time, above all because the partial or total recovery of the weight that had been lost is very common over the long-term 312.
Physical exercise has also been shown to be effective in slowing the progression to diabetes 50. The ADA recommends at least 150minutes/week of moderate to vigorous physical activity, or shorter periods (at least 75minutes/week) of vigorous intensity exercise to prevent or delay the appearance of type 2 DM in high risk populations and/or those with prediabetes 313. Intermittent high intensity training and continuous moderate intensity training have a similar positive effect on functional capacity and cardiometabolic markers in individuals with prediabetes or type 2 DM 314–315.
9Compatibility and incompatibility in living donor KTCompatibility barriers- •
An ABO blood group and HLA compatible transplant offers the best opportunity of success (Quality of evidence: A).
- •
Incompatibility in ABO blood group and the HLA system are independent. Donor and recipient may be HLA identical and ABO incompatible (NG).
- •
We recommend that blood group should be determined at an early stage in the study of a potential KT donor-recipient pair so that expectations can be properly managed and transplant options can be discussed (B).
- •
ABO blood group incompatibility is not an absolute contraindication for transplant, and it can be resolved by paired KT or a desensitizing treatment (A).
- •
To evaluate the transplant options for an ABO incompatible pair it is essential to quantify the isoagglutinins (ABO antibodies) in the recipient, preferably using donor erythrocytes (B).
- •
HLA should be typed using molecular biology methodologies (B).
- •
We recommend that HLA should be typed at allelic level (2 fields) and in the 11 loci HLA, that is: HLA-A, B, C, DRB1, DRB3/4/5, DQA1, DQB1, DPA1 and DPB1, in the recipient as well as in the possible donors (B).
- •
High resolution HLA typing makes it possible to interpret whether the recipient has donor-specific antibodies (DSA), detected using the new techniques for studying anti-HLA antibodies (NG).
- •
The new solid phase techniques have higher sensitivity to detect anti-HLA antibodies in comparison with the classic cellular techniques (A).
- •
The moment for studying histocompatibility should be decided on the basis of the characteristics of the recipient and the potential donors to prevent unnecessary examinations, guiding the expectations of the donor and recipient (NG).
- •
We recommend that a detailed clinical immunological history of the recipient should be prepared, and this should be shared with the histocompatibility laboratory, including their potentially sensitizing events and the relationship between the recipient and potential donors (NG).
- •
It is important to show whether the potential donor is the father of the descendants of a female recipient or whether the donor is the son of a female recipient, given the risk of sensitization during pregnancies.
- •
Changes should be shown in immunosuppression if the recipient still carries a dysfunctioning graft.
- •
We recommend that a crossmatch test should always be performed between the donor and recipient (A).
- •
A virtual crossmatch test will give us an initial approximate idea of donor and recipient compatibility (NG).
- •
A cell-based crossmatch test should be performed before transplantation, with cytotoxicity techniques and flow cytometry between the donor and recipient (NG).
- •
The histocompatibility laboratory or a trained professional in this field should interpret the test data and advise the clinical team on the risk for the donor-recipient pair (NG).
Human KT became a reality in the 1950s after performing the procedures on identical monozygotic twins as donor and recipient 316. The required technical and surgical advances had occurred deces earlier. This underlines the fact that the most important barrier for the survival of a solid organ transplant lies in the potential immunological “incompatibility” with the donor, which may trigger the loss of the graft 317.
All of the molecules which are present in the donor but absent in the recipient are able to cause a potential immune response. Nevertheless, the barriers idntified in clinical practice which clearly generate incompatibility and possible rejection are:
- -
Blood group incompatibility, due to the presence of antibodies which are naturally present in any individual older than 4 months.
- -
Incompatibility in the Human Leukocyte Antigen (HLA) system, due to the presence of antibodies acquired by previous contact with HLA antigens.
More than one third of potential KT recipients have developed anti-HLA antibodies that may hinder compatibility 318. Incompatibility in both of these systems is independent. The gene involved in the definition of the ABO blood group is located in the long arm of chromosome 9, and the genes which encode the HLA antigens are located in the short arm of chromosome 6. This explains the fact that two brothers may be HLA identical and nevertheless also ABO incompatible.
However, it is possible to transplant a non-compatible organ but with different levels of HLA mismatches between the donor and recipient. The probability of rejection is associated with the number of HLA loci mismatches between the donor and the recipient, Greater match between them is related to the increase in graft survival 319.
Compatibility barriers due to ABO blood group360 different blood groups have been recognized on the surface of human erythrocytes 320. Nevertheless, only one of the blood group systems described has been shown to be significant for KT: the ABO.
Characteristics of the A, B, O antigensThe ABO group is one of the simplest blood groups in terms of its structure, as a single monosaccharide at the end of a chain of three or four monosaccharides determines whether it is specifically A, B or O. However, it is also the most important blood group system, which was why it was the first group to be described by Landsteiner in 1901 321. It consists of chains of 4 or 5 monosaccharides on the surface of erythrocytes, usually conjugated with polypeptides to form glycoproteins or with a type of lipid (ceramides) to form glycosphingolipids. The majority of ABO antigens are found in the glycoproteins of the erythrocyte cell membrane.
The oligosaccharides which determine the A, B or AB specificity of the group are synthesized in stages. Over a basic chain of three monosaccharides (fucose, galactose and N-acetyl galactosamine (NAcGlc)) known as antigen H, if a certain glucosyltransferase is inherited then an additional monosaccharide is added. Individuals who inherit the NAcGlc glucosyltransferase add one NAcGlc to substance H. This 4 monosaccharide structure is known as blood group A. Not all of the NAcGlc glucosyltransferases display the same efficiency in transforming substance H into antigen A. One form that is expressed by approximately 80% of A individuals transforms about 85% of substance H into A (of 1,000,000 antigens per erythrocyte). These are group A1 individuals. The remaining 20% of A individuals have a NAcGlc glucosyltransferase that only transforms 15% of substance H into antigen A (about 200,000 antigens) and they are known as group A2 322.
The individuals who inherit galactose transferase add a molecule of galactose and the structure is that of group B. Those individuals who inherit both glucosyltransferases are the AB group. In individuals who inherit glucosyltransferase genes that produce non-functional enzymes, substance H is not modified and they are blood group O. The correct term is the letter “O” and not the number zero 323.
Expression of the A, B, O antigensAs well as in the erythrocytes, the ABO is expressed in many other tissues within the organism, and it is also found as a soluble glycoprotein in bodily secretions. Within the kidneys antigens are expressed in the vascular endothelium (arteries, glomerular capillaries and peritubular) and in the distal tubular epithelium. On the contrary, it is not found in the proximal tubule or in the mesangial cells 324.
The intensity of group ABO antigen expression in the erythrocytes and tissues varies in parallel 324. Thus individuals in group A1 have greater antigen expression (in the tissues and erythrocytes) than those in group A2. An additional factor affects ABO antigen intensity of expression, the secretor gene. The secretor gene makes it possible for antigen H to also be found in bodily secretions, and it makes A and B antigen expression more intense. To detect whether an individual is a secretor or not it is necessary study if they carry the gene (FUT2) using molecular biology or by determining if the individual is a carrier of the Lewis blood group (Le) b in their erythrocytes. Only individuals who have the secretor gene will be Le b positive.
These differences in the intensity of ABO antigen expression are of practical importance in the field of renal transplantation. In the case of A2 group and non-secretor donors, due to the low presence of A antigens in their kidneys grafts to O recipients have been undertaken, without previous conditioning 325.
A, B, O antigen study techniquesThe techniques currently used to differentiate blood groups in human erythrocytes are based on haemagglutination reactions between erythrocyte antigens and antibodies which are read and interpreted manually or automatically.
The significant change in recent years is the support where these techniques are applied. At first, erythrocyte aggregation reactions took place in a test tube, and they were read and interpreted manually by agitating the centrifuged tube against an illuminated background. However, at the end of the 1980s the French scientist Yves Lapierre developed a new technique and commercialized it to detect erythrocyte agglutination 326.
Column agglutination technology (CAT) is based on plastic microcolumns that are full of acrylamide or glass gel microspheres which retain the erythrocyte agglutinates. In comparison with the traditional technique in glass tubes, this technique gives a reaction that is stable once it has occurred. It is easier to read and reproducible, and it even permits automatic reading and interpretation. To detect blood group antigens there are 6 or 8 column cards in the market, depending on the manufacturer, each one of which is able to detect a specific antigen (A, B, AB, RhD), while one of them is used as the control of the agglutination reaction. Automatic devices now exist in the market which dispense, incubate and centrifuge, before reading and interpreting the results.
ABO antibodies: isoagglutininsThe visibility, importance and relevance of the ABO blood group is due to the fact that all individuals have antibodies against the antigen that they lack, unlike the other 357 antigens in which antibodies are only generated when the individual has come into contact with human erythrocytes (transfusion or pregnancy, and they are negative for the antigen in question). The reason why the antibodies are present lies in the fact that ABO antigens are also present in vegetables and many Gram negative aerobic bacteria 327. After 2 or 4 months of life humans develop antibodies against antigen A or/and B, depending on their group. These antibodies are known as isoagglutinins because the erythrocytes agglutinate when human plasma/serum is confronted by incompatible erythrocytes. This was how they were discovered by Landsteiner.
Unlike other immune responses in humans, in which IgM antibodies are generated after an initial exposure, followed by IgG antibodies and the disappearance of the IgM ones, in the case of the ABO the majority of individuals have a significant amount of IgM isoagglutinins in circulation during most of their life. It has been suggested that their continued presence may be due to continuous exposure to ABO antigens in Gran negative bacilli that are present in the intestinal flora.
IgM antibodies are responsible for the agglutination of incompatible erythrocytes due to their pentameric structure, which permits the aggregation of several erythrocytes. They are also effective complement activators, able to bring about the lethal consequences observed in cases of transfusion of ABO incompatible erythrocytes or when an ABO incompatible kidney is transplanted, with almost instantaneous graft loss 328.
Nevertheless, the majority of ABO isoagglutinins in circulation are IgG, Unlike IgM, IgG are monomeric and do not cause direct agglutination. For this to occur it is necessary to add a second antibody against human IgG, which is known as the indirect antiglobulin test (IAT). Not all IgG subclasses are able to activate the complement, only subclasses IgG3 and IgG1 have significant activity 329.
Anti-ABO antibody study techniquesGiven that isoagglutinins are able to agglutinate erythrocytes, the main technique used due to its ease and availability is haemagglutination. The first method used for detection was tube agglutination. To detect the IgM the dilution of erythrocytes is added and the plasma to be tested is centrifuged and read. If it is wished to measure IgG an additional stage is necessary: after adding the dilution of erythrocytes, the plasma and a low ionic strength solution to favour fixation of the IgG, it is incubated for 15minutes. After centrifuging it is washed 3 times and the anti-human IgG is added, it is centrifuged and read. Given that these techniques are routinely used for transfusions, they have the advantage that they are available in the majority of hospitals 24/7.
Column agglutination techniques have now replaced tube techniques, as is the case for blood group study. For IgM the solution of erythrocytes and the plasma to be tested is dispensed, centrifuged and read, while for IgG the same process is used but in a microcolumn which contains the human anti Ig-G, so that it only has to be incubated, centrifuged and read. The introduction of automation has also simplified the execution of the technique even further, and as the reading is also automatic this reduces the variability of interpretation, making it possible to store the reaction pattern for subsequent revision, if necessary.
In the field of ABO-incompatible renal transplantation quantification is more important than the detection of isoagglutinins. Another technique commonly used in immunohaematology to quantify them is dilution. Successive dilutions are made of the plasma stream (1,1/2,1/4,1/8,1/16,1/32/,1/64,/128, etc.) with a buffer solution, and they are confronted with erythrocytes. In general in immunohaematology the inverse of the last dilution to give a positive reaction is reported as the titre (for example, titre 8 if the last positivity occurs in 1/8 dilution. However, in our experience, given the frequent administration of endovenous immunoglobulins in pre-transplantation desensitization, treatments in the case of incompatible ABO which in the columns usually give a weak positivity that hinders readings, we use the final dilution that gave a reaction pattern of +2 as the titre. (The intensity of positive reactions is graded in 4 levels, +1, +2, +3 and +4, depending on how the erythrocytes are distributed along the column).
Another important aspect for consideration in titration of the isoagglutinins is the question of the erythrocytes to be used in the test. Given the direct correlation between expression in tissues and in the erythrocytes of individuals, it is recommended to always use the erythrocytes of the donor for the follow-up of titration pre- and post-transplantation in a recipient of an ABO incompatible kidney.
Compatibility barriers due to the HLA systemCharacteristics of the HLA systemTransplantation success depends on avoiding the allogenic response of the recipient to the differences in the major histocompatibility antigens expressed in the transplanted organ, i.e., the incompatibilities in the HLA system. The immunological alloresponse will be more intense when the difference between the donor and recipient HLA typing is greater.
HLA system antigens are molecules which are expressed in the cell membrane, and they are classified in 2 types:
- -
Class I HLA molecules formed of a single polypeptide chain (bound to Beta2-microglobulin) and codified by 3 gene loci (HLA-A, B, C). They express in the membrane of all cells and their physiological function is to present intracellular antigens (peptides) (e.g., virus) to cytotoxic CD8+ T lymphocytes.
- -
Class II HLA molecules are heterodimeric proteins anchored to the cell surface by their two chains (α and β). There are also 3 types of HLA-II molecules (DR, DP, DQ) which are codified in the gene loci DRA1, DRB1, DRB3/4/5, DPA1, DPB1, DQA1 and DQB1. They are expressed in monocytes, endothelium, B cells and dendritic cells. Their physiological function is to present extracellular antigens (e.g., bacteria) to CD4+ T helper lymphocytes. T helper lymphocytes may also express HLA-II molecules in their membranes after being activated.
One of the main characteristics of the HLA system genes is its complex polymorphism. As they differ between individuals, allogenic HLA molecules are recognized as foreign, and the cells which express them are attacked in a similar way to cells infected by a virus. The antigenicity of HLA system molecules is conditioned by the fact that each individual has several types of HLA antigens with multiple possible different alleles, due to their expression in the cell membrane and their peculiar function as a molecule which presents peptides to lymphocytes.
The aim of the majority of the techniques used in the histocompatibility laboratory is to:
- -
Evaluate the incompatibilities that the recipient will detect in the donor cells. This consists of typing the HLA system in the recipient and the donor.
- -
Identify the presence in a recipient of alloantibodies against polymorphisms in the possible donors. As well as detecting them, it is possible to characterise these antibodies at the level of their target (HLA-I, HLA-II or non-HLA), the type of immunoglobulin (IgG, IgG subclasses, IgM) or their capacity to fix complement.
- -
Identify whether the recipient alloantibodies will react against the polymorphisms expressed in the proposed donor. This is analysed by applying the crossmatch test.
To evaluate HLA compatibility it is essential to typify the HLA of the recipient and all of the possible donors. The majority of laboratories undertake HLA typing studies using Molecular Biology techniques based on PCR (Polymerase Chain Reaction). Depending on the type of technique used it is possible to achieve a low or antigen level of typing resolution (1 field, example A*02) or a high or allelic level of resolution (2 fields or more, example A*02:01). There are multiple types of PCR for HLA typing:
- -
PCR-SSO (Sequence Specific Oligonucleotide probes): the most polymorphic exons in each HLA locus are amplified generically before hybridising the amplified product with specific DNA probes for the polymorphic positions. The Luminex platform is used now, in which the probes are attached to microspheres in suspension.
- -
PCR-SSP (Sequence Specific Primers): this is based on the use of specific primers for a certain region of DNA present only in a specific allele or group of alleles. This enables the use of PCR that will only be positive for each one of the alleles or group of alleles.
- -
PCR-SBT: this is direct nucleotide sequencing of the gene to be analysed. Amplification takes place using specific primers for the most polymorphic regions of the gene (exons 2,3 and 4 in class I HLA and exon 2, and sometimes 3, in class II HLA) before performing the sequencing reaction and electrophoresis of these reactions in an automatic sequencer. The complete nucleotide sequence of the amplified region is compared with the sequences of all of the HLA alleles of the corresponding locus, to thereby determine which alleles are present in a certain individual.
- -
Real time PCR: specific primers and probes are used with no need for any post-PCR processing. A fluorescent substance identifies the presence or absence of the amplified products. Analysing the positive and negative reaction patterns gives the HLA typing.
- -
Second generation or Next Generation Sequencing (NGS): this consists of clonal, massive and parallel sequencing which permits a large number of sequences of each nucleotide in each test. Although several platforms exist, they all use technology that includes amplification of the DNA template (HLA genes), the preparation of libraries, cluster generation, sequencing and data analysis. This technique is highly productive and permits complete gene sequencing, reducing the number of ambiguities in the assignation of the results.
Classically, in candidate patients for KT and their donors, HLA typing was performed in the A, B and DRB1 loci at antigen level (1 field). The association between HLA incompatibilities and renal graft survival has been widely proven in deceased donor KT. In the case of living donor transplantation the risk of graft failure has also been observed to increase by 44% for each mismatch in adult recipients who received a first transplant from a related living donor, and it increases twice if mismatches are 6/6, taking transplanted patients with 0 incompatibilities as the benchmarks. These differences are even more marked if the organ is from an unrelated donor 319.
HLA typing at allele level is advisable (2 fields) and in the 11 HLA loci (HLA-A, B, C, DRB1, DRB3/4/5, DQA1, DQB1, DPA1 and DPB1), in the recipient as well as in potential donors (EFI European Federation for Immunogenetics. Standards for histocompatibility testing. http://www.efiweb.eu/). Not all of the techniques listed above permit high resolution or allele level typing. This is why second generation or NGS technology is becoming predominant in the majority of HLA typing laboratories, as it makes it possible to study all of the HLA genes in high resolution relatively easily and highly productively. High resolution HLA typing of the recipient/donor pair makes it possible to evaluate their compatibility more precisely, thereby gaining better knowledge of the probability of long-term graft survival.
High resolution HLA typing also makes it possible to determine whether the recipient has donor specific antibodies (DSA) using the new anti-HLA antibody study techniques, if there are antibodies against certain alleles but not all of them in a single antigen group (such as A*02:01 positive, A*02:03 negative). Typing all of the HLA genes also makes it possible to determine, if the recipient has anti HLA-C or HLA-DQ or DP antibodies, if they are DSAs or not 330.
Another advantage of high resolution HLA typing is that it enables the evaluation of recipient/donor compatibility in terms of the epitopes that may be recognized by specific antibodies, rather than being based on antigen/allele incompatibilities. These epitopes are determined by polymorphic amino acids on HLA molecule surfaces, and it is possible to determine HLA compatibility at this structural level. The polymorphic residues that can be recognized by antibodies have been entitled “eplets” and, by using the HLAMatchmaker 331, it is possible to study their level of recipient/donor compatibility. Several studies have shown that eplet-level compatibility is clinically relevant 332, as HLA mismatches in renal transplants which are compatible at eplet level have been found to have practically the same survival rate as those in recipients transplanted from zero-mismatched donors according to conventional criteria (at antigen level). Although recipient/donor compatibility is not now routinely evaluated using eplets, it is possible that in the near future this will be a part of clinical practice.
Anti-HLA antibodies: antibody determination techniquesAnti-HLA alloantibodies may be determined by using cells that express HLA molecules on their surface as the target, or by using “artificial” systems which present HLA molecules attached to a solid phase support.
The main cellular technique that has been used to determine alloantibodies is microlymphocytotoxicity or complement-dependent cytotoxicity (CDC): this technique is based on the cytolytic effect of the complement on lymphocytes when a (serum) antigen-antibody reaction takes place on their surface. This study is performed using a panel of cells from different donors 30–60 who are carriers of different HLA antigens. It detects the presence in serum of complement-activating cytotoxic antibodies, chiefly IgG1, IgG3 and IgM. Variations of this technique exist, such as incubation of the cells and serum with human anti-IgG. This variant, which is used the most often in the U.S.A., increases sensitivity and detects IgM, IgG1, IgG3, IgG2 and IgG4 immunoglobulins.
Specificity is assigned by mathematical correlation between the presence of a certain antigen in the lymphocytes and positive and negative reactions of the serum. The CDC test thereby provides three types of information:
- -
Whether or not the recipient has alloantibodies.
- -
The percentage of reactivity or PRA (Panel Reactive Antibody). This represents the percentage of the general population (represented in the panel) with whom the patient is incompatible. This enables prediction of the probability of a positive lymphocyte crossmatch test.
- -
In some cases it identifies the antigens which the alloantibodies are reacting against. In recipients with multiple alloantibodies, determining acceptable antigens using cytotoxicity techniques lacks precision because each cell expresses several alleles. Donors with these antigens have a very high probability of a positive crossmatch test using cytotoxicity.
The use of serological techniques such as CDC to detect anti-HLA alloantibodies is decreasing. They are being replaced by more sensitive solid phase methods which are able to identify specific antibodies more quickly and simply.
The crossmatch test consists of confronting the cells of a specific donor with recipient serum to detect the presence of preformed alloantibodies against the donor. In clinical practice this is performed using two techniques:
- A)
Crossmatch cytotoxicity lymphocyte test or “Crossmatch-CDC” (CDCCM) between the recipient and donor: Based on the CDC technique, the recipient serum(s) are incubated with donor lymphocytes in the presence of complement. A positive reaction is visualized with the aid of a vital colouring agent, which penetrate through the cell membrane if it has been made permeable by the complement. It detects IgG1, IgG3 and IgM antibodies. The reaction is quantified according to the percentage of dead cells, so that the cellular viability of the basal sample is essential. This crossmatch test may be performed using total lymphocytes or cell subpopulations. If the subpopulations of T and B lymphocytes are separated it is possible to differentiate the anti HLA-I antibodies from the anti-HLA-II antibodies. It is possible to treat using DTT (which destroys the IgM) to differentiate IgM lymphotoxic antibodies (generally autoantibodies) from IgG type ones. To discover the approximate “quantity” of anti-HLA alloantibodies in the recipient it is possible to detect the cytotoxicity of the serum at different dilutions (from 1/1 to 1/512). This analysis is of interest if recipient desensitization is intended. Crossmatch by cytotoxicity predicts a high risk of hyperacute rejection, so positivity contraindicates KT. It has a high positive predictive value (PPV) for graft loss (80%) 2.
- B)
Flow cytometry crossmatch lymphocyte test between the recipient and donor (FCCM): the binding of the recipient serum antibodies to the donor lymphocyte membrane is identified using a human anti-immunoglobulin (generally a Fab2-anti-IgG) marked with a fluorochrome that is detectable by flow cytometry. To identify the lymphocyte subtypes against which the serum reacts, monoclonal antibodies marked with other fluorochromes are used. Anti-CD3 is usually used to identify the T lymphocytes, and anti- CD19 is used to identify the B lymphocytes. The (inactivated) T cells only express HLA-I, while the B cells express HLA-I and HLA-II. Thus the anti-HLA-I antibodies will react with the T and B cells, while the anti-HLA-II antibodies will only recognise the B lymphocytes. This also makes it possible to rule out IgM autoantibodies. Positivity is generally calculated based on the Change in the Mid Channel of Fluorescence=[Mid Channel of the problem serum]–[Mid Channel of the negative control]. The positivity cut-off has to be set in each laboratory. The usual cut-off values may be different for T (CD3+) lymphocytes and for B (CD19+) lymphocytes. The cytometry crossmatch indicates an increase in the risk of losing the graft within one year which is low for the first transplantation (10%), although it is higher for second or consecutive transplants (30%) 333.
Solid phase techniques are based on the union to a “solid phase” of purified HLA antigens from multiple individual cells and joined to a plastic surface. ELISA plates can be used (less often), or a set of polystyrene microspheres or “bead arrays” (more commonly). These polystyrene microspheres coated with HLA molecules have an internal fluorescent marker that are be identified by cytometry (Luminex). The reaction is revealed with a secondary antibody, generally human anti-IgG, marked with fluorochrome, so that they detect IgG alloantibodies if they are complement activators (IgG1, IgG3) or if they do not activate it (often IgG2, IgG4). No IgM antibodies are detected (unless an anti-IgM is used as a secondary antibody), and nor are those against non-HLA antigens (such as autoantibodies).
The most widespread solid phase techniques based on Luminex technology are:
- A)
Screening Bead arrays: each microsphere contains natural class I or II HLA molecules from multiple individuals. They make it possible to identify the existence or not (positive/negative) of alloantibodies against class I or II HLA, although they cannot determine their specificity. Some kits also detect the presence of anti-MICA antibodies. These tests are more sensitive than antibody detection using cytotoxicity (CDC) 334,335.
- B)
Microspheres with isolated antigens, or Single Antigen Bead arrays: each microsphere is coated with a single class I or class II HLA allele obtained using genetic recombination, so that they are recombinant antigens. This permits the direct identification of antibody specificity. This is highly useful, especially in hypersensitized patients, as it enables the identification of those antigens that may be considered acceptable even though they are mismatches, i.e., the alleles against which the recipient has no alloantibodies. Based on the positive specificities it is possible to estimate a calculated PRA or PRAc, which has the same significance as the classic PRA by CDC.
There is no single shared criterion among different laboratories defining the level of antibodies (MFI or Median Fluorescence Intensity) which is relevant and which are irrelevant 336. Moreover, the cut-off differs depending on the kit used. Each laboratory therefore has to set its own MFI cut-off to define positivity/negativity. It is important to differentiate between a positive result according to kit manufacturer criteria and positive within the clinical context of establishing an acceptable risk of suffering antibody-mediated rejection.
Comparing the positive alloantibody specificities and the HLA typing of a specific donor makes it possible to obtain the result of what is known as the Virtual Crossmatch, which predicts the result of the cell-based crossmatch. The Virtual Crossmatch has a high Negative Predictive Value in comparison with the cytotoxicity crossmatch, although its Positive Predictive Value is lower (that is, it detects antibodies that are undetectable by cytotoxicity) 325. It has to be taken into account that a positive solid phase virtual crossmatch indicates increased risk of antibody-mediated rejection (which varies according to the study from 5% to 55%) 337,338 although it does not necessarily contraindicate transplantation.
Solid phase methods have the advantage that they do not detect antibodies against non-HLA antigens. Nevertheless, there is a theoretical possibility that in the process of obtaining and joining to the solid phase the HLA molecules may suffer conformational changes which interfere with the union to some alloantibodies, or which give rise to non-specific reactions.
Variants of the standard technique which permit the determination of complement-fixing anti-HLA antibodies have become more relevant over recent years. There are therefore commercial methods to determine whether antibodies fix C1q or C3d. Determination of the capacity of anti-HLA alloantibodies to fix complement may be indicated in patients with acute early antibody-mediated rejection, to determine which treatment will be the most appropriate 339,340.
Other variants of the technique enable the identification of IgM antibodies or the study of the different IgG subclasses (IgG1, IgG2, IgG3 and IgG4). Although IgG3 and IgG1 are known to be able to activate complement, while IgG2 and IgG4 do not, the role of each one of these subclasses in the development of rejection has not yet been sufficiently elucidated to use these determinations in clinical practice.
Planned study of the donor-recipient pairRecipient HLA sensitization evaluationIt is fundamental within the study for LDKT to carry out a detailed immunological clinical history of the donor and the intended recipient in the initial assessment. We recommend that this history should include:
A) Potential sensitizing events in the recipient 329
The following HLA-sensitising events should be included in the clinical history:
- -
Pregnancies (including gestation to term and abortions)
- -
Transfusions
- -
Previous transplantations
- -
Tissue implants (ventricular assistance devices, biological valves
Some consensus documents also recommend giving detailed information about inflammatory events which may reactivate the pre-existing alloimmune memory, such as: major surgery, severe infections or recent vaccination 263. It is also relevant to include information on treatments with biological agents which may generate false positives when cellular techniques are used (anti-CD20 antibodies, anti-IL6, etc.)
B) The relationship between a recipient and the potential donors
It is useful to know the relationship between the recipient and donor, to offer an initial approximation of the level of compatibility, and it may also be an essential decision-making tool when there are several potential donors, or when recipients have a high level of HLA sensitization.
The following data are of especial interest in clinical practice:
Siblings: the possibility of an identical D/R HLA pair. This is associated with longer graft survival. This is especially interesting for highly sensitized recipients (e.g., in case of re-transplantation).
Parents/children: they will share at least one haplotype.
A woman whose donor is the father of her children: there is a possibility of HLA incompatibility due to recipient exposure to donor HLA antigens during pregnancies.
This information should be shared with Histocompatibility laboratories to ensure correct interpretation of the results of the immunological study.c) Study of anti-HLA antibodies (“PRA” report)
Study of a KT recipient includes the determination of HLA antibodies. HLA antibody detection techniques allow us to measure the level of recipient sensitization: a panel of cytotoxic antibodies or PRA CDC (using lymphotoxicity techniques) or calculated PRA (PRAc) (using solid phase techniques - Luminex®). The PRA will inform us about the possibility of access to transplantation for a specific recipient, and it will be a necessary step in the study of a donor-recipient pair.
The immunological study of the recipient and their immunological follow-up are beyond the scope of this chapter. The most up-to-date recommendations for study of the recipients are those published in the latest KDIGO Guides on the assessment and management of KT recipients and those of the STAR project 263,341.
HLA typing and compatibilityThe recipient and all potential donors should be typed using molecular biology techniques. Serological methods are not recommended, as they do not offer sufficient resolution to assign potential antibodies which are directed against a specific allele.
HLA allele typing (two digits) is currently recommended for at least HLA-A, -B, -C, -DR and -DQ loci 137. The most recent KDIGO guides recommend typing the loci that enable correct interpretation of the recipient's HLA antibodies (for example, in recipients with antibodies against HLA-DP, it is recommended that this locus should be typed in the recipient and their potential donors) 329. However, it is best to type 11 loci: HLA-A, B, C; HLA-DRB1,3, 4, 5; DQB1, DQA1; DPB1 and DPA1 (9). This information will be essential for the correct interpretation of the level of D/R compatibility and HLA antibodies analysis, preformed as well as de novo after transplantation.
The number of incompatibilities (mismatches) between donor and recipient may be relevant, especially if there is more than one potential donor, or in young recipients with a high probability of needing a new renal graft in their lifetime. It is also a risk factor for the de novo development of donor specific antibodies, and it therefore affects the development of antibody-mediated rejection and graft survival 333.
- -
HLA matching at antigen/allele level. - The level of HLA compatibility between donor and recipient is expressed in the number of HLA specificities for each donor locus that are absent in the recipient. Advances in HLA typing have led to improvements in the analysis of D/R matching, which used to be determined in the HLA-A, -B and -DR loci at antigen level, as HLA matching is now measured in 11 loci at allele level.
- -
HLA compatibility at epitope level. - The introduction of molecular typing techniques and knowledge of the three-dimensional structure of a HLA molecule has enabled the identification of antibody recognition patterns at the level of how amino acids are arranged within these molecules. This HLA compatibility is based on the fact that each HLA molecule is structurally unique, and that there cannot be an immunological response to self-proteins 342,343. Different software systems have been developed based on these principles, to establish the compatibility between donor and recipient at epitope level. The first to be developed was HLAMatchmaker 16, which is a software algorithm to calculate the number of mismatched eplets between the donor and recipient. Other algorithms were subsequently developed, such as Pirche-II 31 or HLA-Emma 344.
These programmes are not currently in routine use in Histocompatibility Laboratories to examine HLA mismatching at epitope level for live donor transplantation.
Compatibility studies between a recipient and potential donorsAs well as discovering the level of HLA compatibility between donor and recipient, the immunological study of a pair for LDKT includes pre-transplantation crossmatch tests to confirm the absence or presence of specific anti-donor alloantibodies.
The virtual compatibility test [virtual crossmatch (VCM)] is defined as immunological evaluation based on the alloantibody profile of the recipient compared with donor HLA antigens. This virtual VCM is increasingly important and accepted within transplantation programmes, and it allows us to take the first steps in an immunological approach to the study of live donor transplantation. Nevertheless, given that LDKT is planned, applying only the virtual compatibility test is not recommended 331.
The recommendation for LDKT study is always to perform a direct compatibility study with a cell-based crossmatch test, using CDC (CDCCM) techniques and/or flow cytometry (FCCM), before transplantation. The recommendation is to perform a final crossmatch test 14 days before the planned date of transplantation 28.
There are no recommendations on which type of crossmatch test (CDCCM vs FCCM) should be used in the study for a LDKT. The KDIGO guides recommend using a flow cytometry or cytotoxicity crossmatch test, without preferring one or the other 263. The British guides recommend using a flow cytometry crossmatch test in recipients with preformed antibodies against class I HLA (using donor T lymphocytes) and/or class II (with donor T and B lymphocytes), as well as performing a CDC crossmatch test with T and B lymphocytes with untreated recipient serum and serum treated with DTT that will give us more information for appropriate classification of the immunological risk 137.
Nor are there any studies that measure the number of crossmatch tests that should be carried out in a pair. New compatibility studies should be performed if the recipient has experienced any sensitizing event or if there is discordancy between test results. Given that LDKT is a planned procedure, it seems reasonable to perform a cell-based crossmatch test using cytotoxicity and flow cytometry techniques to ensure a correct approach for the immunological risk, given that both tests supply complementary information 338.
Although it is infrequent (<20%) discordant results may arise between both types of crossmatch tests. It is fundamental that these tests are correctly interpreted by HLA laboratories and transplantation teams to determine the level of immunological risk 337 (Table 10).
Interpretation of possible discordant results between both types of crossmatch test by HLA Laboratories together with transplantation teams, as this is fundamental to determine immunological risk.
| Virtual crossmatch test | Physical crossmatch test | Interpretation |
|---|---|---|
| - | - | Concordant |
| + | + | Concordant |
| - | + | Discordant:- Consider false positive*,a- Cutoff MFI high- Specific antibody or allele not tested in SAB- Non-HLA antibodies |
| + | - | Discordant:- Low level of HLA Ab- Antibodies against denatured antigens- Viability of donor cells |
When we start the immunological study of a donor/recipient pair, the first thing to do is to study the immunological history of the recipient and their level of sensitization. Based on this information, we can classify them as:
A male recipient without sensitizing events. - In the first stage of the study we will be able to estimate that the VCM with the donor is negative. We will therefore perform recipient and donor HLA typing, as well as the cell-based CM at more advanced stages. Only when there is more than one potential donor will we consider typing the recipient and the potential donors in an initial phase, to discover the level of compatibility between the recipient and potential donors, as this will be a relevant tool when deciding which donor to accept. In the final stage of the study we will perform crossmatch tests using CDC and flow cytometry, together with typing if this had not been carried out beforehand.
Recipient with sensitizing events. - We initially propose performing a VCM test to evaluate compatibility and immunological risk. For this purpose we will type the donor and recipient as well as the study of HLA antibodies in the recipient's sera.
If the VCM is positive with preformed antibodies against the donor's HLA which have a very high MFI and the cell-based crossmatch test is positive, direct transplantation without desensitizing treatment will be ruled out due to the high risk of early antibody-mediated rejection. We would suggest a paired transplantation, given the low possibility of achieving HLA desensitization. If the MFI of the DSA is low, we would consider HLA desensitization strategies and/or inclusion in a paired KT scheme.
If the VCM test is negative, we will be able to wait until a final phase before performing a cell-based CM test.
Estimation of immunological risk [Figure 10]The results of the different techniques used will allow us to estimate the immunological risk between the recipient and the intended donor. This is essential to accept a potential donor, to decide the immunosuppression strategy for the recipient and even to estimate outcomes 263. Figure 10 shows the level of immunological risk according to virtual and cell-based compatibility test results.
Recipients with a positive CDC crossmatch test and/or cytometry together with a positive virtual crossmatch test are at very high risk of developing hyperacute rejection (above all in patients with a positive CDC crossmatch test) 317 and acute early antibody-mediated rejection 345. Direct KT is not advisable in these cases, and the approach is to include the donor/recipient pair in paired KT programmes.
If the virtual crossmatch test is positive but the CDC and flow cytometry crossmatch tests are negative, then the immunological risk is high 346 and we should consider desensitization strategies to overcome the HLA incompatibility barrier in patients with antibodies against the donor who have medium to low MFI levels 347. Nevertheless, if the MFI of these antibodies is very high, with little probability of success in desensitization to achieve relatively safe levels to perform a direct KT, then we can decide on paired el KT.
Potential KT recipients who have negative virtual and cell-based crossmatch tests will be candidates for direct KT with a high level of immunological risk (in patients with a high level of sensitization and high PRAc) or a standard level of risk (recipients with a low PRAc). We should take into account that there is a potential latent immunological memory response in recipients who have previously received a transplant, when there are HLA mismatches between the donor and the recipient, or in female recipients who have previously been pregnant, when the donor is the father of their children 341. Careful monitoring or close vigilance after transplantation in this type of recipients will allow us to anticipate the development of this potential response, which would lead to an antibody-mediated rejection. The immunological risk will be very low in the case of HLA-identical siblings.
Recommendations for the management of HLA incompatibility (Figure 11)The new techniques used to detect anti-HLA antibodies are more sensitive, and it is increasingly common to find living donor and recipient pairs who are HLA incompatible, especially in individuals with a very high cPRA, which makes it very difficult to find a compatible donor 348. For such individuals, and also for any potential recipient with anti-HLA antibodies, we suggest using this workflow for the recipient together with the potential living donors to achieve a KT from the living donor who is the best option in terms of quality. It is also an opportunity to evaluate the risk in detail, making it possible to fully plan the transplantation and the corresponding treatment.
To summarise, it is essential to have a good characterization of the immunological risk and the opportunities for the recipient, through a good immunological history. Individuals with a high level of HLA sensitization would benefit from the possibility of donation by a HLA-identical sibling, even if they were ABO incompatible, because as we will see in the specific chapters, ABO desensitization treatment is more effective than HLA desensitization. If they have no identical siblings, the possibility of having a higher level of HLA compatibility (a lower level of incompatibility) would lie in genetically related family members. If although no compatible living donor is found there is the wish to donate, the intensity of donor-specific anti-HLA antibodies in the recipient should be evaluated and the options of paired KT or HLA desensitization should be considered. It is sometimes possible to study a potential living donor while the patient is included in the deceased donor waiting list, to increase transplant options for a sensitized patient.
10Living donor nephrectomyThe concept of minimally invasive living donor nephrectomy has been developed over the last 20 years. Due to the increased experience in laparoscopic surgery, laparoscopic nephrectomy in living donors is now the technique of choice in the majority of transplantation hospitals 349. Laparoscopy has given rise to a revolution in LDKT, as it has made donation far more attractive due to its few complications, short hospitalization, shorter convalescence and better cosmetic results 32. Innovations such as LESS (laparoendoscopic single side Surgery), surgery through natural orifices, retroperitoneoscopy and robotic surgery are currently carried out in several hospitals. Open surgery using a mini-incision is still practiced in some centres.
Surgical innovation and increased experience in transplantation hospitals have increased the number of organs available for transplantation. Donors with a complex vascular pedicle, lithiasis and non-standard risks are now considered valid.
This guide describes the recommendations for donor selection and planning the nephrectomy, applied in different special donor situations. It will also make recommendations for different surgical techniques and their possible complications.
Donor selection and planning surgery- •
Left nephrectomy is usually preferred in live donors, although right nephrectomy may also be performed, with similar complications (Quality of evidence: NG).
- •
Living donor nephrectomy when there are multiple vessels or anatomical alterations should take place in more experienced hospitals (NG).
- •
We recommend that donors with widespread atheromatosis or fibromuscular dysplasia of both renal arteries should be rejected (NG).
Once the viability of donation has been confirmed on the basis of medical workout, the donor should be physically examined and the results of their angiotomography should be thoroughly reviewed. It is important to know the surgical history of the donor, to prevent possible complications during the operation. Donor weight and more specifically their physical examination will be important when selecting the surgical technique. If the aim is to perform a transvaginal nephrectomy, a digital vaginal examination should be performed prior to the surgery to confirm good elasticity that will permit the safe harvesting of the organ. In the case of a LESS nephrectomy the donor should be thin and without a history of major abdominal surgery (because it may have created adherences).
The anatomical evaluation of donors should include angiotomography with arterial, venous and nephrographic phases to identify any possible alterations in the urinary tract, and/or the vessels. This exploration has a sensitivity/precision of over 95% in the detection of renal veins and arteries 350,351. The radiologists will be in charge of supplying exact anatomical information, based on their own experience.
If both kidneys are similar, it is preferred to collect the left one as it has a longer vein, making it easier to engraft. The laparoscopic harvesting of a right kidney is not a contraindication nowadays, and it accounts for 15%-20% of nephrectomies in the majority of series. A higher rate of graft thrombosis in right living donor nephrectomies was described in the past. Several subsequent studies have shown similar results for organs from both sides (2D) 352. To facilitate renal transplantation with short veins, a transposition of the iliac vein in the recipient may be performed, as this reduces the space between veins in the anastomosis 353.
Variants and vascular alterationsDuring the study of a living donor we may find different situations that have to be evaluated separately, while also taking into account the characteristics of the recipient. One such situation consists of complex bilateral renal pedicles (multiple arteries and veins, fibromuscular dysplasia of the intima, calcifications in the arterial ostium and even aneurisms).
Kidneys with multiple pedicles are now accepted in the majority of hospitals, and they have been shown to give good functional results 354,355. In cases where there are multiple arteries it is preferable to obtain a single anastomotic opening by bench surgery on the graft whenever this is possible. When the arteries have similar calibres they should be joined laterolaterally in a “shotgun barrel”. When the arteries have different calibres, the smaller ones are anastomosed to the larger one (terminolaterally) using discontinuous or continuous stitches in Prolene 7/0 (Figure 12A). If the anatomy makes this necessary, it is possible to do independent anastomoses. In those cases where the renal artery has been sectioned after the bifurcation, so that we have short vessels, the hypogastric artery and its branches in the recipient may be used to obtain a viable vessel (Figure 12B).
A: Shotgun barrel anastomosis and terminolateral anastomosis in a kidney from a living donor. B: Use of the hypogastric artery to obtain a single artery. C: Atheroma plaques in the ostium. D: Fibromuscular dysplasia of the unilateral intima. Bench surgery repair with a cadaveric graft. E: Repair of arterial aneurism by means of cadaveric arterial graft in bench surgery. F: Exeresis of a tumour in bench surgery.
When there are upper polar vessels, these can be eliminated when they are small (<2mm) as they do not represent a significant loss of renal function. This is more controversial for the lower polar vessels, as these irrigate the urethra and ligating them may cause urinary tract ischemia. To avoid this tract ischemia an anastomosis can be done between the urethra of the donor and the recipient's one.
In the case of multiple veins, one of them can be ligated if they are of different diameters. When the veins are similar we should anastomose them to each other, ensuring good venous drainage. As older donors are now accepted atheromatosis at the level of the renal artery ostium is frequently observed. When this atheromatous plaque is large and bilateral, the donor should be rejected (Figure 12C). If these plaques are unilateral, the kidney can be collected while taking special precautions during the harvest. It is important to place clips with sufficient separation to border the plaque.
Fibromuscular dysplasia may affect the renal arteries. This consists of a heterogeneous group of changes in the arteries that are neither atherosclerotic nor inflammatory, and they cause a certain degree of stenosis, occlusion or vascular aneurisms which may restrict the renal flow. Donors will be rejected if they have bilateral involvement. They may be accepted if the dysplasia is unilateral, with replacement of the affected area in bench surgery using a cadaveric tissue graft (Figure 12D).
Renal artery aneurisms are rare, and they generally affect the arterial division. They are at risk of rupture when they are larger than 1.5cm and not calcified. Nephrectomy in a donor with an aneurismatic artery is therapeutic. The affected kidney may be repaired in bench surgery using a cadaveric tissue graft or the internal iliac of the recipient (Figure 12E).
Lithiasis- •
Accepting donors with a history or presence of lithiasis should be based on the risk of its recurring after donation (NG).
- •
After donation they should follow the specific guidelines set for the general population to prevent recurrence (NG).
- •
It is safe and reproducible to extract lithiasis in bench surgery (NG).
In 5% of donors angiotomography shows the presence of asymptomatic lithiasis356. These donors are currently considered to be valid in certain circumstances. The clinical history of the donor must be taken into account, as well as whether they have suffered renal colic previously or have been treated for lithiasis. These donors are accepted on the basis of the risk of the lithiasis recurring 357 and their awareness of the post-donation consequences. Donors who are younger than 40 years old have a high risk of recurrence, as do those with a family history of renal lithiasis and those who have suffered frequent lithiasis episodes. If they have no history of lithiasis and it is unilateral, with a correct metabolic study, then these donors may be considered to be valid. They should be followed-up according to the lithiasis management guides to prevent recurrence.
Once they have been accepted it is necessary to decide which kidney to harvest, with different options. If unilateral microlithiasis is detected, we will be able to harvest either kidney without the need to treat the said lithiasis. There is minimum risk of future problems for the donor as well as the recipient. If single small unilateral lithiasis (<1cm) is detected, the kidney will be collected after extracorporeal lithotrity, or removed using ureteroscopy in bench surgery 358.
Renal tumours- •
The kidneys of a donor with an incidental low grade renal tumour smaller than 4cm may be accepted after tumorectomy, informing the donor and recipient of the risk (NG).
- •
We recommend that the donor and recipient should be subjected to regular oncological check-ups (NG).
As donors are becoming older we find incidental small renal masses359. These tumours of less than 4 centimetres in size are usually of low risk and with low metastatic potential. The treatment of choice is partial nephrectomy, which has been found to give the same oncological results as in the general population 360. Those kidneys (non-standard risk) are considered to be valid for transplantation on condition that they are suitable for the recipient and that the patient and donor accept the risk 150,361,362.
The tumour will be resected in bench surgery and a pre-operative biopsy will be used to detect the margins and characteristics of the tumour. A histological analysis of the tumour prior to transplantation should be done to obtain information on the aggressivity (grade) of the tumour, and take the final decision whether to transplant (if it is low grade) or not (Figure 12F) 151,152,363.
Surgical technique- •
Laparoscopic nephrectomy of a living donor is the technique of choice as it causes less postoperative pain and results in a shorter hospitalization and convalescence than open surgery (NG).
- •
Living donor nephrectomy by lumbotomy may be indicated when the donor has had multiple abdominal interventions and intra-abdominal adherences (B).
- •
Living donor nephrectomy using a mini-incision is a valid option for donation (NG).
- •
LESS or transvaginal living donor nephrectomy should be performed in experienced hospitals (NG).
- •
Robot-assisted living donor nephrectomy gives similar results to laparoscopic techniques, although it is more expensive (NG).
The experience of the surgeon should be taken into account when selecting a surgical technique, to minimise complications and obtain a good quality organ. Traditionally the surgical technique of choice for living donor nephrectomy was open surgery through a lumbotomy, which was associated with a high rate of morbidity and a high risk of eventration. Increased experience in laparoscopic surgery means that this technique is now used in the majority of hospitals with good results for donors and recipients, minimising complications 364,365. Due to this, laparoscopic nephrectomy of the donor is now the technique of choice 366.
All of the minimally invasive techniques require that the donor be in lateral decubitus, with 3 or more trocars in place through the abdominal cavity. The path for renal harvesting will vary depending on the technique used, and it may be a periumbilical incision, a pfannestiel, in the iliac fossa or transvaginal.
Live donor nephrectomy by lumbotomy is not considered a technique of choice, as it requires a larger incision and therefore involves more postoperative pain and longer hospitalization. Living donor nephrectomy through a lumbotomy may be considered in certain circumstances, when the donor has had multiple abdominal interventions and has intra-abdominal adherences.
Different techniques have been developed as alternatives, using an anterior mini-incision, in the side, subcostal or posterior, giving simple access to the kidney 367. Randomized studies which compared surgery using a mini-incision with classical open surgery and laparoscopic surgery found that surgery takes longer with the mini-incision than it does with classical lumbotomy, although it causes less morbidity in the donor (with less need for analgesics and a faster recovery). In comparison with laparoscopic surgery, donors require more analgesia and recovery time is longer. No significant differences were found in terms of the functional results of the graft between any of the three techniques 368,369. The micro-incision technique makes it possible to perform a nephrectomy with low morbidity and good functional results, so that it is a good alternative in certain circumstances when laparoscopy is still not viable 370.
Laparoscopic living donor nephrectomyLaparoscopic living donor nephrectomy is the technique of choice due to its advantages in comparison with open surgery. Several systematic reviews which compare the open technique with laparoscopy conclude that the results in terms of donor safety and graft functioning are similar. It should be underlined that these works found open nephrectomy to have a shorter surgical procedure time, it also involved greater loss of blood. Additionally, laparoscopy led to less postoperative pain and shorter hospitalization and convalescence 371–374.
A variation on pure laparoscopic surgery is hand-assisted laparoscopic nephrectomy, which was developed to shorten the learning curve for pure laparoscopic surgery. It consists of the insertion of the non-dominant hand of the surgeon during the whole operation, to facilitate the surgical manoeuvres and increase safety. Randomized trials which compared pure laparoscopy with this hand-assisted variant in living donor nephrectomy found no significant differences between them in terms of complications, conversion rate or length of hospitalization, with less warm ischemia in the case of hand-assisted surgery 375,376.
Living donor nephrectomy by retro-peritoneoscopyThis technique was developed to avoid invading the peritoneum and reduce possible complications at this level. The retroperitoneal approach involves performing surgery in a small space with an anatomical vision different from the one we are used to, which would be the transperitoneal view, increasing the difficulty of this approach. The possible disadvantages associated with this technique are the risk of causing pneumomediastinum, pneumothorax, pneumopericardium or air embolism 377.
One benefit of this technique is that it avoids contact with the intestine, and it may be positive for donors with previous surgical operations. A meta-analysis undertaken to compare living donor nephrectomy using laparoscopy and hand-assisted retroperitoneal technique, no differences were found in terms of blood loss, hospitalization or graft survival. On the other hand, the retroperitoneal technique took less time and caused less warm ischemia 377. Retroperitoneoscopy has recently been suggested as the treatment of choice in cases of right renal donors 378.
Robot-assisted living donor nephrectomyIt is possible to perform robot-assisted living donor nephrectomy. Robotic surgery permits more subtle movements, with greater mobility than conventional laparoscopy, although it is more expensive. A meta-analysis which compared robotic surgery with laparoscopy found shorter operations, less warm ischemia and less bleeding with conventional laparoscopy. Nevertheless, there was less postoperative pain with robotic surgery. No differences were found between both techniques in terms of the duration of hospitalization or creatinine levels 379.
Living donor nephrectomy through a single incision (LESS)Surgery using the LESS technique involves making a small incision to insert several laparoscopic instruments to perform intraabdominal surgery, giving the same surgical results as conventional laparoscopy but with less morbidity and better cosmetic results 380–386. This is an attractive alternative, as surgery takes place through a single incision through which the organ is collected.
Two randomized clinical trials 387,388 and cohort studies 389–391, compared LESS with conventional laparoscopy, without finding differences in terms of complications or conversion to open surgery. Although the LESS technique was associated with less bleeding, it was also linked to longer surgical time.
Assisted living donor transvaginal laparoscopic nephrectomyThe assisted transvaginal approach permits assisted laparoscopic living donor nephrectomy through a transvaginal opening through which the kidney is collected. This technique gives better cosmetic results and reduces morbidity, as it reduces abdominal scarring 392. There are no studies that compare this technique to conventional laparoscopy.
Clipping systemsA controversial aspect of living donor laparoscopic nephrectomy is the type of clipping system used on the renal artery. There are two types of clip: staples and clips 393. Metal staples were used at first, and these were not free of mechanical problems (stapler malfunction), causing a shortening of the graft artery due to its width, and they are expensive. To overcome these difficulties Hemo-o-lok-type clips came into use more than two decades ago. These are simpler to apply and make it possible to obtain an artery that is a few millimetres longer, as there is no need to clip the proximal part. The current practice of renal extraction teams in Europe shows that the use of Hem-o-loks can offer good results in living donor nephrectomy. However, both the FDA and the manufacturer contraindicate them for such use due to the exceptional death of donors after hemorrhage 394,395.
11Recipient surgery in living donor KTLDKT surgery is technically highly complex, and it includes the creation of vascular and urinary tract anastomoses. It is undertaken by dedicated KT surgical teams. In Spain, KT is an area of recognized expertise within the scheme of the Urology speciality. Although in the majority of hospitals which perform LDKT it is carried out using open surgery (the “gold standard”), laparoscopy has also been explored experimentally 396, and there are now groups which have published their experience using robotic kidney transplant (RKT) 397. In spite of this, EAU (European Association of Urology) Guidelines do not routinely recommend these techniques due to lack of evidence in this field 398.
In this section we will describe the open or robotic surgical technique used in the recipient, before analyzing the results and complications. Finally, we will analyze procedures in special situations, such as transplantation into complex recipients: 3rd - 4th transplantations, patients with arteriosclerosis and KT in patients with urinary tract disorders.
Conventional open surgery- •
If a right kidney is used with a very short vein, we recommend that bench surgery should be used to create an optimum length renal vein by reconstruction and lengthening techniques with saphena and gonadal vein (Quality of evidence: B).
- •
We do not recommend routinely administering thromboembolic prophylaxis to all recipients using low molecular weight heparin (C).
- •
We recommend that single dose antibiotic prophylaxis should be administered (A).
- •
We recommend that the lymph glands should be ligated or coagulated to prevent the appearance of lymphocele. (A).
- •
We recommend that the kidney should be kept cold during surgery, wrapping it in a gauze containing crushed ice, or placing it in two gauzes with ice above and below the kidney (B).
- •
We recommend that the shortest possible length of urethra should be used for anastomosis in the bladder, while keeping the periurethral fat to ensure good irrigation (B).
- •
We recommend that a Lich Gregoire-type extravesical re-engraftation should be used in a double J (A).
- •
We recommend that a prophylactic mesh should be used in obese and diabetic patients, and/or patients treated with mTOR inhibitors (A).
Recipient surgery commences when the surgeon receives the kidney recently collected from the donor. The renal artery is located on a table prepared for bench surgery and perfusion is started 399. This perfusion continues until clear fluid emerges from the renal vein and approximately 500 cc-1000 cc has passed through. Bench surgery then commences, in which the following steps should be followed in order:
Freeing the perirenal fat to check that the correct perfusion of the graft, verifying the absence of traumatic or tumoral macroscopic lesions.
Dissection of the artery and vein until an appropriate length for anastomosis is achieved. The artery will have no aorta patch so that its ostium will be smaller. In the case of double or triple arteries the surgeon will have to decide whether to create an anastomosis between them and how: terminolateral, laterolateral or separately.
Vascular surgery techniques may be used in special cases, using the saphena or a segment of internal iliac of the patient 400,401. The right renal vein is usually short, although it is sufficiently long for anastomosis. If the renal vein is too short, there are reconstruction techniques using the saphena vein and gonadal vein to achieve an optimum length 402,403. Transposition of the artery and iliac vein is a manoeuvre that may be useful to facilitate venous anastomosis 353.
The recipient is catheterized in a sterile field, and previously will have received thromboembolic prophylaxis with low molecular weight heparin if they are a patient at risk, and a single dose of antibiotic prophylaxis according to the specific protocol of each hospital 404.
A perirectal incision in the right iliac fossa is generally used. The peritoneum is dissected and separated from the retroperitoneal structures medially and cranially until the iliac vessels are identified, before creating the appropriate space to make the transplantation.
The iliac vessels are then dissected and freed in the zone where the anastomoses will be performed. A point must be chosen where the vessels are not bent. The anastomosis should be made away from zones with vascular calcifications and atheroma plaques so that the anastomosis is not made on them, preventing possible arterial dissection. We recommend ligating or coagulating the lymph glands during these manoeuvres to prevent the postoperative appearance of lymphoceles. After placing a clamp on the vein of the recipient to isolate it from circulation the venotomy is performed, the recipient vein is irrigated with heparin and a venous terminolateral anastomosis is made using continuous monofilament 5/0 or 6/0 suture. A clamp is placed on the artery and the arteriotomy is performed. The recipient and donor intima should be examined before starting the anastomosis to ensure that there is no break. The terminolateral arterial anastomosis is then made using continuous 6/0 or 7/0 monofilament or single stitches. When engrafting a LD kidney the arterial anastomosis is the most delicate moment of surgery, as the artery has no patch and it may sometimes be very narrow calibre.
The whole operation takes place while keeping the kidney chilled in ice. It may be wrapped in one gauze that contains ice, or by placing it in two gauzes with ice above and below it.
Renal perfusion commences once both anastomoses have been completed. In LDKT the kidney usually immediately takes on a pink colour. If this does not occur then the vein and artery are inspected by touch, and the anaesthesiologist is asked to check that the arterial pressure parameters are correct. Likewise, if there is any doubt an intraoperative Doppler ultrasound scan can be performed. The urethral re-implantation is then performed. The shortest possible length of urethra should be used, keeping the periurethral fat to ensure good vascular irrigation in the urethra and thereby reduce the probability of postoperative urethral stenosis and urinary fistulas. An extravesical Lich Gregoire-type re-implantation is usually performed, leaving a catheter in double J 405.
At the end of surgery a drainage is put into place and the wound is closed. Given that it is possible that an incisional hernia will appear after the operation or during follow-up, it is recommended that a prophylactic mesh should be put into place in the cases with the highest risk: patients who are obese, diabetic or in treatment with an mTOR inhibitor (imTOR).
The drainage will be removed on the 2nd-3rd day depending on the volume drained, and the bladder catheter will be withdrawn in 5-7 days.
Surgical complications- •
Immediate surgical exploration is recommended in case of an arterial thrombosis, and that depending on macroscopic appearance, consider disassembling the arterial anastomosis, extracting the coagulation, perfusing the kidney and performing a re-anastomosis (B).
- •
Surgical exploration is recommended immediately in case of the diagnosis of a venous thrombosis. If the graft is viable a venotomy may be performed with thrombectomy (B).
- •
We recommend that significant stenosis of the renal artery should be treated with angioplasty and the insertion of a vascular stent. In the case of a very recent transplant, a long stenosis or failed angioplasty, surgical treatment can be used (B).
- •
We recommend that lymphoceles should be treated by inserting a percutaneous drainage. If the lymphocele is large and symptomatic then laparoscopic marsupialization would be indicated (B).
- •
We recommend that initial low volume urine leakage should be treated conservatively, with a urethral catheter and nephrostomy, or a double J catheter (B).
- •
We recommend that if conservative treatment of the urine leak fails or the leak is massive and very early, then surgical treatment should be selected: re-implantation or urethral anastomosis to the native urethra (B).
We recommend that in case of urethral stenosis >3cm or the failure of minimally invasive treatment then re-implantation surgery or urethral-urethrethrotomy should be used (B).
In spite of the good results which are now achieved 406,407, LDKT is not free of surgical complications. The most frequent complications are (Table 11):
The main postoperative complications in kidney transplantation.
| Complication (%) | Symptoms | Diagnostic technique | Treatment |
|---|---|---|---|
| Haemorrhage (0.2-25)398,408 | Haematoma | CT | Revision surgery if active bleeding. |
| Arterial thrombosis (0.5-3.5)398,409 | Raised creatinineAnuria | Doppler ultrasound scan | Immediate surgery. |
| Venous thrombosis (0.5-4)398,410 | PainRaised creatinineAnuria | Doppler ultrasound scan | Immediate surgery. |
| Arterial stenosis (1-25)398,411 | Raised creatinineRefractory AHT | Doppler ultrasound scanCT-Angiography | <50% asymptomatic: observation>50%: stent, surgery |
| Lymphocele (1-26)398,412 | PainRaised creatinineFever, infection | Ultrasound scanCT | Percutaneous drainageMarsupialization |
| Urinary leakage (0-9.3)398,413 | Increase drainage volume | Increased creatinine in drainage liquidCTPyelography | Low volume: Nephrostomy, double JHigh volume: Surgery |
| Urethral stenosis (0.6-10.5)398,414,415 | Dilated urinary tractRaised creatinine | NephrostomyAnterograde Pyelography | <3cm. Percutaneous dilation>3cm. Surgery |
| Haematuria (1-34)398 | Haematuria | Clinical | Vesical washingEndoscopic examination |
| Wound infection (4)398 | Pain, exudate, infection | Clinical | Topical dressingsAntibiotics |
| Eventration (4)398 | Opening of woundBreakage of aponeurosis | Clinical | Repair with mesh |
Haemorrhage (0.2%-25%): this usually takes the form of an asymptomatic haematoma which does not require any action. Larger haematomas which may compromise vessels, with haemodynamic instability and when computed tomography (TC) shows active bleeding will require revision surgery 398,408.
Arterial thrombosis (0.5%-3.5%): this is usually the result of a technical error in making the anastomosis, although atherosclerosis, rupture of the intima, acute rejection, compression caused by haematomas or lymphoceles, hypercoagulability states, severe hypotension and immunosuppression may also participate. This manifests in anuria and worsening of renal function. It is diagnosed by Doppler ultrasound scan. Immediate surgical exploration is usually recommended, although there is little probability of finding a viable graft. If this occurs then the arterial anastomosis is disassembled to extract the coagulate before perfusing the kidney and re-anastomosing it 398,409.
Venous thrombosis (0.5%-4%): this is one of the most important causes of graft loss during the first month. It may be caused by technical errors, difficulties during surgery, or a state of hypercoagulability in the recipient. Doppler ultrasound scan will show the absence of venous flow, with an abnormal arterial signal. Surgical exploration is recommendable even though the graft is lost in the majority of occasions. If the graft is viable venotomy may be performed with thrombectomy after clamping the iliac vein. Alternatively, the graft may be taken out to extract the coagulation, after which it is perfused and re-engrafted 398,410.
Arterial stenosis (1%-25%): This is usually late-occurring. Risk factors are donor arterial atherosclerosis, donor artery trauma during harvesting, the absence of an aorta patch, suture technique (with single stitches or continuous suture) and iliac artery lesion during transplantation. Arterial stenosis may also be suspected in case of hypertension refractory to medical treatment, or an increase in creatinine. It is diagnosed by Doppler ultrasound scan and in case of doubt with angiotomography or magnetic resonance imaging technique. Stenosis above 50% is usually associated with worsening renal function. If there is stenosis <50% without clinical or analytical repercussion close monitoring is another option. If there is significant stenosis >50% the first line treatment will be angioplasty with the insertion of a vascular stent. In the case of very recent transplantation, very long stenosis or failed angioplasty then surgical treatment may be used 398,411.
Lymphocele(1%-26%): simply puncturing and emptying this relapses in 95% of cases and adds the risk of infection. Placing a percutaneous drainage may resolve the problem in 50% of cases. If the lymphocele is large and symptomatic then laparoscopic marsupialization will be indicated 398,412.
Urinary leakage (0%-9.3%): urinary leakage may be urethral or vesical. Failure of the re-implantation suture or urethral necrosis are the most common causes. Advanced recipient age, acute rejection and vesical anomalies have also been associated with this complication. The suspicion arises when the drainage volume increases and the liquid has increased creatinine. If the leakage is initial and low in volume it may be treated conservatively (with urethral catheterization or nephrostomy and double J catheter). If conservative treatment fails or leakage is massive and very initial, surgical treatment is advisable, with re-implantation or urethral anastomosis to the native urethra 398,413.
Urethral stenosis (0.6%-10.5%): early cases of stenosis (in the first 3 months after surgery) are considered to be due to defects in techniques or ischemic problems. If stenosis occurs late (>6 months after surgery) then infections, fibrosis, vascular disease or rejection may participate. Diagnosis will be based on the presence of urinary tract dilation and renal function alteration. The first step will be to place a nephrostomy catheter and perform an antegrade pyelography. Stenosis <3cm may be treated using percutaneous dilation or incision. This conservative treatment resolves the problem in 50% of cases, with the best results in stenosis of less than 1cm. In the case of stenosis >3cm or failure of minimally invasive treatment then surgery will be indicated: re-implantation, pyelovesicostomy, re-implantation with a psoic bladder or Boari Flap or urethral-ureterostomy 398,414,415.
Haematuria (1%-34%): this is usually caused by bleeding of the vessel that accompanies the urethra. Careful haemostasis during re-implantation prevents this complication. Continuous vesical washing usually resolves this problem. In the case of major bleeding the patient will be taken to the operating theatre to remove coagulations and coagulate the bleeding vessels 398.
Surgical wound infection (4%): the most frequent predictive factors for this are: obesity, age>60 years, anaemia, hypoalbuminemia and prolonged surgery. Topical daily dressings and antibiotics if there is associated cellulitis resolve the infection 398.
Eventration (4%): this occurs more often in patients who are obese, diabetic or have a history of haematoma, graft rejection, repeated surgery and use of imTOR. Repair of the hernia with the placement of a mesh will be the usual treatment 398.
Robotic KT- •
Robotic KT gives good results over the medium term and in the near future may be an alternative to open surgery (NG).
- •
RKT is restricted to selected recipients as it has a higher rate of retarded graft functioning and arterial thrombosis, and it cannot be used in patients with advanced atherosclerosis (NG).
In 2015 two hospitals carried out the first completely robotic transplants in Europe, and since then many experiences in specialized hospitals and multicentre series have been published 416,417. Robotic KT is an alternative to open surgery, and it is feasible, reproducible, safe and has surgical advantages when performing vascular anastomoses and urethral re-implantation. This is partly due to the increase in vision and great freedom of movements offered by the robot. It has also been suggested that this technique may reduce complications, the length of hospitalization and postoperative pain, while giving better cosmetic results than open surgery 418,419.
RKT involves complex surgery with multiple steps, and it requires the complete domination of robotic surgery. Surgical time should not be excessively prolonged, and proper fluid management is essential, with appropriate intra-abdominal pressures. This makes it possible to prevent the potential reduction in renal flow and pneumoperitoneum, as well as prolonged surgery 420–422. Some groups have described good results during the first year of development 423–426.
Surgical techniqueThe patient is placed in Trendelenburg 20°-30° position and sterile catheterization is performed. Five ports are introduced, one of them for the camera, and the arms of the robot are connected. Transperitoneal surgery then commences by preparing the iliac vessels and the bladder for subsequent re-implantation. A peritoneal flap is created in this first phase to retroperitonealize the kidney at the end of the procedure. Covered in a gauze with ice the kidney is inserted into the patient through a periumbilical 6cm incision that will be covered by a device (Gelpoint). The Gelpoint makes it possible to insert ice around the kidney during surgery, and also to insert the trocar of the camera through it. After clamping the iliac vein with bulldogs, a venotomy is made together with a terminolateral continuous venous anastomosis with Goretex-6/0. The manoeuvre is repeated with the artery, making a continuous terminolateral anastomosis with 6/0 Gore-Tex. The extravesical re-implantation is made following the Lich-Gregoire technique, and finally the kidney is retroperitonealized and fixed, thereby reducing the risk of pedicle torsion.
Finally, a hypogastric drainage is put into place and the periumbilical wound is closed. The drainage will be removed on the 2nd or 3rd day, depending on volume, and the vesical catheter will be removed after 5-7 days.
Complications with RKTIn spite of its good results, drawbacks have also been found with this technique, such as the impossibility of using it in patients with severe atherosclerosis, its higher-than-expected rate of retarded graft functioning and the development of arterial thromboses, even in selected patients. Early complications do not seem to be any greater than those with conventional engraftation techniques 397.
Re-transplanted recipients- •
We recommend that the free iliac fossa should be used for second transplantations, usually the one on the left side (B).
- •
We recommend that transplantectomy should be performed for a 3rd or 4th transplantation, either before or simultaneously with the engraftation (B).
A relevant percentage of recipients will require a second KT. This KT takes place in the free iliac fossa, usually the one on the left side, and technically it is no more difficult. Re-transplanted patients are known to evolve better than those who return to dialysis, in a similar way to patients transplanted for the first time.
Few series show the results of 3rd and 4th transplantations. The 3rd or 4th transplantation involves a re-transplantation in an occupied renal fossa that had been operated previously, implying increased technical difficulty: longer surgical time, an increase in bleeding and a second warm ischemia, increasing the risk of an arterial or venous lesion due to post-surgical adherences and a higher risk of repeat surgery. Depending on the location and size of the malfunctioning graft the transplantation may take place into more distal iliac vessels or in the common iliac and iliac-cava sector. For a 3rd or 4th transplantation it is usually necessary to perform a transplantectomy that may be carried out before or during engraftation (in the majority of cases). Some authors perform the 3rd or 4th intraperitoneal transplantation in the medial line using more proximal vessels, and they thereby more away from the occupied renal fossa 427. Likewise, if both iliac fossae are occupied, then an orthopaedic transplant may be indicated 428. The 3rd or 4th transplants are a valid option with acceptable results, although they are usually a surgical and medical challenge due to the high rate of complications 429,430.
Recipients with severe arteriosclerosis- •
We suggest that an aortofemoral prosthesis should be considered in recipients with very severe arteriosclerosis 6 - 12 months before transplantation, or an orthotopic transplant (C).
Severe arteriosclerosis and peripheral vascular pathology are risk factors associated with graft loss, and they also hinder surgery. It is also known that patients with chronic renal failure have thicker sclerotic plaques than the general populations, as well as thicker intima and media, especially after long periods of dialysis.
The detection of patients with vascular problems commences when they are outpatients. Patients with intermittent claudication and without pedal pulses should be evaluated by the vascular surgeon. Pre-transplantation CT-angiography will be of key importance when evaluating potential surgical difficulties 431. In patients with calcifications the surgeon seeks a segment of artery that is free of them and makes the transplantation. If any difficulty in placing the proximal and distal clamps for the anastomosis is foreseen, it will be possible to use an arterial angioplasty balloon introduced percutaneously to occlude the circulation proximal and distal to the zone selected for anastomosis.
In spite of proceeding with precaution, sometimes when making the arteriotomy a certain degree of displacement of the intima may be noticed. In these cases it is necessary to affix it with a stitch to prevent arterial dissection during reperfusion. In very severe cases it may be necessary to make an endarterectomy, fixing the distal intima to the wall 431.
Sometimes the common iliac and external vessels are completely occupied by calcifications. There are two options in these cases: to use an aortofemoral prosthesis, or to make an orthotopic transplantation. A vascular prosthesis is usually put into place from 6 to 12 months before the transplantation, and the arterial anastomosis will be made over this prosthesis. Although it is possible to make the aortofemoral by-pass and the transplantation during the same procedure, this involves long surgery and risk 431. It may be indicated in LD transplantations when the vascular and urology teams know each other and have prepared for the surgery. Orthotopic transplantation is another option in the case of diffuse calcifications 428.
Recipients with problems in the bladder and urinary tracts- •
We recommend that patients with the suspicion of functional vesical problems should be subjected to a pre-transplantation urodynamic study (B).
- •
In the case of bladders with high pressure where vesical augmentation is indicated, it is recommended that this procedure should be carried out prior to transplantation (B).
The main urological alterations which cause renal failure are vesicoureteral reflux, urethral valves, a neurogenic bladder 10%-37% and Prune Belly syndrome. The majority are therefore anomalies which appear at paediatric age, and patients will usually be transplanted before adult age 432.
On the other hand, up to 20% of adult patients who are candidates for KT will have urinary tract anomalies. The following patients will be at risk of having urinary tract anomalies: those with a neurogenic bladder, myelomeningocele, spina bifida, spinal trauma, multiple sclerosis, central nervous system degenerative diseases, cerebral vascular accident, dysfunctional bladder, bladder with fibrosis (due to radiotherapy, tuberculosis, vesical tumour), urethral stenosis, urinary derivations and infravesical obstruction 432. The pre-transplantation study of these patients should include a good interrogation, physical examination and evaluation studying vesical capacity, accommodation and emptying. The following studies will be indicated for this evaluation: 1) retrograde and urinary urethrogram, offering information that is basically about the anatomy of the urethra, vesical capacity and the presence of reflux, and 2) a urodynamic study to give information about the filling phase (vesical capacity and accommodation), the emptying phase (detrusor contraction and infravesical obstruction) 432.
Depending on the result of these examinations a post-transplantation strategy can be prepared, which may run from observation to self-catheterization or surgery to remove an obstruction. In the case of high-pressure bladders that require vesical augmentation, this will preferentially be carried out prior to transplantation 432.
In KT with an augmented bladder re-implantation will be attempted in the vesical part, making a submucosal tunnel or a Lich Gregoire-type re-implantation. In continent reservoirs using colon a submucosal tunnel will be made. In patients with an ileal conduit, the kidney will be engrafted inverted, so that the urethra is proximal and close to the urinary derivation. Bricker-type re-implantation will be used in these cases. In patients with a Mitrofanof to the navel the arrangement of the derivation should be taken into account when making the engraft 398.
12Immunosuppression in LDKTThe majority of studies in the field of KT immunosuppression have included deceased and living donors without differentiating between the results, and very little specific research has been undertaken on LDKT recipients. There is insufficient evidence to indicate whether the origin of the graft in terms of donor type modifies the risk of acute rejection or loss due to immunological reasons 433. Based on current knowledge immunosuppressant therapy during induction and maintenance should therefore be based on similar criteria in living and deceased donor KT.
Selection of immunosuppressant therapy during induction as well as maintenance will chiefly depend on the risk that the recipient will develop acute rejection (immunological risk). The KDIGO guides consider patients with one or more of the following characteristics to be at high risk of suffering acute rejection 434: one or more HLA antigen incompatibility, young or old recipients, Afro-American ethnicity (in the U.S.A.), more than 0% PRA, presence of DSA, ABO incompatibility, retarded graft functioning or more than 24hours cold ischemia. Patients who do not have any of these factors are considered to be at low immunological risk.
Immunosuppression induction therapy in LDKTInduction therapy in patients at low immunological risk- •
We suggest that induction and maintenance immunosuppressant therapy should be selected on the basis of similar criteria for LDKT and deceased donors, as there is no evidence that the origin of the graft modifies the risk of acute rejection or loss of the graft (Quality of evidence: C).
- •
We recommend that induction therapy should be combined with conventional immunosuppressant drugs, as the former is only superior to therapy with conventional drugs in reducing renal graft rejection and graft failure (A).
- •
We recommend that basiliximab should be used for induction in LDKT recipients at low immunological risk, due to its better efficacy and safety profile (B).
- •
We suggest that induction therapy with lymphocyte-depleting agents or interleukin-receptor antagonists should not be used in HLA-identical LDKT recipients (C).
- •
We suggest that at least one dose of intraoperative steroids should be administered, even in LDKT between twins without induction therapy (D).
Given that the risk of graft loss is especially high in the first months after transplantation [3] induction therapies using biological agents have been developed. There is insufficient evidence to suggest that using biological agents as induction therapy is superior to using standard triple immunosuppressant therapy in terms of patient or graft survival and long-term renal function. Nevertheless, a large number of controlled trials and meta-analyses across the whole spectrum of immunological risk suggest that the combination of induction therapy and conventional immunosuppressant agents is better than therapy solely with conventional agents in reducing rejection and failure of the renal graft 435–438.
The efficacy of induction with basiliximab 439 in patients with low immunological risk was tested in a Cochrane systematic review of 71 controlled studies and 10,537 patients 440 and in systematic reviews. Compared to a placebo, lIL-2 receptor antagonists reduced the rates of rejection and graft loss at one year, although they did not modify long-term patient and graft survival; compared with other mono- and polyclonal antibodies they were found to have a better safety profile 441. Thus in spite of the variable results obtained respecting their efficacy with thymoglobulin 442–449 and alentuzumab 450,451, thymoglobulin is associated with an increased risk of adverse effects such as leukopenia, thrombocytopenia, CMV infection and infection-associated mortality 452–454. Alentuzumab has a higher rate of acute late rejection 453, it is not available in many hospitals, and it has been associated with the risk of inducing autoimmune diseases such as thrombocytopenia and autoimmune thyroid diseases 455,456.
When the evidence in low immunological-risk recipients of living renal donors is analysed specifically, although thymoglobulin seems to be beneficial in some cohort studies, when it was compared with basiliximab 457,458, two recent prospective studies showed similar results with both of them 459,460, with a higher rate of infections by the BK virus in the thymoglobulin group 459.
A reasonable exception to the use of induction therapy would apply to recipients with an especially low immunological risk, such as those who receive a renal transplant from HLA-identical living relative. When the donor and recipient are identical twins good results have been reported after interruption of immunosuppressant therapy 461, although this treatment is maintained in many patients 462; in any case, it is recommended to administer at least one intraoperative dose of steroids to prevent the risk of immune response activation induced by ischemia-reperfusion 463. Several studies also suggest that it is also possible to avoid the use induction therapy when donor and recipient are HLA-identical but not twins, although there are no prospective trials which would show the best immunosuppressant strategy 464–466.
Induction therapy in high immunological risk patients- •
We recommend that thymoglobulin induction should be used in high immunological risk LDKT recipients (A).
- •
We recommend that basiliximab should be used as induction therapy in high immunological risk recipients when the use of thymoglobulin is not advisable (B).
At a general level there is substantial evidence that rabbit antithymocytic globulin (ATG) is superior to placebo, IL-2 receptor antibodies and horse antilymphocytic immunoglobulin (Atgam®) in patients at high immunological risk 439,445,467–470. Alemtuzumab is similar to thymoglobulin in the prevention of early acute rejection, but it obtains worse results in the rate of late acute rejection 450, patient and graft survival and the development of chronic graft nephropathy 450,468,471,472. Its lack of availability and the possibility of its being associated with the appearance of autoimmune diseases has restricted its use 452,453. Rituximab does not seem to add any benefit in the prevention of acute rejection in the first six months 473, although this has been observed in high immunological-risk patients 474. Higher mortality was shown to exist at three years 475, principally cardiovascular in origin, and this may be due to the antiatherogenic effect which may be induced by Rituximab-depleted B cells 476. Its most proven use is in ABO-incompatible transplantation, based on a systematic review of 45 studies which show results comparable to splenectomy 477.
Studies undertaken in high immunological-risk LDKT recipients also describe the superiority of thymoglobulin over anti-L2 antibodies and placebo 456,478,479. Nevertheless, in patients in which thymoglobulin is considered to be unadvisable (due to severe leukopenia or plateletopenia, for example) substitution with basiliximab may be considered in combination with tacrolimus, mycophenolate and corticoids 459.
Although the optimum dose of thymoglobulin has not been established, to ensure sufficient efficacy in the prevention of acute rejection and reduce the risk of infections or lymphomas, the total dose may be from a total of 3mg/kg to 6mg/kg, administering 2mg/kg on the first three days or 1mg/kg -1.5mg/kg during five to seven days 480–484.
Maintenance immunosuppressant treatment in LDKT recipientsInitial maintenance immunosuppression in high risk patients- •
We recommend that triple immunosuppressant maintenance therapy should be used during the first year after transplantation (A).
- •
We recommend that maintenance immunosuppression should consist of a combination of a calcineurin inhibitor (cyclosporine or tacrolimus), an antimetabolite (mycophenolate mofetil or enteric-coated mycophenolate sodium) and glucocorticoids (A).
- •
We recommend that tacrolimus should be used rather than other calcineurin inhibitors, including cyclosporine (B).
- •
We suggest that cyclosporine should be used when necessary to prevent the side-effects of tacrolimus (C).
- •
We recommend that mycophenolate should be used rather than other antimetabolic agents such as azathioprine (B).
- •
We recommend that mycophenolate should not be used in women who intend to become pregnant because of its teratogenicity (B).
- •
We recommend that azathioprine should be used as an alternative to mycophenolate in women who intend to become pregnant (C).
The most widely used immunosuppressant therapy regimes combine several drugs with different mechanism of action, to optimise efficacy and minimise side effects. Most hospitals combine a calcineurin inhibitor (cyclosporine–CsA- or tacrolimus), an antimetabolite (mycophenolate mofetil–MMF- or enteric-coated mycophenolate sodium -MPHS-), and glucocorticoids, chiefly prednisone 485–487. Some time after transplantation, in case of toxicity or due to the appearance of a neoplasia, mammalian target of rapamycin–mTOR are used, such as rapamycin/sirolimus or everolimus. However, from the first some hospitals use a combination of a calcineurin inhibitor and an imTOR. Belatacept may be used as the patient progresses in case of toxicity or poor adherence to treatment 486–488.
The effectiveness of triple immunosuppressant maintenance therapy in preventing acute rejection and preserving graft survival has been shown by several randomized controlled trials and meta-analyses 448,487,489–490, as confirmed by the KDIGO guides 434. Several studies have proved the benefit of combinations which use mycophenolate in comparison with those that include an imTOR 491,492. Use of the latter has been associated with early complications after transplantation, such as delayed graft functioning, problems with scarring and increased incidence of lymphoceles.
Of the calcineurin inhibitors, tacrolimus is clearly preferred over cyclosporine 493, even though patient and graft survival are similar, because with tacrolimus the rates of acute rejection are lower 487,494,495, and graft renal function seems to be better with tacrolimus at 1 and 2 years 455. There are some differences in their adverse effects 488,496,497, so that to avoid the side effects of tacrolimus it has sometimes been replaced by cyclosporine in a reasonably safe way 498, or by belatacept or an imTOR, although there is little evidence in favour of use the latter.
There is little evidence that delaying the introduction of a calcineurin inhibitor reduces the incidence of retarded graft function 448,499. The KDIGO guides recommend not waiting until the graft starts working to administer the calcineurin inhibitor 434.
Of the antimetabolite agents, although some cohort studies found milder gastrointestinal effects with MPHS than they did with MMF 500,501, clinical trials show that both agents are similar in terms of efficacy and safety 502,503. The 2009 KDIGO guides recommend them over azathioprine 434 as they are better to prevent acute rejection and have a better side-effects profile 504.
Although persistent diarrhea may lead to the interruption of mycophenolate, reducing or suspending this drug has been associated with a higher risk of graft rejection and failure 505,506. It is contraindicated during pregnancy as it is teratogenic, and in women who want to become pregnant it is preferable to use azathioprine, which seems to affect neither fertility nor pregnancy 507.
Respecting the glucocorticoids, in the field of KT there is no agreement on the optimum dose or the maintenance regime, which are set by each hospital depending on patient characteristics 508–512. However, Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients (OPTN/SRTR) data show that glucocorticoids are not suspended in the majority of patients during the first year 513. Suspending them in comparison with continuing at azathioprine, MMF or MPHS 514 and be linked with an increased risk of a recurrence of glomerulonephritis 515.
Initial maintenance immunosuppression in low-risk patients- •
We suggest that it is possible to suspend maintenance immunosuppression during the first year after renal transplantation between identical twins (C).
- •
We suggest that, if it is decided to minimise immunosuppression, that corticoids and/or calcineurin inhibitor drugs should be eliminated in kidney transplantation between identical twins (D).
- •
We suggest that the monozygotic nature of the donor-recipient twin relationship should be verified before deciding to minimise immunosuppression therapy (C).
- •
We suggest that calcineurin inhibitor drugs should not be used in long-term maintenance, keeping MMF and/or corticoids, in HLA-identical LDKT recipients who are not twins (C).
- •
We suggest that corticoids should not be completely suspended in low immunological risk non-HLA identical LDKT recipients, as this may be associated with a higher risk of rejection (C).
There is no agreement on the need for maintenance immunosuppression in transplantation between identical twins, in which genetics suggests there will be no recipient immune response. Registry studies show that immunosuppression is maintained in from 1/3 to 2/3 of patients, and that patient or graft survival is similar in those who maintain immunosuppression and those who suspend it 461,462. In any case, the monozygotic nature of the twins should be verified before deciding to minimise immunosuppression therapy, as 25% of non-monozygotic twins may be HLA-identical, 516 and the existence of different phenotypes between twins should not rule out the possibility of their being monozygotic, because their degree of similarity is influenced by other factors 517. The most widespread tendency in the literature when minimising immunosuppression is to eliminate the corticoids and calcineurin inhibitors to prevent nephrotoxicity 433.
Reduced immunosuppression has also been suggested in Caucasian patients who receive a living donor graft from a HLA-identical non-twin relation 465,466,518,519, chiefly based on monotherapy with mycophenolate and the suspension of corticoids. In these patients a greater graft loss due to chronic nephropathy has even been observed since calcineurín inhibitors came into use 464. Nevertheless, suspending tacrolimus and continuing with everolimus and corticoids does not seem to be acceptable, as this has been associated with a high rate of rejection 520.
Finally, in patients with low immunological risk where there is no donor-recipient HLA-identity, the use of induction therapy has been suggested, together with the suspension of prednisone during the first week after induction. This would have the purpose of avoiding its adverse effects 434, but no good quality trials are available which evaluate the long-term effect of this suspension, and some studies describe a possible association with a higher probability of graft rejection and loss 521.
Alternative drugs for maintenance immunosuppression- •
We suggest that imTOR should substitute calcineurin inhibitors when patients cannot receive the latter (C).
- •
We recommend that belatacept should be given to patients who cannot receive calcineurin inhibitors (B).
- •
We suggest that belatacept should be restricted in patients with positive Epstein-Barr virus serology (C).
Some trials suggest that the imTOR give better renal function at one or two years, although they do not improve graft survival 522,523. It also offers less long-term risk of malignity 524. Other studies associate the imTOR with increased risk of death due to a cardiovascular or infectious cause 524, dyslipidaemia, lymphoceles, medullar toxicity 522 and acute rejection 524. Although it seems to be safe when used as an alternative in patients who react to cyclosporine as toxic 525, administering it instead of tacrolimus is associated with an increase in the rejection rate 526, which has also been confirmed in LDKT 527,528.
Although Belatacept is associated with better long-term graft functioning than cyclosporine, it has a higher rate of acute rejection and lymphoproliferative disorders after transplantation, especially in patients with previous negative serology for EBV 529–531. In comparison with tacrolimus it has a higher acute rejection rate with a variable effect on long-term renal functioning 532,533, although it is an alternative in case of toxicity with calcineurin inhibitors 534,535.
13Living donor paediatric recipientsDemographic aspects of paediatric KT- •
We recommend that related LDKT should be the renal replacement therapy of choice in children, and it is recommended that it should be considered at an early stage, to avoid the transitory stage of dialysis (Quality of evidence: B).
KT is the ideal universally accepted treatment for ESRD in children, with countless advantages over other alternative renal replacement therapies such as haemodialysis in hospital or at home, or peritoneal dialysis. Paediatric nephrologists agree that early KT should take place to avoid the transitory phase of dialysis; this makes it possible to minimise the impact of renal disease while reducing institutional as well as community healthcare costs. It also has countless social and familial advantages. It is exceptional for a child to be definitively excluded form a KT scheme, and when this does occur for medical, surgical or psychosocial reasons, it is always temporary.
In spite of the above considerations, early KT is only achieved in our context in 40% of patients, and overall LDKT represents 20%-30% of all paediatric transplantations 29.
Different registries show a worldwide tendency for KT to be the most widespread paediatric therapy. Nevertheless, this varies between countries due to differences in per capita income and national policy respecting preference for donation to children 536. Thus with little variation between countries, the average incidence in Europe of replacement renal therapy in children stands at 6 new patients per year per million inhabitants younger than 14 years 537. However, the rate of KT varies quite widely: in the Scandinavian countries, France, Austria and Spain more than 85% of their population under 14 years old and in renal replacement therapy have received a KT, and this datum is harder to obtain in registries that do not include the whole national population 538,539. Europe in general has a policy of giving priority to paediatric transplantation over dialysis 540,541.
Advantages of living donation for paediatric recipients- •
We recommend that LDKT in childhood is the option which gives the best results in terms of survival in the treatment of CRD (B).
The widespread belief that KT is the best renal replacement therapy in children and should be the first choice means that the ideal donor has to be found. In general related LDKT, especially from parents to children, is normal practice in transplantation programmes worldwide, and it is almost exclusive in some countries in comparison with deceased donor transplantation. In terms of efficacy, the ONT describes LDKT as “the therapy which offers the best survival results in the treatment of chronic renal failure”542. Its use in children is justified by two needs; firstly there are too few deceased infant donor organs to meet existing paediatric demand, and there is also no preferential option for deceased adult donor KT, and secondly it is necessary to ensure the best results over the short to medium terms. Living donation must be supported by an organizational system which guarantees the absence of coercion or economic reward for donors, although these problems do not arise in donation by parents to their children.
The existence of a good national system to govern living donor programmes also makes paediatric paired KT possible, when due to donor-recipient incompatibility transplantation is impossible with the selected pair. In these cases donor-recipient pairs can be matched within a single hospital or within a strict national scheme which enables simultaneous transplantations 543,544. Apart from immunological compatibility, other advantages of living donation are that transplantation can be planned to take place at the best moment for the recipient, and donor-specific desensitization can take place for recipients with anti-HLA or another type of antibodies. Finally, it ensures better graft survival in children with anatomical vascular or urological difficulties that would make re-transplantation option far more complex or even impossible. The main contraindication against living donation is the potential risk for the donor, or the risk of relapse of the primary disease in the graft.
Paediatric recipients of LDKT- •
We suggest that the recipient should be informed about the transplantation process in a way that is suitable for their age, obtaining their informed consent and taking their opinion and desires into account (NG).
- •
The greatest difficulties in paediatric transplantations arise at the age limits of the same (new-born babies and adolescents), which are intrinsically special risk groups (NG)
Potential paediatric LDKT recipients should be treated in a paediatric hospital by specialists who are aware of all of the specific aspects associated with the primary disease as well as those intrinsic to infancy. The latter are physical (growth, development and maturing) as well as psychosocial factors which are fundamental for the transplantation process (socioeconomic status, degree of dependency, adherence to therapies, etc.). Paediatric recipients should also be informed in a way suitable for their age about the transplantation process, so that they are able to give their informed consent, while taking their opinions and desires into account.
The extreme paediatric ages (new-born babies and adolescents) are a priori the ones which give rise to the greatest difficulties when carrying out a transplantation, and they are groups at special risk 545–547.
Very young recipients of living donor transplantation- •
LDKT in extremely small recipients (in terms of their age or weight has a higher rate of primary graft failure, chiefly due to vascular factors (arterial and venous thrombosis) (A).
- •
We recommend delaying transplantation until the recipient is two years old or 10Kg (B).
- •
It is exceptionally possible to perform a KT in recipients weighing from 6 to 10 Kg in experienced hospitals, after careful study of the balance between risks and benefits (D).
Children under the age of 2 years suffer the highest rate of morbimortality in all forms of renal replacement therapy 545,548. Commencing replacement therapy during the first year of life is associated with a mortality-independent relative risk 3 to 6 times higher than other age groups 545,548.
In the paediatric population starting replacement therapy with KT has better results over the short to medium term than dialysis. However, this finding has not been completely corroborated in children under the age of two years 548,549. KT is not used universally for children under the age of 2 years and most especially those under the age of 1 year; 99% of these patients start treatment with dialysis, especially peritoneal dialysis 545,548–550.
Commencing KT programmes in extremely small recipients is associated with a higher number of vascular complications, especially arterial and venous thrombosis, as well as primary graft failure. These complications are especially frequent when the donor too is very young, and many programmes reject such young donors for small recipients 551.
Surgical considerations and perioperative and postoperative management are fundamental when performing a KT in a patient less than 2 years old or less than 10 Kg in weight 536,550,552–554. In these patients a suitable policy of haemodynamic monitoring and supply of colloid and crystalloid fluids are associated with good results, preventing early graft loss due to technical causes such as vascular thrombosis, lack of primary function or acute tubular necrosis 550–554. The few experiences when recipient weight is lower than 6 kilogrammes are generally negative 551–552.
We can therefore definitively consider recipients over 2 years old and 10 Kg in weight to be suitable for KT; until then, the best replacement therapy is peritoneal dialysis, and KT should be postponed until the above weight and/or age have been reached, if circumstances make this possible. Exceptionally (in case of peritoneal or vascular access failure or other types of risk) it will be possible to perform a KT in recipients weighing from 6 to 10 Kg in experienced hospitals, after carefully balancing the risks and benefits. Given the major risk of graft loss, KT should not be performed in recipients who weigh less than 6 Kg. Finally, performing KT in very small recipients requires a multidisciplinary team that includes highly experienced surgeons and/or urologists, anaesthetists, nephrologists, intensive care specialists, nutritionists and wide-ranging support by other paediatric specialists. For technical reasons this type of transplantation should be performed in paediatric referral hospitals.
Adolescent LDKT recipients- •
We suggest that a multidisciplinary approach should be used in patients aged from 13 to 18 years, chiefly in the process of supplying information before and after KT, to reduce the risk of non-adherence to treatment (D).
The paediatric population from 12 to 18 years old who are candidate KT recipients are also going through the gradual process of becoming independent and responsible; this makes this population especially vulnerable to non-adherence to therapy and a permanent risk of rejection 553,554. Registry data also show an increase of graft loss due to non-adherence to therapy, reaching a maximum at from 13 to 21 years old, after which it falls 555.
Establishing a temporary contraindication against LDKT in an adolescent who does not adhere to treatment would seem to be obvious. Nevertheless, prolonged treatment with dialysis does not seem to mitigate the subsequent risk of failure to comply with therapy 556. In such cases the process of informing the patient before, during and after transplantation and the support of social workers and psychologists who specialise in adolescents is useful in preventing a lack of adherence to therapy 555,556.
Given that the highest percentage of graft losses occurs during the transfer process, it is fundamental to ensure a smooth transition to adult units for these recipients.
KT from an adult donor to a very young recipient (donor-recipient disassociation)- •
Living donation by a parent to a very young baby involves a reduction in blood volume of from 33% to 50%, and the surgical approach and perioperative management are fundamental (B).
KT from an adult donor to a very small recipient is a habitual practice which requires initial higher pressure perfusion, and it may lead to a 33% to 50% reduction in blood volume, so that surgical, perioperative and anaesthesia management are fundamental 550,557,558.
Surgical approach: an adult kidney for a paediatric recipient, and most especially if it is for a very small child, will involve a size discrepancy between the donated kidney and the abdominal cavity of the recipient. This may lead to compromise of the space, hindering the creation of the vascular anastomoses and closure of the abdomen after positioning the graft, with compartmental syndrome. The extraperitoneal approach using Gibson's incision is the procedure of choice in KT to babies, for living as well as deceased donors. Nevertheless, doubts may arise when the recipient is very small or has abdominal problems of another type. An intraperitoneal approach is recommended when the recipient weighs less than 15 kilos, with the advantage of offering a larger surgical field and wider exposure of the major vessels, although it also has the disadvantage of possible intestinal obstruction 559. In any case, the approach should be selected on the basis of the conditions of the recipient and the experience in each hospital.
Vascular anastomosis: the size discrepancy is also found between the renal hilus vessels and those of the recipient, so that the best locations for anastomosis should be selected on the basis of this disparity. The arterial anastomosis may be terminolateral in the aorta, in the common iliac or in the external iliac, and terminoterminal to the internal iliac. When the recipient weighs less than 20 kilogrammes we recommend making the anastomosis in the aorta and inferior vena cava 558. If they weigh from 20 kg-30kg, the recommend site is the iliac artery and common iliac vein. When the graft has a double artery, if both are separated then both anastomoses are independent, but if they are close, they will be joined in bench surgery, suturing the accessory artery to the main artery.
Abdominal wall closure: closure of the abdominal cavity after transplantation may be compromised and require delayed closure in 48 or 72hours. When the disassociation between the donor and recipient is very striking, closure of the wall may cause an increase in abdominal pressure which may collapse the inferior cava and hinder the venous return from the graft. This may progress until thrombosis occurs (compartmental syndrome). In these cases positioning a Gore-Tex sheet sutured to the edges of the wound may prevent this complication, permitting the gradual closure of the wall on subsequent days 558,559. This gradual closure of the surgical wound edges will take place under Doppler ultrasound scan monitoring.
Perioperative management: to maintain correct perfusion pressure and reduce the risk of vascular thrombosis, perioperative management should include the administration of intravenous fluids (crystalloids and/or colloids) and inotropic drugs, together with correct haemodynamic monitoring 552:
- -
Monitoring central venous pressure with a central venous catheter (12-18cm H2O) and direct monitoring of arterial pressure.
- -
Due to the risk of retarded graft functioning, the average arterial pressure obtained after 10minutes of graft reperfusion should not be lower than the pressure when the clamps were removed.
- -
The ratio between the average arterial pressure and weight should be greater than 4.3 in patients who weigh from 13 to 21 Kg (56–90mmHg), and ideally greater than 2.5 in patients who weigh more than 22 Kg.
- •
Although graft survival 5 years after LDKT from a donor over the age of 50 years is less than that of transplants from young living donors, it is better than any graft from a deceased donor, including young ones (B).
- •
We suggest that living donors over the age of 50 years should be assessed on an individual basis for a paediatric recipient, as the graft survives for a shorter time, even though this survival time is longer than that of any graft from a deceased donor, including young ones (D).
A tendency has been observed in international registries over recent years for more renal transplants to be used from donors over the age of 50 years for paediatric KT 560. Although survival at 5 years is less than that corresponding to young LDKT, it is better than that of any graft from a deceased donor, including young ones 554,557,561–563. Long-term graft survival becomes poorer to the degree that donor age increases over 50 years 554,557,563.
Complex vascular anomalies in LDKT and paediatric recipients- •
Occlusive thrombosis of the inferior cava vein and mid aortic syndrome require planning of the transplantation which includes selection of the optimum donor and planning of the surgical approach, which has to be adapted to the anatomy of the recipient (D).
Of the vascular anomalies which compromise transplantation into a paediatric recipient, occlusive thrombosis of the inferior cava vein is one of the most challenging situations, and although rare, it is the most frequent such anomaly 564–566. The other complex vascular anomaly is mid aortic syndrome, which is usually progressive during infancy but tends to stabilise during puberty, so that transplantation should be delayed as far as possible or may not even be feasible. Planning a transplantation should include the selection of an optimum donor and plan the surgical approach, deciding on the best anatomical site for anastomosis based on recipient anatomy.
All KT candidates must be subjected to previous vascular evaluation by iliac-cava Doppler ultrasound scan. Those patients with a suspicion of venous thrombosis or arterial anomalies should also be subjected to other more specific imaging tests such as CT-angiography, magnetic resonance angiography or transjugular retrograde venography to permit planning of the surgery. It is also important to determine whether any risk factors for hypercoagulability are present.
In inferior cava vein thrombosis the veins which are collateral to the thrombosis should be avoided as venous graft drainage may be compromised, favouring the thrombosis. Possible vascular structure are: the ovarian vein, the left renal vein, the superior or inferior mesenteric vein, the portal vein or even deeper veins such as the lumbar veins. As the first option, and on condition that it is thrombus-free, we should use the infrahepatic cava vein, placing the graft in orthotopic position on the left side 565, performing left nephrectomy and using the native renal vein for the anastomosis to the renal vein of the graft, with anastomosis of the renal artery of the graft directly to the aorta. If the renal vein of the donor is short, a lengthening procedure may be used; either with the cava of the donor or with a segment of gonadal vein 564,565. The urethra will be re-engrafted using modified Lich-Gregoir technique if we have sufficient length, or by means of urethra-ureterostomy between the donor urethra and the native one, if the first is too short 558,559,567.
During the postoperative phase anticoagulation should be used with heparin, followed by platelet antiaggregation, as well as ensuring venous permeability by Doppler ultrasound scan immediately after the operation and magnetic resonance imaging and/or vascular tomography 3 months after the transplantation.
Complex urological anomalies and treating them in LDKT and paediatric recipient- •
We recommend pre-transplantation urological evaluation using cystourethrography to identify anomalies and treat them before engraftation (B).
Patients with a neuropathic bladder, posterior urethral valves, complex urological malformations and urological problems associated with anorectal malformation, such as cloacal fistula are common in KT candidates. These anomalies may endanger graft survival, due basically to the vesical dysfunction which accompanies them. Pre-transplantation urological evaluation is therefore a requisite for a LDKT, allowing us to identify problems and duly treat them prior to engraftation 568. Cystourethrography will be the first test used in this evaluation.
If there is a suspicion of vesical dysfunction we will perform a urodynamic study to confirm whether the bladder is dysfunctional and identify the type of problem (hyperreflexia, low accommodation or myogenic failure). High grade urethral vesical reflux in native kidneys favours repeated post-transplantation infections and should be treated, surgically or endoscopically, before transplantation 558,559,567,568.
Correction of vesical dysfunction will require specific treatments prior to transplantation, such as the use of anticholinergic drugs and/or intermittent catheterization, the injection of botulinum toxin, vesical augmentation or continent urinary diversion.
Vesical hypoplasia or urethral agenesis and atresia require reconstruction of the urinary tract before transplantation, constructing a neobladder from a colon or ileal segment, and a Mitrofanoff-type drainage tract 564,569,570.
Cancer in the paediatric recipient- •
The time during which LDKT is contraindicated in a malign process should be evaluated jointly with Oncology depending on the type, grade and extension of the tumour (D).
There is no evidence or agreement on how long the waiting time should be before receiving a transplantation after the complete cure of a neoplasia. The most advisable strategy is currently therefore to evaluate the risk of relapse together with oncologists, according to the type, grade and spread of the malign process, deciding the necessary waiting time on an individual basis 571. Modern genetic studies are now very useful aids in distinguishing between different biological subtypes and thereby help in decision-making.
Wilms’ tumour is an exceptional case, as the transplantation candidate may be considered to be a valid recipient immediately after a complete cure, without having to wait the traditional 1 - 2 years 567.
Intellectual retardation and the paediatric recipient- •
LDKT is not contraindicated in recipients with a severe intellectual deficit, as there are no differences in graft survival between them and other patients. The final decision will be based on agreement between the transplantation team and the parents (NG).
The decision to perform a KT in a paediatric recipient with severe psychomotor retardation is always difficult, and it should be adopted by all of the members of the multidisciplinary transplantation teams and the parents, who are responsible for the patient's independence 572. Given that patient and graft survival are similar to those for other patients, the fact that a severe intellectual deficit does not contraindicate receiving a KT is perfectly valid 573. Absolute contraindications derive from the association between psychomotor retardation and other concomitant situations which in themselves are each a complete restriction 571–576.
Obesity and the paediatric recipient- •
We suggest that LDKT should be delayed in recipients with a BMI higher than 35 Kg/m2 due to the increased risk of graft loss, stimulating weight loss by means of habits that modify their lifestyle (D).
Data from the European registry indicate that Spanish children who receive KT are often overweight and obese 577, due in part to the changing Western lifestyle. Certain hereditary pathologies such as Prader-Willi or Bardet-Biedl syndromes are associated with renal failure subsidiary to replacement therapy and specific problems with obesity which have to be evaluated, defined and treated before transplantation.
Potential recipients should be assessed before transplantation in nutrition units, changing their diet and stimulating physical activity and sport. Recipients with a BMI>35 Kg/m2 are at increased risk of graft loss ten years after transplantation 578, so that they should be encouraged to lose weight. The decision to perform a KT in an obese paediatric recipient should be based on team decisions.
ABO incompatible living donor KT and paediatric recipients- •
Although it is ideal for a paediatric recipient to receive an isogroup donation, ABO incompatible LDKT in a group O or B paediatric recipient with low isoagglutinin levels is an option that can be considered (C).
Although there is no evidence which specifically supports the paediatric indication, ABO incompatible LDKT in a group O and B paediatric recipient with low isoagglutinin levels against the potential donor, and using plasmatic exchanges, immunoabsorption or rituximab, it is a reasonable option in cases with large increases in waiting list time 579–581. In recent years it has been found that patients with low isoagglutinin titres (<16) can receive an ABO incompatible transplantation without the need for plasma exchanges 582, although there is no evidence to indicate which isoagglutinin levels permit transplantation without previous treatment 583–586.
Living donor KT in highly sensitized paediatric recipientsThe presence of preformed anti-HLA antibodies drastically worsens the result of KT in children as well as in adults 562,563. Although desensitization programmes give good results over the short to medium terms, the long-term prognosis is still disappointing. Several countries have developed national or international programmes with the aim of prioritizing transplantation in these patients by using a virtual crossmatch 587–591.
LDKT in highly sensitized children is no different from the situation in adults, and it would be the therapy of choice if the potential donor had no antigen prohibited by recipient antibodies. Otherwise, a child with the presence of a titre above 98% against the panel could be included in the national scheme of highly sensitized patients 591, while those patients with preformed antibodies but with a lower titre could be included in the deceased donor waiting list, while evaluating the possibility of paired LDKT or even using a specific desensitization protocol, depending on the patient's situation and how long they have spent in dialysis 592,593.
Absolute contraindications in paediatric recipientsThere are few absolute contraindications against performing a KT in the paediatric age 567,573–576: malign neoplasia in the previous 12 months, pulmonary disease which requires continuous oxygen therapy, irreversible heart failure, active infection, a refusal to complete the normal vaccination schedule or inappropriate social support for post-transplantation treatment.
Considerations on choice of a live donor for childrenLaparoscopic harvesting has less morbidity than open nephrectomy for the donor, and the functional results of the graft are the same (B).
Age and social aspects: 90% of living donations in childhood are made by the parents, and the factors limiting age are described in a previous section. The selection of one or the other parent if they are equally compatible and healthy is their own choice after they have been informed of their level of compatibility. All of the psychosocial and economic factors which influence donation should also be taken into account 594. In living donation for a paediatric recipient other family donors or donors with emotional ties are also accepted: compatibility must be taken into account, and the advantages must be weighed in comparison with a deceased donor transplantation 563,593. Although donation by a young sibling (under the age of 25 years) is not contraindicated, this requires a far more exhaustive evaluation to prove their maturity. Sometimes we find single-parent families without any social support, and these situations would lead us to advise against LDKT. In any case, it is fundamental to offer psychological support before and after donation, and although LDKT may strengthen positive emotions between the donor and recipient, it may also lead to tensions and anxiety, above all in adolescent recipients 594.
Aspects of surgery and the harvesting: the main limitation of LDKT for paediatric recipients is the size discrepancy between donor and recipient vessels. To guarantee a suitable flow to the kidney the recipient artery for the anastomosis should be twice the width of the renal artery of the donor, or at the least the same size 595. In children who weigh less than 15 kilogrammes it is often necessary to wait for a time before being able to perform the transplant with assurance of success. The presence of vascular anomalies or multiple pedicles increases the risk of graft thrombosis; thus when there is a double artery and it is close to the main artery, the accessory artery anastomosis to the main one should be terminolateral, with a single anastomosis in the recipient. If both arteries are a certain distance apart it will be necessary to make the anastomoses separately. There is no established limit on the maximum number of arteries over which a kidney should be rejected. The surgeon's criterion and experience will decide if a transplant is possible. Renal angiotomography reconstruction in three dimensions, including the excretory phase, is the method of choice for evaluation of the arterial and venous anatomy and the parenchymal and collector system of a potential living donor 596.
The left kidney is usually collected, as if they are both equally suitable this usually facilitates the vascular anastomoses, while otherwise the rule is that the best kidney should be left in the donor. Laparoscopic nephrectomy is preferred over open surgery for renal harvesting from a living donor 597, as it causes less donor morbidity and has similar functional results. The greatest problem is that it is harder to obtain renal vessels of an appropriate length, above all the renal vein, and this occurs more often with the right kidney. A hand-assisted laparoscopic approach improves traction on the kidney and obtains a more suitable vessel length. Positioning the graft inverted in the recipient may also facilitate the vascular anastomoses in a graft with short vessels.
14LDKT with HLA incompatibilityKT is the best therapeutic option for chronic renal failure 598, and LDKT offers better results than those made from a deceased donor 599, nevertheless, the number of LDKT has fallen in Spain in recent years 600.
The number of recipients who have been highly sensitized by pregnancy, transfusions or previous transplants is now increasing in Spain 601,602. This tendency is similar to the reported trend in countries such as the U.S.A. 15, while it is less pronounced than is the case in Korea 603 or the United Kingdom 604. The definition of a sensitized patient is not precise, although the presence of antibodies against the donor is considered to be a risk factor for the development of antibody-mediated rejection, making transplantation unadvisable 317. Less than 30 years ago candidates with more than 50% PRA were considered to be hypersensitized 605. Currently in the U.S.A. figures above cPRA>80% are defined as highly sensitized, while >99% is defined as very sensitized 606; the corresponding figure in Eurotransplant is 85% 607 and in other countries it stands at around 80% 608–610. In Spain there is a national scheme for hypersensitized patients which sets a minimum level of 98% cPRA for inclusion 602.
The transplantation options for highly sensitized patients vary, depending on the availability or not of a living donor. If there is no incompatibility then transplantation will proceed. If on the contrary there is incompatibility between the donor and recipient then the options include inclusion in the waiting lists for paired donation, desensitization therapy or inclusion in specific deceased donor waiting lists. Desensitization protocols have been designed in the last two decades based on treatment with intravenous immunoglobulin (IVIG), different apheresis techniques and B lymphocyte depletion agents or plasma cells. Recently new desensitization methods have emerged, including complement blocking agents, or Imlifidase (endopeptidase) which is capable of cleaving G immunoglobulins, that are able to reduce the incidence of acute rejection, although the mid-term prognosis is unknown 348.
Desensitization for HLA-incompatible LDKT- •
We suggest that transplantation candidates with more than 80%-85% of cPRA antibodies against potential donors should be considered sensitized (Quality of evidence: C).
- •
We suggest that LDKT candidates and their donors should be informed of the different options for HLA-incompatible transplantation (paired, desensitization or specific lists with preference for a deceased donor) (C).
- •
The decision to perform an HLA-incompatible transplantation by using a desensitization protocol should be considered depending on the characteristics of the recipient, the donor and time in the waiting list, in comparison with the increased risk of antibody-mediated acute rejection and other complications due to over-immunosuppression. (NG).
- •
We recommend a desensitization protocol which includes rituximab, IVIG and apheresis techniques (immunoadsorption or plasma exchange) (B).
- •
We recommend adding anti-CD20, as it increases the probability of accessing a transplantation (A).
- •
We do not recommend substituting rituximab with bortezomib, due to its toxicity and poorer results (B).
- •
As there are no studies which compare different IVIG preparations (non-specific or CMV hyperimmune) it is not possible to recommend one or the other (NG).
- •
The inclusion of eculizumab in the usual desensitization protocols reduces the incidence of short-term antibody-mediated acute rejection (B).
- •
Imlifidase makes it possible to transform a highly positive crossmatch tests into a negative one in 6hours (C).
- •
We suggest that LDKT HLA-incompatible candidates who are not suitable for desensitization should be included in specific deceased donor transplant lists for highly sensitized candidates (C).
Desensitization consists of removing pre-existing antibodies from the body using apheresis techniques, blocking existing ones or their actions by using IVIG, preventing the formation of new ones by blocking B-cell proliferation and preventing recognition and an immune response by means of immunosuppressant therapy. There are several desensitization protocols, and in general they are based on the use of rituximab, IVIG and apheresis techniques (2,611) New drugs have been added in recent years, including eculizumab and imlifidase, and these reduce the incidence of acute rejection. A conditioning regime is shown in Figure 13.a) B-cell depletion agents
The most widespread form of desensitization. Rituximab commences 31 days before the planned date for transplantation. From 7 to 14 days before transplantation the patient is admitted to hospital and PPHS or IA commences until the crossmatch is negative. 100mg/kg of CMV specific Igs is infused after each session of PPHS/IA. Immunosuppression also commences with tacrolimus, mycophenolic acid derivates and prednisone. Prophylaxis is given after transplantation, with valganciclovir, septrin, itraconazole or inhaled amphotericin. Basiliximab or thymoglobulin is given for induction, and PPHS takes place on days +3,+5 and +8. (Ref: 614).
The most widely used regime at the present time consists of using rituximab for one month prior to commencing apheresis. It functions 2-3 after infusion, with a peak at from 2 to 4 weeks, and this effect may be maintained for up to 12 months 612. The dose used in the majority of protocols is 375mg/m2 body surface, and premedication with paracetamol, steroids and antihistamines to prevent adverse secondary reactions. Some authors use bortezomib, a plasma-cell depletion agent, with different results 611, although with a high incidence of side effects 613.b) Immunosuppression
Immunosuppression starts one or two weeks prior to transplantation, with tacrolimus (0.15mg/kg to achieve levels of 8-12ng/ml in the first month), MMF at a dose of 1g every 12hours or 720mg every 12hours of MPHS and 20mg/day prednisone 614.c) Apheresis
In patients with no vascular access (pre-dialysis or peritoneal), it is necessary to catheterize a central vein (preferably the internal jugular vein) 615 for apheresis with or without dialysis. If the technique of centrifugal plasma exchange is used then this can be applied using a peripheral vein. The apheresis techniques used are PPF or plasma exchange, double filtration PPF and immunoadsorption (IA). The advantage of IA over PPF is that it does not remove plasma, so that plasma proteins including albumin and coagulation factors are not lost in the exchange, so that replacement is not required, as it is with PPF 616. The drawback is that it filters IgG 3 less efficiently and it is also more expensive; both techniques are usually combined 617. The volume of plasma used in the exchange tends to vary at from 1 to 2.5 volumes per session, while the number of sessions varies and generally depends on the degree to which the patient is sensitized. When the crossmatch tests with the donor for CDC and CF are negative and the NFI of the donor-specific antibody or antibodies falls below 1000 the patient is considered to be desensitized and transplantation takes place 614,618.d) Immunoglobulins
After each apheresis session in which some antibodies have been removed, IVIG is administered with the aim of neutralising the antibodies and modulating the immune response 619. IVIG reduces the incidence of acute rejection and graft loss in sensitized patients 620,621. Two versions are marketed, non-specific IVIG and hyperimmune IVIG CMV. There are no studies which compare the efficacy of both versions. One study compared historical series of candidates treated with high doses of IVIG or low doses plus PPF, and the results of the latter group with PPF were better 622. Non-specific IVIG is used in doses of 500mg/kg weight - 2000mg/kg weight 623, with a maximum dose of 150g, and a larger volume is required for administration. Anti-ABO isoagglutinins are usually included in its presentations, and premedication is required to prevent adverse reactions 624. CMV-specific IVIG is used in a 100mg/kg dose 625–627, so that the volume administered is considerably smaller. It is highly purified and has CMV and Epstein Barr virus neutralising antibodies, so that it may offer beneficial results in terms of the short-term prevention of opportunistic infections 628. In economic terms the results seem to favour using non-specific IVIG, although no cost-effectiveness studies have been performed.e) Eculizumab
Eculizumab is a humanized monoclonal antibody against the C5 fraction of the complement, preventing it from cleaving and blocking the opsonizing and inflammatory capacity of C5a and the formation of membrane attack complex C5b-9 629. Use of Eculizumab during 4- 9 weeks is associated with a reduction in the incidence of antibody-mediated acute rejection 630,631, although an increase in the MFI of the DSA has been detected, together with a higher frequency of transplantation glomerulopathy. This drug is tolerated well, although patients have to be vaccinated beforehand against meningococcus, pneumococcus and haemophilus.f) Imlifidase.
Imlifidase (endopeptidase) is a drug collected from Streptococcus pyogenes, and it functions like scissors, cutting the bonds which join the different chains of the immunoglobulins, turning a positive crossmatch test into a negative one in 6hours. Data from the first international studies show that it is possible to transplant into sensitized patients with a reduced incidence of rejection in comparison with the usual protocols 632,633.g) Others.
Tocilizumab is a monoclonal antibody against the IL-6 receptor, and in initial studies it improved the results in comparison with previously used desensitization protocols, although we do not have enough information about its use in clinical practice 634.
Other drugs are under development 635, and these may improve current results. These drugs include the administration of a complement C1 fraction inhibitor (C1Inh) although it is still in phase I/II 636.h) Induction
Once successful desensitization is considered to have been achieved then the transplantation takes place. Induction therapy with basiliximab or thymoglobulin is administered, depending on the protocol and groups 614. Some protocols include the use of alemtuzumab instead of thymoglobulin 623,637.i) Prophylaxis
The aim of desensitization protocols is to reduce the immune response, so that opportunist infections may increase. In spite of this the prophylaxis does not differ very much from what is used in transplantations without desensitization: cotrimoxazol during 3-6 months and valganciclovir during from 3 to 6 months, basically in seropositive donors and CMV seronegative recipients. Antifungal prophylaxis will follow local practice; some hospitals use oral itraconazole or inhaled amphotericin during 3-4 months 614.
Patients who receive eculizumab should be vaccinated against meningococcus, haemophilus and pneumococcus, and they should receive oral penicillin until at least one month after treatment has ended, as recommended for patients with aHUS 638,639.
Results- •
The incidence of antibody-mediated acute rejection is high in patients who receive a transplantation after desensitization (B).
- •
Survival is lower in patients transplanted after desensitization than it is in patients with a compatible LDKT (B).
- •
Survival is higher in HLA-incompatible patients transplanted after desensitization than it is when they remain in dialysis and are transplanted afterwards, or when they remain in dialysis and the waiting list (B).
A retrospective multicentre study of hospitals in the U.S.A. found that the results in terms of transplanted patient survival after desensitization are better than those corresponding to patients who remain in dialysis before transplantation, and those who remain in dialysis and do not receive a transplantation 640. Other studies do not find these differences between the groups of those who were transplanted or in the waiting list 604. This may be explained by differences in definitions, desensitization, the method used to select the control group, the population and mortality in dialysis, which is higher in the U.S.A. than it is in Europe 49. In waiting list patients with high cPRA, this is an independent risk factor for mortality in transplantation candidates 641,642.
The probability of accessing transplantation after desensitization varies from 37% to 96%, depending on the protocol used, the level of sensitization and group experience 611,614,622,623,630,643–649. A review found that the probability of transplant was 90.7% when PPF was used, 95.5% when IA was used, 73% with the use of high dose IVIG without apheresis and 30.8% with bortezomib 611. The most experienced group with desensitization is probably the Cedairs Sinai group, Los Angeles. Vo et al. have transplanted 45/56 (80%) of the waiting list patients with PRA >80% with a living donor using desensitization, as opposed to only 2% per year in the waiting list without desensitization. They were unable to transplant 9/56 (16%) of the candidates 623. There is little information on the frequency of transplantation in patients where desensitization had failed.
When bortezomib is used, the probability of transplantation varies from 43% to 84% 613,650,651. It was necessary to suspend bortezomib due to adverse effects associated with bortezomib in 11.5% of patients, and in 15.4% without association with bortezomib.
Survival and rejectionIn successive series patient survival is higher than 90% at 1-5 years and 80% at 8 years, while graft survival is higher than 90% at one year and from 60%-70% at 5-8 years, in spite of high acute rejection rates 611,614,618,623,630,637,643,646,652–655.
Recent publications show the results of using endopeptidases (Imlifidase) in 46 patients, the majority with a deceased donor (n=39), although patients who had received a LDKT were also included (n=7). 85% of patients had a positive crossmatch test, DSA in 93% of cases and cPRA >97%. The protocol also includes the use of rituximab and IVIG. Patient and graft survival are 100% and 96% at the 6th month, respectively, with a 27% incidence of antibody-mediated rejection 632,633. Adding eculizumab to the usual desensitization protocol makes it possible to reduce this rejection rate to 12%-15% in the first year, with renal survival higher than 90% 630,654.
In an experience of the Mayo Clinic in patients with DSA MFI >1000 and negative CF, 75% with LDKT, shows a 0% incidence of antibody-mediated rejection. The incidence of chronic antibody-mediated rejection was 17% and patient survival was 96.3%, while graft survival (death censored) was 86% after an average follow-up of 4 years 637. These patients were not subjected to desensitization, so that the authors consider that certain patients could benefit from a transplantation, especially one from a living donor, rather than remaining in the waiting list for a donor with a completely negative crossmatch test (negative DSA, MFI<1000).
It is possible to apply desensitization protocols in deceased donor KT with IA in the immediate postoperative period. If the crossmatch test was positive pre-IA but negative post-IA, the patient was transplanted. 101 patients were transplanted with this protocol, 27 of them were CDC positive pre-IA and negative post-IA. The incidence of antibody-mediated rejection stood at 41%. Death censored renal survival was worse in the patients with DSA than in the controls without DSA 656.
Some authors have reported the results of a combination of desensitization and paired LDKT, with a small number of patients, although they were able to transplant candidates with 100% cPRA 657,658.
Table 12 shows a summary of the latest publications on the results of desensitization.
Summary of living donor desensitization protocols.
| Author | YEAR | N°. (follow-up) | AAMR (%) | ATMR (%) | PAT. S (%) | RENAL S (%) | Method |
|---|---|---|---|---|---|---|---|
| Stegall (Mayo Clinic)1 | 2006 | 61 (12) | 37 | 93 | 82 | CDC | |
| Magee (Boston)2 | 2008 | 28 (22) | 39 | 42 | 96 | 89 | CDC/FC |
| Thielke (Illinois-Chicago)3 | 2009 | 51 (23) | 24 | 43 | 91 | 81 | CDC |
| Rogers (Adelaida-Australia)4 | 2011 | 10 (12) | 0 | 30 | 90 | 90 | CDC/FC/Luminex |
| Montgomery (Baltimore)5 | 2011 | 211 (96) | 86 | ||||
| Morath (IA) (Heidelberg. Germany)6 | 2012 | 10 (19) | 30 | 20 | 100 | 90 | CDC/Luminex |
| Vo (Cedairs Sinai- L.A.)7 | 2013 | 46 (48) | 22 | 29 | 96 | 92 | CDC/FC/Luminex |
| Kute (BORT) (India)8 | 2011 | 29 (10) | 10 | 24 | 100 | 88 | CDC/FC |
| Riella (Boston)9 | 2014 | 39 (60) | 61 | 23 | 84 | 86 | CDC/FC/Luminex |
| E. De Sousa-Amorin (Hospital Clinic)10 | 2015 | 24 (37) | 41 | 21 | 86 | CDC/FC/Luminex | |
| Okada (Japan)11 | 2019 | 15 (48) | 60 | 93.3 | 85.6 | CDC/FC/Luminex | |
| Jordan (IdeS) (LA+Lund +Paris)12 | 2018 | 35 (6) | 25 | 100 | 94% | CDC/FC/Luminex | |
| Winstedl (IMLIFIDASE) ESOT 201913 | 2019 | 46 (6) | 27 | 100 | 93.5 | CDC/FC/Luminex | |
| Marks (Eculi vs PP+Igs)14 | 2019 | 102 (36) | 11,8vs29,4 | 98 vs 98 | 91.8 vs 78.5 | CDC/FC/Luminex | |
| Fernandez (A Coruña)15 | 2017 | 23 (43) | 8,6 | 8,6 | 91.8 | 85 | CDC/FC/Luminex |
| Kim (Korea)16 | 2019 | 17 (37) | 47 | 100 | 85 | CDC/FC/Luminex |
N (FOLLOW-UP): No. of patients included and (months of follow-up). AAMR: acute antibody-mediated rejection. ATMR: acute T-cell mediated rejection. PAT. S: patient survival. RENAL S.: graft survival, Method: Methodology used to measure sensitization: CDC Antibody-mediated cytotoxicity, FC: flow cytometry, Luminex
Stegall MD. Am J. Transplant. 2006; 6: p. 346-341.
Magee C. Transplantation. 2008; 86: p. 92-103.
Thielke JJ. Transplantation. 2009; 87: p. 268-273.
Rogers NM. Transplant Int. 2011; 24: p. 21-29.
Montgomery, RA. Am J. Transplant 2010; 10: 449–457
Morath C. Transplant Internat. 2012; 25: p. 506-517.
Vo AA. Transplantation. 2013; 95: p. 852-858.
Kute VB. Saudi J Kidney Dis Transpl. 2011; 22(4): p. 662-669.
Riella LV. Transplantation 2014; 97: 1247-1252.
De Sousa-Amorim E. Transplant Proc. 2015; 47: 2332e2335.
Okada D. Transplant Internat. 2018; 31: 1008-1017.
Jordan S. NEJM. 2017; 377: 442-453.
Winstedt L. Transplant Int. 2019; 32(S2): 62.
Marks WH. Am J. Transplant. 2019; 19: 2876-2888.
Fernández C. Nefrologia. 2017; 37(6): 638-645.
Kim DG. BMC Nephrology. 2019; 20: 456.
- •
Desensitization protocols may expose patients to a higher incidence of infections and other adverse effects (NG).
- •
The costs of HLA-incompatible LDKT are higher than those for a HLA-compatible donor, although it is cost-effective in comparison with remaining in dialysis (NG).
The number of infectious complications (CMV, BK, urinary infection) is slightly higher in the group of sensitized patients than it is in those who are not sensitized. Few data exist on the incidence of EBV, and it is even possible that IVIG has a protective effect 603,614,632,640,643,646,655,659–664 (Table 13).
Main infections after desensitization.
| Autor | CMV (%) | Urinarias (%) | BK (%) |
|---|---|---|---|
| Khwaji (L.A.)1 | 15 vs. 10 | 34 vs. 31 | 10,6 vs. 5,5 |
| Jeong (Korea)2 | 5,3 | 10,5 | |
| De Sousa (H Clinic)3 | 13 | 85 | 4,3 |
| Morath (Heidelberg)4 | 10 | 20 | 20 |
| Thielke (Illinois)5 | 7 | 4,9 | |
| Niederhaus (Wisconsin)6 | 15 | 0 vs. 5,6 | 21 |
| Okada (Japón)7 | 20 vs. 11 | 0 vs. 1,6 | |
| Jordan (USA+Suecia)8 | 0 | 0 | |
| Fernández (A Coruña)9 | 26 | 33 | 4 |
| Toyoda (L.A.)10 | 16 vs. 25 | EBV: 2,9 vs. 11,3 | 11,2 vs. 13 |
| Amrouche (Necker)11 | 9 | 75 | 10 |
| Kim (Corea)12 | 64,7 vs. 48,7 vs. 13,9 | 23,5 vs. 51,5. 7,7 | 0 vs. 5,1 vs. 6,6 |
Kahwaji J. Transplantation. 2011; 91: 225-230.
Jeong JC. Medicine. 2016; 95(5): e2635.
De Sousa-Amorim E. Transplant Proc. 2015; 47: 2332e2335.
Morath C. Transplant Internat. 2012; 25: 506-517.
Thielke JJ. Transplantation. 2009; 87: 268-273.
Niederhaus SV. Transplantation. 2011; 92: 1-17.
Okada D. Transplant Internat. 2018; 31: 1008-1017.
Jordan S. NEJM. 2017; 377: 442-453.
Fernández C. Nefrologia. 2017; 37(6): 638-645.
Toyoda M. J Immunol Res. 2017; 2017:5672523.
Amrouche L. Transplantation. 2017; 101:2440-2448.
Kim DG. BMC Nephrology. 2019; 20: 456.
Other complications have been reported in connection with desensitization protocols that are especially linked to the use of apheresis techniques and other medications 639.
HLA-incompatible LDKT is more expensive than a compatible KT, due to the use of B cell depletion agents, apheresis, IVIG, induction, immunosuppressant medication and the time spent in hospital 665. Although its total cost is higher, LDKT is cost-effective in comparison with remaining in dialysis 623,666. The costs of additional medication such as eculizumab have not been evaluated.
ConclusionsTo summarise, desensitization now makes it possible for 70-90% of the patients who commence the protocols to access transplantation, with high patient and renal survival, in spite of an increase in the incidence of antibody-mediated rejection, which may be reduced by using Imlifidase or eculizumab.
The possibility of a highly sensitized recipient accessing a HLA-compatible transplant by means of paired transplanted would be an excellent option. Nevertheless, the probabilities of transplantation are low, especially for those with a cPRA of 97% or more 667. Specific KT programmes for hypersensitized patients at a national level make it possible to transplant many patients who cannot find any possibility of accessing paired transplantation and who also cannot be desensitized. The possibility of using this option falls considerably when the cPRA is above 99.9%.
Deciding on one option or the other basically depends on weighing up the particular situation of a candidate and their donor in terms of blood group and cPRA, as this will influence the time spent in the paired donor scheme waiting list, or the special deceased donor list. With desensitization there is a higher probability of transplantation within less time, in spite of a high incidence of rejection. The British guides contain a simulator where depending on the blood group of the donor and recipient, the state of sensitization and age, the probability of a paired transplantation is calculated depending on the level of HLA sensitization and ABO group, with predicted survival (2). Software has recently been developed to aid the management of a paired donation 668.
Finally, desensitization is not possible in a considerable percentage of patients due to their high cPRA or MFI. Moreover, due to their high cPRA it is hard to find a suitable donor in the lists for paired KT or even in the deceased donor lists with specific programmes. The inclusion of compatible donors in the paired donation list, the acceptance of A2 groups for O recipients, the reduction in the unacceptable donor threshold and transnational programmes all increase the possibilities. Another option could be to desensitize patients to try to reduce their cPRA and have more options for transplantation. The results with Imlifidase are hopeful, with the aim of achieving desensitization that is 100% effective.
15Living donor with ABO incompatibilityOf the different options for renal replacement therapy, LDKT offers the highest patient and graft survival rates, a better long-term quality of life and lower economic cost 669. In the donor-recipient selection process, blood group incompatibility (ABOi) has traditionally been considered to be an absolute contraindication for donation. However, it has been known for a number of years that transplantation from an ABOi donor gives excellent results. Unlike HLA incompatibility, in an ABOi transplantation (once the desensitization process has been successfully completed) clinical evolution is similar to that of ABO-compatible transplantation (ABOc).
The effectiveness of ABO-incompatible LDKT- •
We recommend ABOi LDKT as it gives results that are comparable to those achieved by ABOc LDKT (Quality of evidence: B).
- •
We suggest that the option of ABOi transplantation should be considered in hospitals that perform LDKT to increase the opportunities for transplantation (NG).
The rule that donation should be avoided in case of blood group incompatibility has been abandoned and successfully surpassed 670. Poor initial results 671 meant that this option was side-lined until the 1990s, when different groups, especially in Japan 672,673 reported excellent results in series with a large number of patients. This led to its use by groups in the U.S.A. 674,675, France 676 and Sweden 677,678. In Spain the first ABOi LDKT took place in 2006, in the Hospital Clínic, Barcelona, and the good initial results were confirmed by other groups 679–683. The Hospital Universitario in A Coruña 684 soon tried this option, followed by several other hospitals 685.
What do we need to study to carry out an ABO-incompatible LDKT?- •
We suggest that ABOi transplantation should be considered an alternative when there is no ABO-compatible donor (D).
- •
We suggest monitoring titres of the IgG and IgM isoagglutinins. The importance of the role of both types of immunoglobulin in transplantation is controversial (D).
The initial acceptance criteria are the same as for an ABOc LDKT (9), to which we add the possibility of determining antibodies against A or B incompatible antibodies, known as isoagglutinins. The titre of IgG and IgM isoagglutinins should be the same or less than 1/8 for transplantation to take place. The next requirement will therefore be to apply one of the apheresis treatments used to reduce the isoagglutinin concentration, if necessary (it always is necessary, except for very low basal titres <1/8). Normally from 3 to 5 apheresis sessions are required to reduce the titres to a safe level for transplantation. If the basal titres are very high (>1/512) this increases the risk that the apheresis treatment will not achieve an effective reduction in the same, so that donation is ruled out. This gives rise to an enormous economical and emotional cost 686,689, so that many hospitals recommend or contraindicate desensitization treatment when the basal titres are very high (9,686,687). There is no agreement on whether a low titre is simultaneously necessary for both Immunoglobulins (IgM and IgG) 690,691, or if this is necessary for only one of them. Some groups believe that it is crucial to reduce the IgG titre, while others hold the contrary opinion. On the other hand, highly experienced hospitals accept transplanting patients with higher titres (1/32) at the moment of KT 692,693.
When the incompatible blood group of the donor is A2, given the low antigen expressivity some hospitals do not formally consider this to be an ABO incompatibility if the titres are low, and they go on to transplant directly as if the donor were ABO-compatible 694.
How should isoagglutinins be measured?This aspect is described in detail in section 9.
Desensitization techniques (Table 14)Desensitization for an ABOi KT requires two essential actions: eliminate isoagglutinins from the circulation and inhibit their synthesis. The treatment can be complemented with the administration of IVIG, which may have an immunomodulating effect, as well as making it possible to restore the protective antibodies lost in apheresis. Nevertheless, these preparations also contain isoagglutinins, which may be a confusion factor when interpreting how titres evolve during treatment 686,695.
Sensitization techniques and their objectives.
| Technique | Variants | Objectives | Remarks | Evidence quality |
|---|---|---|---|---|
| Plasma exchange | Single filtrationDouble filtration | To reduce circulating isoagglutinins | Possible complications with post-transplantation haemorrhage due to alterations in the coagulation system | B |
| Immunoadsorption | SpecificNon-specific | To reduce circulating isoagglutinins | Specific immunoadsorption is more efficient in reducing IgM titres | B |
| Immunoglobulins | Low dosesHigh doses | Replacing immunoglobulins and a possible immunomodulatory effect | Administered at high doses it may contain isoagglutinins that interfere with the titration result | C |
| Splenectomy | Depletion of B cells | This is no longer routinely practiced | C | |
| Rituximab | Conventional doses vs. low doses | Depletion of B cells | There is no agreement on dose or frequency of administration | C |
To centre on the different apheresis techniques, many different forms have emerged in recent years (53,683,696-701). Basically there are three techniques, each with its own versions: plasma exchange, specific immunoadsorption and non-specific immunoadsorption. When we are dealing with low titres, any technique will be effective. To effectively reduce high titres, no technique has been proven to be any more effective than the others, although some authors state that specific immunoadsorption would be the best. Economic costs should be taken into account as well as efficacy, together with logistic aspects and side effects, especially in connection alterations in coagulation factors, reactions of intolerance the risk of infections 688,698,699. The immunoadsorption techniques are tolerated the best, making it possible to treat a greater volume of plasma in each session without altering the coagulation factors. An interesting logistic aspect is the possibility of simultaneously carrying out haemodialysis and apheresis.
Respecting the blocking of isoagglutinin production, splenectomy was used in the first Japanese experiences 671,672. The chief drawback with this, apart from possible surgical and complications and infections, was the need for routine vaccination against encapsulated bacteria over an indefinite period of time. Replacing splenectomy with treatment using rituximab not only proved to be more effective and safer, as its effect reduces after a few months. Although it is not supported by any clinical trials, it is now the treatment of choice 676,678,682,684,697,698,702–704. However, there is no agreement on the number or size of doses, how many days it should commence before the start of desensitization or other aspects of administration criteria. Some groups consider a single dose given 3-4 weeks prior to transplantation to be sufficient; others prefer two doses, the first 3-4 weeks before transplantation and the second immediately beforehand, seeking to combine the immediate and deferred pharmacological effects 678,682,684,692. Opinions also differ regarding the size of the dose. At first the same dose as the one used in lymphoma treatment was given, 375mg/m2 once or twice 473, but after studies were published indicating that a lower dose was enough to remove CD19-positive B lymphocytes from circulation guidelines were established that varied from 50mg to 400mg 678,705–707. Several meta-analyses associate the excessively prolonged use of this drug (but not the size of the dose) with higher mortality due to infections 697,698,700,701,708,709. Thus these findings and the improved efficiency of apheresis techniques have led some groups to decide against systematically administering rituximab, and they now only give it in certain cases 710,711.
Bortezomid would be included among the other therapeutic options for blocking isoagglutinin synthesis. It has been trialled as a desensitization therapy in ABOi LDKT in cases refractory to rituximab or to substitute it 680,712. Eculizumab has also been trialled for usefulness, and it was able to prevent antibody-mediated rejection in four patients with no apheresis treatment 713,714. There is interest in knowing the possible efficacy of Imlifidase, which is capable of cleaving all circulating IgG in two steps and a few minutes after single dose. A limiting factor would be its lack of efficacy against IgM antibodies.
Finally, many hospitals routinely use IVIG. This would have two uses: it restores the immunoglobulins eliminated by apheresis, for which administration at low doses of 100mg/kg - 200mg/kg would be sufficient, or it could be given at high doses (1g/kg - 2g/kg) to achieve a multifactorial immunomodulating effect, most especially providing anti-idiotype antibodies which would facilitate the phenomenon of accommodation 715. Three factors arise against the systematic use of IVIG: it is unnecessary to replace idiotype antibodies when specific IA is used; its efficacy has yet to be proven, so that it may constitute an unnecessary cost, and finally its isoagglutinin content may distort the results of titration 677,682,683,690,695.
How do the isoagglutinins behave after transplantation? Is it correct to monitor titres and no intervene while they remain low?
- •
We suggest practicing one or two apheresis sessions after transplantation as the protocol, although as no clear recommendations exist, other groups prefer to wait for a possible rise in the titres (C).
Isoagglutinins must be at a safe level before practicing an ABOi LDKT. Anti-A or B antibodies may bounce back after transplantation to levels even higher than they were at the start of desensitization therapy 687,706. This potential rebound may lead to an early rejection, although in the majority of cases when an increase in titres is detected without any alteration in graft functioning, biopsy of the same shows the absence of immunological damage except for the usual presence of C4d deposits 716,717. A low level of isoagglutinins in the peripheral blood may indicate that most of them have been absorbed by the graft, so that circulating anti-A or B antibodies would have less avidity for donor antigens, reducing their pathological involvement 718.
In any case, after transplantation some groups believe it to be necessary to recommence apheresis sessions if the isoagglutinin titres increase during the first two weeks. Other groups only do this if there is renal dysfunctioning or a biopsy shows signs of rejection other than C4d deposits. Some hospitals prefer to include one or two apheresis sessions in their protocol, in the attempt to prevent a rebound in the titres or even to prevent antibody-mediated rejection. It should not be forgotten that this rejection may arise even if the patient has not yet shown a rise in titres 680–682. On the contrary, other hospitals consider that post-transplantation apheresis sessions only cause problems (with haemorrhaging, logistical problems and unnecessary expense) without any benefits 672,684,698,719.
Is patient and graft survival the same as with ABOc LDKT?
The published results of series of hospitals report similar results or slight differences in patient and graft survival (C).
- •
Meta-analyses or analyses of registries are able to detect poorer patient and graft survival due to infections in the first year of evolution (B).
- •
We recommend closely monitoring complications with infections in ABOi recipients due to certain experiences that have reported increases in mortality secondary to infections (B).
The meta-analyses that report the results of patient survival after receiving an ABOi LDKT do so by comparing this with the results of ABOc transplantation. The majority show that there are no differences, or that any differences are small 53,696,697,703,704 and that they disappear over the long-term. Even when the potential increased risk of complications due to infection caused by desensitization therapy is taken into account, ABOi LDKT would be better in any case than deceased donor KT or remaining in dialysis 720. On the other hand, nor was it found to have higher mortality when it was compared to paired donation 721.
In terms of graft survival, many groups have reported similar results over the short to medium term and even over the long-term 682,692,693,700,708,722. Other series found poorer results, and data from meta-analyses and registry studies underline shorter graft survival than in ABOc LDKT at one, 3 and 5 years, although there were no long-term differences 53,697,703,704,722.
Although ABOi transplantation is considered to be a reasonably safe procedure, the use of powerful immunosuppressant drugs, and especially rituximab, is associated with increased infections 700,708,709. Several series report a higher frequency of viral infections 723,724 as well as pneumonia, urinary infections and infections of the surgical wound 724,725. Not all of the series agree with these findings, and moderating the use of rituximab as suggested by Japanese groups has been accompanied by a significant fall in infection by CMV 693. Moreover, with this moderation and other anti-infection prophylactic measures, rates of urinary infection and pneumonia have recently been reported that are comparable with those corresponding to ABOc transplantation 698,723.
Unlike infections, the incidence of tumours does not seem to increase in ABOi transplantations, although it is true that there is not so much literature on this subject 726,727.
What occurs with acute rejection?
- •
The incidence of acute rejection, and particularly antibody-mediated acute rejection, is higher in meta-analyses of ABOi transplantations, although many hospitals report comparable results (B).
The initial Japanese series reported a significant incidence of graft loss due to hyperacute or accelerated rejection, and in fact this may be the main or perhaps the only difference between ABOi and ABOc transplantations. This would be due to the swift development of acute rejection mediated by antibodies against A or B incompatible antigens. On the contrary, the initial Swedish series described a very low rejection rate, cellular as well as humoral, which it attributed to the important amount of immunosuppressant medication administered. Other works underline a higher incidence of mild cellular rejection 679,728,729.
Accommodation and rejection mechanisms
A phenomenon involving possible immunological accommodation in a situation of ABO incompatibility arises in almost all ABOi transplantations: the presence of high titres of isoagglutinins, AB antigen expression in the endothelium and C4d positivity in peritubular capillaries, in the absence of other signs of rejection and with conserved renal function (B).
- •
Early losses due to accelerated rejection are rare and are usually accompanied by thrombotic microangiopathy (C).
- •
We suggest that prophylactic anticoagulation should be administered in the first weeks after transplantation, due to the susceptibility of the ABOi endothelium to thrombotic phenomena in the case of any immunological or non-immunological insult (C).
The most intriguing aspect of rejection mediated by antibodies against ABO antigens is that it rarely appears after 2 - 3 weeks post-transplantation, in spite of isoagglutinins being detected, even at high titres, and signs in protocol biopsies that the complement had activated. Several different mechanisms may intervene in accommodation, and they may affect antibody synthesis, exposure to antigens and the development of active protection measures. To establish accommodation it is necessary to overcome the first phase successfully, in which the complement together with the initial damage caused by the antibodies would activate a repair and protection response against the cytotoxicity which over the long-term will favour the acquisition of activate cellular and tissue protection mechanisms 64. If in the first phase ultrastructural alteration of the plasma membrane had occurred, with regional changes in platelet aggregation and neutrophil recruitment, this intrinsic resistance against cytotoxicity would be lost and graft thrombosis could occur in the first hours 718. It is therefore recommended to administer prophylactic anticoagulation during the first week.
What role would the complement and C4d deposit in transplant biopsy?
The systematic presence of C4d in protocol biopsy stains in ABOi transplantations is well-known, although its exact significance is not known, although it tends to be understood as an accommodation phenomenon 713,730,731. Patients with an ABOi transplantation with C4d deposit but no other histological abnormalities do not usually develop glomerulopathy of the transplant or chronic graft dysfunction. In fact, when they have a persistent diffuse C4d deposit they have less chronic damage after the first year. Pharmacological blockage of complement activation may be of interest to prevent or treat humoral rejection, as well as to prevent thrombotic microangiopathy phenomena associated with endothelial damage. There are few experiences, which nevertheless were successful, in the prophylactic use of eculizumab in transplantations of this type.
16Paired kidney transplantation and altruistic donorsLDKT proposes interchanging donors in different donor-recipient pairs, which due to biological incompatibility or another reason decide not to transplant directly but rather to create new donor-recipient pairs for direct transplantation.
Biological incompatibility between donor and recipient is a barrier for LDKT. 35% of patients are estimated to be incompatible with their potential live donor 732, due to blood group incompatibility (ABOi) or the existence of preformed antibodies in the recipient against the HLA system antigens of their potential donor, giving rise to a positive crossmatch test (HLAi). The possible options for treatment therefore include (9): searching for another living donor; including the pair in a paired KT scheme; considering an incompatible transplantation with desensitization; or remaining in the waiting list for a deceased donor.
Paired LDKT was suggested by Rapaport in 1986 733, and for this the author considered it to be fundamental to guarantee the anonymity of the members of the new pairs which were formed. Subsequently Park's group 734 carried out the first paired donation between two recipients with incompatible donors in South Korea, in 1991. Switzerland (1999) and the U.S.A. (2000), designed paired LDKT programmes at hospital level. The Netherlands started the first national scheme in 2004, followed by the United Kingdom [2007], Canada [2009] 735–738 and Spain [2009] 543. The Spanish scheme, which led to 203 transplantations from 2009 to 2018, is now the second in terms of activity, after the United Kingdom 51.
The results obtained have increased the number of transplantations 15,51,667,739–743 with similar results in graft and patient survival to those of direct compatible LDKT 744,745, and better results than direct HLA-incompatible LDKT 6,54,746,747.
Foundations of the paired KT scheme in Spain- •
We recommend the option of paired KT over HLA-incompatible transplantation due to the improved survival it achieves, so that this information should be given to paired with HLA incompatibility (Quality of evidence: B).
- •
Patients with very few transplantation options (blood group O and highly sensitized) should receive an explanation of the possibility of adding treatments such as desensitization to paired KT cruzado, to increase their possibilities of transplantation (NG).
The main aim of the Spanish paired transplantation 543 is to increase the possibilities of LDKT in those cases without donor-recipient compatibility, due to ABOi blood group or a positive crossmatch test, as well as other circumstances in which, although transplantation within the pair is not impossible, there is a real benefit associated with a paired KT procedure (such as a reduced difference in donor-recipient age or donor anthropometric characteristics that better fit the needs of the recipient).
The regulation which established the procedure for paired transplantations was developed and published by the ONT in 2009, in collaboration with a multidisciplinary committee of experts in different aspects of the living donor KT procedure: nephrologists, immunologists, urologists, transplantation coordinators and experts in bioethics and lawyers.
Once the scheme was under way a continuous improvement mechanism was established, based on the creation of a workgroup and a scientific committee (whose members are drawn from the workgroup). The workgroup is composed of the individuals in charge of the scheme in the ONT and in each participating hospital. It is convened by the ONT once a year to analyse the results of the scheme and establish modifications in different areas to achieve continuous improvement. The functions of the scientific committee include evaluating complex cases, as well as drawing up suggestions for improvements.
The scheme has thereby gradually improved, by including new hospitals, unifying clinical and immunological criteria, updating registry information, expanding donor typing, including chains of altruistic donors, creating a compatibility algorithm, the recent inclusion of the possibility of performing ABOi transplantation in the scheme for potential recipients with HLA sensitization, the priority score, the search for renal transport schemes which reduce times and are sustainable, and the broadening of pairs and HLA diversity by making the scheme international. In fact, three international paired transplantations have now been performed, between Spain and Italy and between Spain and Portugal 748.
The scheme contains 3 elements: the requisites which transplanting hospitals have to fulfil to take part, the computing tool which contains the donor and recipient database, a selection algorithm for possible exchanges, and a tool for optimising detected cycles, to maximise the number of transplantations and increase the options for recipients who are “hard to match”, and a working protocol which describes the procedures for registering pairs, creating pairs (or matching-run) and the procedure which leads to transplantation.
Exchange options within the scheme (figure 14):An exchange of donors between donor-recipient pairs previously registered in a database, to form cycles of two and three KT.
The donors and recipients in these cycles form new pairs which are “more” compatible in immunological terms or, if they were compatible beforehand, ones who are more suitable in anthropometric terms or age. Thus for a cycle to be formed it has to be bidirectional. That is, all of the donors in the cycle should be compatible with the recipient selected for them. It is a legal imperative that the members of the new pairs must not know each other 41.
The transplantation procedures are executed simultaneously, to minimise the risk of one or several recipients not receiving a transplant after the nephrectomy of their incompatible donor.
The inclusion of an altruistic donor in the scheme, thereby commencing a KT chain. Compatibility in this option is one way, that is, it is only necessary for the donor to be compatible with the next recipient in the chain, facilitating the creation of the latter 749.
In this case it is not necessary to carry out all of the transplantations at the same time, as if an unexpected event occurs which causes a recipient in the chain to not receive their corresponding KT, their donor will still not have made their donation. Being able to create the transplantation chain in several phases also makes it possible to add links, increasing the number of transplantations. This option is known as a transplantation chain with a bridging donor. The final link in the chain is a patient in the waiting list of the hospital which evaluated the altruistic donor 750.
Given that transplantation chains are characterized and differentiated by including an altruistic (Good Samaritan) donor, they are described in a specific chapter.
Participating hospitalsHospitals that wish to take part in a paired KT scheme must have the necessary infrastructure and the agreement of all the clinical departments involved to fulfil the conditions of the scheme. The transplantation teams must also have experience in the LDKT procedure. This point is of vital importance, as donors will be exchanged between different hospitals and there must be trust in how all of them will work.
Due to the above reasons, the national paired renal donation protocol sets the following requisites:
There is a criterion which sets the minimum amount of activity. This currently stands at having carried out 15 LDKT in the three years prior to joining the scheme.
Minimally invasive techniques must be used for nephrectomy 32.
The conditions set by the scheme for carrying out diagnostic tests (such as broadening donor typing) must be accepted.
An individual in charge of the scheme must be designated at hospital level.
Participation in the scheme must be approved by the corresponding health authority 543.
The scheme currently has 26 participating hospitals and 19 histocompatibility laboratories (one of which is the reference laboratory for two Madrid laboratories and 6 others in Catalonia) in 12 autonomous communities. This is an important datum, given that countries such as the Netherlands base some of the success of their scheme on the existence of a central histocompatibility laboratory 751. In spite of the increased difficulty of managing immunologically complex patients in different laboratories, Spain has obtained excellent results. The English scheme is organized in a similar way to the one in Spain, with 20 different histocompatibility laboratories and 23 transplanting hospitals, and the United Kingdom scheme is now one which has performed the highest number of paired KT in Europe 51.
Hospitals which are not members of the scheme may include donor-recipient pairs through hospitals which are members. Autonomous Community Transplantation Coordinators establish agreements and procedures for this 543.
Thus all renal patients with a potential incompatible donor (or a compatible one, but who could benefit from an exchange) will therefore have the opportunity of taking part in the programme, thereby guaranteeing fair access to it. This opportunity for patients and their potential donors involves the right which all of them have to be properly informed about the paired LDKT Scheme 2,9.
Computer toolThis online application can only be accessed by hospitals which are members of the scheme and the personnel in charge of the scheme in the ONT, by entering their user name and password, to comply with the data protection law. The search for possible exchanges may be carried out manually, although the number of pairs and their complexity makes it recommendable to use the system automatically to gain good results. This computer tool includes 543,752,753:
A database of donor-recipient pairs, where the demographic and clinical data of the patients and their possible donors are registered and kept updated, as required for matching:
A patient may be registered with more than one donor. A recipients may form a pair for exchange with as many donors as they supply, which increases their chances of finding a match 752,754. From 2009 to 2018 656 donor-recipient pairs joined the Spanish scheme for paired renal donation (557 recipients with a donor, 36 recipients with two donors and 9 patients with more than two donors).
There is no upper or lower age limit for the inclusion of a recipient. The donor has to comply with the requisites established by Spanish law respecting minimum age (≥18 years) and there is no upper age limit, on condition that the donor has been considered to be suitable by the participating hospital. The donors and recipients registered in the scheme have a similar average age, at 50 years and 46.3 years, respectively.
The scheme permits patients to register with their potential a priori biologically compatible and incompatible donors, although they must also wish to make an exchange, to find a younger donor, or one of a more suitable size, improve HLA compatibility or for simply altruistic reasons 755,756. The reasons for inclusion in the scheme were ABOi in 43% of cases, the presence of DSA in 51%, ABO and HLA incompatibility in 4% and 12 pairs were compatible, amounting to 2% of the registered pairs.
The database permits the registration of altruistic donors to build a transplantation chain 51.
The application automatically associates a numerical code with each registered donor and patient, which will allow the members of the new pairs formed to remain anonymous, without the process losing traceability.
It contains a compatibility algorithm, which searches for all of the possible options for exchange within the donor-recipient pool, forming cycles of 2 or 3 transplantations and chains which commence with an altruistic donor. There are two rules:
ABO compatibility and HLA compatibility: using a virtual crossmatch test, it selects those recipients who do not have preformed antibodies against the different HLA antigens of the donor (for class I HLA as well as class II) 736,740,746.
Exclusively HLA compatibility, in this case permitting ABOi KT 697,757.
A selection tool for the detected cycles to maximise the number of transplantations and increase the options of the recipients who have most difficulties in finding a compatible donor, such as those with group O and/or highly sensitized patients 543. To this end the system has:
A score which takes into account level of immunization (cPRA), blood group, time in dialysis, donor and recipient age, the length of time a recipient has been included in the scheme and, in case of a draw, geographical distribution. Given the exceptional nature of a compatible pair and that this increases the options for transplantation, these are given priority 51.
An optimization model, which attempts to maximize:
The number of transplantations carried out.
Transplantations into patients with difficulties to find a compatible donor.
The cycles selected, which involves obtaining more two-transplantation cycles than it does those with three transplantations (on condition that the total number of transplantations is maximized)
The number of robust cycles. When cycles involving three transplantation cycles are selected, if we have to choose between two cycles that share one or two pairs and have the same score, the one that can be shortened to a two-transplant cycle will always be selected (which facilitates its eventual execution) 752.
The compatibility algorithm and the selection tool are always applied together, seeking an optimum set of possible combinations. Two programming models are currently used in Europe for this 752:
Construction of a compatibility graph, assigning as score (known as weight) to each one of the members of a pair, based on criteria set by the programme (age and level of immunization, etc.) This is the model used by the Spanish scheme.
Formulation of the different exchanges using integral programming.
Action protocolBefore including donor-recipient pairs in the paired transplantation scheme and continuing with the pairing dynamic, the pairs have to be informed of how the scheme works and other aspects of it.
Information for the pairsWe recommend the option of paired KT rather than HLA-incompatible transplantation due to its better survival, so that this information should be given to the pairs in which direct transplantation will not be possible (B).
The recipient and their potential live donor should be given information about the paired scheme, with the existing scientific evidence for the same as well as comparison with other therapeutic options. The probability of receiving a paired transplantation should also be explained, depending on their characteristics (blood group and level of immunization) (NG).
The donor and recipient in a compatible pair who could benefit from a paired transplantation should receive all of the available information on the same to decide whether to participate (NG).
The participation of pairs in the paired donation scheme requires signing a specific consent form (B).
Donors should be informed about the regulations governing the confidentiality and anonymity of the procedure, when it is paired as well as in the form of a chain or by direct altruistic donor, ensuring that the donor accepts this before continuing (NG).
The donor should be informed of the risks and benefits which arise from transporting the kidney instead of the donor travelling, and the possibility of the kidney being transplanted into another person (NG).
The donor and recipient have to sign a specific consent form 2,9,543 to participate in the scheme, which means that they have understood the responsibilities they have towards the other pairs if they are selected in a transplantation cycle or chain. For this they must be been previously informed about the risks and benefits of LDKT, the options offered by this scheme, the procedure respecting the deceased donor waiting list, the obligation to keep maintain the anonymity of the members of the new pairs which are formed, the probability of success of the transplantation according to their characteristics (blood group and level of immunization, etc.), and the possibility, if applicable, of receiving desensitization therapy together with paired transplantation, to increase the chances of success of the transplantation, as well as the possibility of an unexpected event occurring (see the section on transportation logistics). Therefore:
The information supplied to a transplantation candidate and their potential incompatible living donor must include the scientific evidence for the results of a compatible transplantation, incompatible transplantation, and the results of both in comparison with remaining in the waiting list. Group O recipients, highly sensitized patients or those with both conditions must be informed of the lower probability of receiving a transplantation in this scheme (9). The recipient must be informed that if they are in the deceased donor waiting list they will be able to remain in both waiting lists, but that they will leave the deceased donor waiting list if they are selected for paired transplantation. The information supplied to each pair must always be comprehensible and accessible.
ABOi and/or HLAi donors and their recipient must be informed of the existence of the paired transplantation scheme, as well as the different options for treatment which exist, the risks of each option and their probabilities of success 2,9.
Donors must know that a compatible transplantation has a higher probability of success than direct HLAi transplantation, with higher short- and long-term risk, if one is suggested for them 2,6.
If a pair is compatible and decides on inclusion in the paired transplantation scheme to obtain other benefits such as reducing the age differences with their living donor, improving HLA compatibility or a more suitable weight, they must receive all of the relevant information to decide on their inclusion.
Paired transplantation- •
We recommend registering the donor-recipient pair in the paired KT scheme only after the end of the clinical study and when the immunological screening is complete (B).
- •
We recommend that the individual in charge of the paired KT scheme in each participating hospital should review and update the information corresponding to the registered pairs before creating each pair (B).
- •
We recommend describing the possible factors that may limit donor acceptance (surgical complexity, age, etc.) at the moment when a pair is included in the paired KT scheme (B).
- •
We suggest that patients should be excluded from the deceased donor waiting list when they are selected in a paired transplantation cycle. Meanwhile they can be active in both waiting lists (NG).
As a general rule, the scheme plans to perform three rounds of paired transplantations per year, that is, one every four months, so that the transplantation teams have enough time to review and update their pairs. It is possible to create an extra round under special circumstances, such as that of an altruistic donor whose study has finalized.
In the Netherlands or the United Kingdom rounds of paired transplantations are created quarterly, and in the UNOS scheme they are made weekly 51. Nevertheless, there is no evidence that a higher number of rounds gives rise to a higher number of transplantations. The number and characteristics of the registered pairs determine the number of possible exchanges 758,759. In fact, the number of exchanges detected increased in Spain when the number of active pairs increased. The average number of active pairs for each round of paired transplantations in Spain was 120 over the past 6 years, with 60% of new pairs in each round.
Three weeks before each round the ONT sends out an email showing the specific date of the round, so that hospitals can include new donor-recipient pairs and update the registered information. A donor-recipient pair cannot be included until their study has finished and immunological screening is complete and has been updated, to ensure that if they are selected, it will be possible to follow the steps in the process within the set times. Otherwise potential cycles with a high risk of not being able to perform the transplantations would be created, with negative consequences for the selected patients and for the working of the Scheme 756,760. A similar recommendation was published in the latest edition of the British LDKT guide 2.
It is also necessary that the information is kept up-to-date, to prevent the detection of cycles which are then found to be impossible to implement, due for example to the appearance of new unregistered donor-specific antibodies, or the medical contraindication of a recipient or donor to continue with the procedure 746,760. In fact, 50% of the reasons why a transplantation cycle is not implemented can be avoided by reviewing the registered information (for example, the registered DSA), giving information on possible restrictions for the acceptance of a donor for specific patients (for example, not accepting a donor aged over 70 years for a recipient aged under 40) and if the status of donors and recipients is updated before each round (to detect possible medical contraindications in the donor as well as in the recipient).
Patients can be active in the deceased donor waiting list and in the list of the paired transplantation scheme at the same time, thereby increasing their possibilities of transplantation. However, patients should be excluded from the deceased donor waiting list when they are selected for a cycle or chain, and they will be temporarily excluded from the deceased donor waiting list while the paired transplantation procedure continues. If the cycle or chain fails, then they will become active against in the waiting list 543.
Results of paired transplantation roundsOnce the possible combinations have been selected the ONT issues a report on the results for the hospitals which are involved, using a secure information exchange space. After this moment the individuals in charge of each one of the cycles at hospital level will evaluate the information received (age, blood group and HLA typing), sharing the clinical reports of the donors and agreeing on the execution of crossmatch tests with immunology laboratories.
A 7 day deadline is set for sharing the clinical reports, plus 15 days more to carry out the crossmatch test. As a general rule, this test will take place in the histocompatibility laboratory of the hospital which registered the recipient, so that the donor and recipient hospital laboratories will agree on a date and means of sending the samples. This test will be take the form of complement mediated cytotoxicity and/or flow cytometry.
Some patients are immunized to such a high level that it is improbable that they will find a donor for whom they lack DSA. In these cases a combined strategy of desensitization and paired transplantation may be used. That is, the transplantation team may, after considering this with the immunology department and agreeing it with the recipient, decide not to register in the database the DSA which the patient has at low titres. If they are selected for exchange with a compatible donor who has one or several of the unregistered DSA in the application, then desensitization and transplantation may take place. 55,761,762.
If the results of the crossmatch tests in the same cycle are negative, the hospitals will share angiotomography images of the renal anatomy of the donors and agree the date for transplantation.
As is the case in any directed LDKT, the ethics committee should evaluate the procedure, and the donors will have to give their express consent to donation before the examining magistrate of the court corresponding to the town in which the nephrectomy or transplantation will take place 41.
In the 9 years this scheme has been working, in Spain a total of 29 rounds of paired transplantations and 203 transplantations have been carried out, distributed in 42 cycles of 2 transplantations, 27 cycles of 3 transplantations and 14 chains of transplantations that commenced with an altruistic donor 763 (Figure 15). 32.8% of the patients were transplanted in this scheme, unequally distributed according to donor and recipient blood group. The highest probability corresponded to those pairs in which the donor was group B and the recipient was group A (Table 15). Altruistic donation made 43 transplantations possible, so that without this, the rate of transplantations within the scheme would have been 26.1%.
Number of 2 transplantation rounds, 3 transplantation rounds and chains initiated by an altruistic donor. Spain 2019-2021 763.
Percentage of patients transplanted in the scheme according to blood group and average calculated PRA (PRAc), with Interquartile Range (RIC). Spain 2009-2018.
| GS | N transplanted/N included | Median PRAc (RIC) of those included |
|---|---|---|
| D0-R0 | 26/111 (23.4%) | 88 (56-99) |
| D0-RA | 47/77 (54.5%) | 79 (11-94) |
| D0-RB | 9/22 (40.9%) | 88 (51-98) |
| D0-RAB | 3/9 (33.3%) | 100 (92-100) |
| DA-R0 | 42/194 (21.6%) | 0 (0-54) |
| DA-RA | 19/77 (24.7%) | 86 (44-99) |
| DA-RB | 20/42 (47.6%) | 0 (0-48) |
| DA-RAB | 2/12 (16.7%) | 97 (90-99) |
| DB-R0 | 15/48 (31.3%) | 0 (0-70) |
| DB-RA | 16/24 (66.7%) | 0 (0-36) |
| DB-RB | 2/12 (16.7%) | 75 (34-92) |
| DB-RAB | 1/5 (20%) | 96 (89-100) |
| DAB-R0 | 3/11 (27.3%) | 56 (0-99) |
| DAB-RA | 4/9 (44%) | 56 (0-99) |
| DAB-RB | 0/4 | 48 (0-98) |
- •
We recommend performing donor nephrectomy in their own hospital and transporting the kidney to the recipient's hospital for transplantation (B).
Although the scheme includes the possibility of the donor travelling to the hospital where the recipient of their kidney will receive it, the recommended option is for nephrectomy to take place in the donor's hospital and for the kidney to be transported. Nephrectomy in their own hospital permits the donor to be in contact with their family and allows them to remain in known surroundings, being cared for by the professionals who have studied them, all of which reduces the stress for the donor and their family. Moreover, if the donor does not travel to the hospital where the recipient is, it will be easier to maintain their anonymity. In Spain, 97% of paired transplantations have involved transporting the kidney (using an ambulance for this, or a high-speed train or aircraft) with an average time in cold ischemia of 5.6 (2.8) hours.
Although renal transport necessarily involves the kidneys being transplanted after a time in cold ischemia, it has been found that cold ischemia times <12hrs. do not affect the incidence of retarded renal function or graft and patient survival over the mid-term 744,745,764. The KDIGO guides suggest informing donors that they have the option of travelling to the recipient's hospital if they wish, while also explaining that the results of renal transportation are excellent 9.
As a general rule, the means of transport to be used is the one which makes it possible to keep the duration of ischemia below 8hours in a cost-effective way 543. Transport, if necessary, may be terrestrial (in an ambulance, hired car or high-speed train) or by air (in a private or commercial flight). The ONT has specific agreements that permit the transportation of a graft in the aircraft cabin, looked after by the crew, at no cost to the healthcare system.
The procedure also stipulates that the nephrectomies from all of the donors in the same cycle without an altruistic donor will take place simultaneously, except in exceptional cases. This condition was established to minimise the risk that one recipient in a cycle would not receive a kidney after their original donor had been nephrectomized.
In any case, the scheme provides for different scenarios of unexpected events:
The kidneys in one cycle have been collected and one engraftation cannot take place: the kidney will be transplanted into a patient in the waiting list of the hospital where the nephrectomy took place and the hospital will return one kidney to the recipient's hospital. The recipient who has not been transplanted will subsequently receive priority in the waiting list.
If the unexpected event (during donor nephrectomy, bench surgery, transport or other circumstances) invalidates one of the kidneys, the other transplantations will take place as planned. This circumstance will be considered an adverse event and the biovigilance system will be notified (the coordinators of the autonomous communities involved will be informed) and the case will be investigated, to discover the origin of the fault and establish preventive measures. In this case the recipient who has not been transplanted will subsequently receive priority in the local waiting list.
In the case of an altruistic donor chain with a bridging donor who does not proceed with the donation after this has already taken place in part of the chain (due to a change of mind or a health problem while waiting, etc.) the recipient who is left without having received a transplant will receive a deceased donor kidney from the hospital of the bridging donor.
Altruistic donors- •
All altruistic donors should be assessed using the same criteria as any other living renal donor, and the exploration of their motivations, expectations and social and family support will all be especially relevant (NG).
- •
It is recommendable that all altruistic donors should initiate transplantation chains, as this offers the greatest benefit in terms of the number of transplantations. Nevertheless, they should be informed of the possibility of donating directly to the waiting list (B).
The terms “altruistic donor”, “Good Samaritan” or “non-directed donor” are used to refer to those individuals who donate an organ to somebody they do not know who needs a transplant. In this document we refer to them as “Altruistic Donors” 172.
In Spain, after being fully informed altruistic donors may decide to donate in two different ways:
By donating an organ to someone in their local waiting list (anonymously to someone they do not know). In some countries such as the United Kingdom they firstly seek a compatible recipient among those with national priority, and if one cannot be found, by default all altruistic donors then initiate a chain of transplantations 2.
In paired renal donation the final recipient at the end of the chain is also selected from the local waiting list. However, the expected benefit in terms of the number of intermediate transplantations performed is greater. The KDIGO guides recommend informing altruistic donors of the greater benefit in number of transplantations when they start a chain 9.
National and international dataAltruistic renal donation is increasingly accepted as a strategy to overcome the lack of organs for transplantation 765,766. From 11% to 54% of people in the world are estimated to be prepared to make a living renal donation to patients with ESRD with whom they have no previous tie 767,768.
However, altruistic renal donors are especially relevant when they are included as complements in Domino Renal Paired Donation (DRPD) 649. This is why different countries, such as South Korea 769, the United States 749, Canada 769,770,771, the United Kingdom 772, the Netherlands 773 and Spain have developed altruistic renal donation schemes with excellent results 49.
To centre on European countries, and more specifically on those which have obtained the best results, altruistic donation rates in the past 6 years have accounted for from 8% to 12% of total living donor activity in the United Kingdom and the Netherlands, respectively, while this percentage in Spain accounted for less than 1% 774,775. Nevertheless, it should be underlined that the Spanish scheme has been able to multiply the benefits of altruistic renal donation, obtaining a rate of efficacy for each altruistic donation of 3 transplantations, as well as including long chains through the use of bridging donors. From 2010 to 2018 14 chains started by an altruistic donor have taken place in Spain: 6 with two links, 3 with three, 4 with four and 1 with six links, adding 43 transplantations to the paired renal donation scheme, increasing its efficacy from 27% to 33%.
Action protocolThe protocol for the evaluation of a potential altruistic renal donor was published by the ONT in 2010, conjointly with a multidisciplinary committee of experts in different aspects of living donor KT procedures: nephrologists, immunologists, urologists, transplantation coordinators and experts in bioethics and law 172.
Evaluation of altruistic donorsAll altruistic donors should be evaluated following the same criteria as any other living renal donor (9), as described in the corresponding section of these recommendations.
Some national guides, such as the one for the United Kingdom, emphasise the need for all altruistic donors to be assessed by a qualified mental health evaluation professional prior to donation (2). Nevertheless, in Spain this is a requisite sine qua non for all renal donors 41.
The peculiarity of the Spanish scheme lies in the fact that the assessment of each altruistic candidate donor is divided into three consecutive phases, all of which have to be passed in order to continue with the process. All three phases are coordinated by the ONT, except for in Catalonia where this function is performed by the Organització Catalana de Trasplantaments (OCATT)172:
An initial semi-structured interview in the ONT/OCATT or in a hospital with experience in paired LDKT. This has two aims, firstly to inform the candidate about the process and, above all, to gather data on their sociodemographic, motivational and health variables, before finally referring the candidate to the authorized hospital(s) in their Autonomous Community, if they pass this initial triage.
The first hospital assessment, in a hospital that is experienced in evaluating living renal donors. This assessment has the purpose of ruling out clinical contraindications for donation.
The second hospital assessment, which will take place in a hospital that is authorized in the Paired LDKT Scheme. This has the aim of ruling out surgical and psychological contraindications, as well as ones in connection with their social and work-related support.
Specific considerations to be taken into account during assessmentWe recommend exploring the underlying motivation, which has to stem from altruistic eagerness (B).
We suggest informing potential young donors that they could consider donating at a later stage of their life (NG).
We suggest waiting for a reasonable time after the first interview, encouraging the candidate to reflect and consider the information that they have received (NG).
The whole process must guarantee (ethically and legally) that the donation is selfless and that the candidate donor seeks no economic benefit, publicity or personal promotion. In case of suspicion, it is especially important to examine the altruistic past of the potential donor, as well as their family support (which should be strong) and their economic situation (which should be sound) 172.
InformationApart from the information which all living renal donors should receive, in their first contacts with the donation network potential altruistic donors should also be informed of the following points:
That for the process of study and assessment they will have to travel to a hospital with experience in paired renal donation, and that this may be some distance away from where they live. Nevertheless, the convenience for a potential donor will always be taken into consideration when there are several options 172
The regulation governing the confidentiality and anonymity of the procedure, ensuring that the donor accepts this before continuing 9,41
The risks/benefits of transporting the kidney and the safety mechanisms in place to ensure that an collected kidney is transplanted, even though adverse events may occur 9
That they will not be able to select a specific date for donation, although all necessary efforts will be made to respects the time periods which the candidate says are preferable. 2,172
That participation in the scheme requires their specific informed consent9,172,543, for which they must have been previously informed and have understood all of the specific questions described in this section.
Recommend a period of time after the first interview, encouraging them to think about all of the additional information they have received and to reflect on their final decision. This is considered to be good practice, and in Spain donors are able to take the time they need, although in the majority of cases an answer is received in less than one month (2).
ExpectationsIn the case of altruistic donors, there are particular considerations in connection with their lack of any ties with the recipient, and this question has to be examined meticulously during the assessment process 743. They have to be prepared for possible situations such as having no information after donation about how the recipient is progressing, or hearing that the transplantation had not succeeded. Although no specific data exist in Spain, in other countries it has been observed that donors need to know that the recipient is doing well 776.
The candidate should be informed about the hypothetical possibility that in the future they may need the kidney that they currently wish to donate, for somebody important and close to them: a child, their partner or a brother, etc. or that they may need it themselves 172.
MotivationThe study of altruistic donors has removed many pre-existing doubts about their motivations 777. Nevertheless, to prevent any future problems in the mental health of altruistic donors, their underlying motivation has to be studied, verifying that it stems from altruistic eagerness and that it results from their positive aspects rather than any motivation based above all on psychopathological tendencies. It will therefore be useful to explore the altruistic actions of the individual in the past 172. Some questions that may help to explore this facet are shown in Table 16.
Questions to explore the motivation and altruistic history of a potential donor.
| Do you consider yourself to have been altruistic in your life? |
| If so, could you tell me about it? |
| Have you ever known anyone who had a renal disease and needed a transplantation? What did you feel? |
| How did the idea arise of donating a kidney to somebody you do not know? How long have you been thinking about doing this? |
| If you were asked what had led you to contact us, could you describe it? |
As is the case in Spain, in other countries donors have been found to be essentially motivated by a desire to help others, and that they think that they will only experience minimum drawbacks.
AgeAnother subject that is discussed in the field of altruistic donation is the age of the donor, particularly in the case of young adults. The majority of concerns here arise in connection with whether the youngest donors are more likely to regret their decision. Although there is no evidence to suggest that this is the case, it may be useful to include questions for younger donors about why they wish to donate now, and whether they could consider doing so at a later stage (2).
Family and social supportAnother aspect where altruistic and conventional donors seem to differ consists of their social support and the perception that they need help and attention. Some altruistic donors do not tell their loved ones about their decision, as they do not feel that they are sufficiently understood by those close to them. Nevertheless, a lack of social support could finally lead to the donation not occurring (2). This is why it is important to identify their relationship with their family at an early stage, and it is recommendable to encourage them to let their loved ones know about their decision. This will allow them to gain their help during convalescence after nephrectomy, preventing them from abandoning the process once it has begun due to pressure from those close to them. 754
RegistrationAfter approval by the hospital ethics committee, the individual in charge of the scheme in the hospital informs the ONT of the altruistic donors whose assessment has ended, and they are registered in the paired renal donation database 172.
Renal assignation of an altruistic donorIf the donor has decided to donate directly to the waiting list, the hospital which assessed them will select one of its recipients, based on the waiting list recipient selection criteria.
If the donor has decided to initiate a chain of transplantations the procedure will be similar to the one established for paired renal donation, as described above, with the following differences:
The other hospitals taking part will have to be informed at least 15 days beforehand that a new extraordinary round of paired donations will take place, due to the entry of a new altruistic donor into the scheme (if it is to take place outside the established three annual rounds). The purpose of this is so that the other hospitals will be able to add new pairs which have been completely studied, so that they can benefit from this option.
Inform the hospital which brings the altruistic donor that it has to select a recipient from its waiting list for the end of the chain.
There is no limit to the number of links in altruistic donor chains. For chains involving more than four pairs, or in those when the starting times agreed with the altruistic donor are delayed, one of the donors may be proposed as a bridge, so that the chain will go forwards in several phases. This option should be considered from the start in the hospitals involved, so that they are able to agree on it with the affected donors. It is recommendable that the different phases should not be more than one or two weeks apart.
17Analysis of the risks for living renal donors over the medium and long-termsCardiovascular risks for renal donorsThe long-term post-donation risk of hypertension is higher than it is for an equally healthy population which has not undergone nephrectomy (Quality of evidence: B).
- •
The risk of mortality due to any cause and of cardiovascular events over the long-term does not differ from that of the healthy control population (B).
- •
We recommend informing donors of the risks of renal and cardiovascular disease associated with obesity, actively recommending changes in their lifestyle together with weight control (B).
- •
Preventing cardiovascular risk factors during post-donation follow-up will contribute to maintaining the health of the donor over the long-term (NG).
- •
Knowing the potential risks over the medium and long-term for a potential donor and informing them accordingly is fundamental in guiding their decision-making and the process of study and selection by the transplantation team. Studies with a long-term follow-up and a large number of cases (given the scarcity of expected events) are necessary to know the long-term risks that can be attributed to renal donation. On the other hand, donors are a rigorously selected group in very good health. The control population will therefore have to be comparable, not only in terms of demographic factors but also medically, to prevent under- or over-estimation of the risks in donors 778.
Most of studies are retrospective, observational and based on single centre or national registries. 95% of them are observational and half of them had short follow-up times (≤1 year), and most of them refer to results in connection with the GFR, arterial pressure and peri-surgical complications 779.
Four studies analysed mortality and cardiovascular events over the long-term with a prolonged follow-up and a representative sample size. They use a selection of individuals with similar health criteria to the donors. Three of these studies found no increase in mortality or cardiovascular events in the donors 120,780,781. Although the remaining study contradicts the other three 37, it has two limitations: (a) the control population is almost 10 years younger on average than the donor population, indicating a distortion in pairing and a clear advantage for the controls (as age is a factor that is associated with mortality), and (b) there was a large percentage of cases without information on variables such as their BMI or smoking, and the analyses were performed with and without data imputation. The results for cardiovascular mortality vary depending on whether cases with or without imputation are used, weakening the final result. A systematic review found no evidence which suggest an increase in the risk of overall mortality, cardiovascular disease or hypertension in living donors compared with non-donor populations 782. Based on current information there is no evidence of any greater risk of mortality and cardiovascular events over the long-term in donors. Nevertheless, further studies are required with a follow-up longer than 20 years to analyse the impact of donation over the very long-term. In any case, the absolute risk of mortality and cardiovascular events is very lower, lower than or similar to that of the general population 33,783,784 and healthy controls 120,780–782.
Both of the analyses which included a large number of donors and controls show a risk of hypertension that is significantly higher in the donors after follow-up periods of 6 785 and 11 years 786. In the first analysis, the appearance of hypertension in the control group was associated with a greater than expected annual fall in filtration, while in the donors it was associated with a halt in the foreseen rising curve for filtration after donation. In a series of 3,700 renal donors who were normotensive before donation, after an average of 16 years follow-up 25% had developed hypertension 787. The associated factors were higher BMI, smoking, dyslipidaemia, familial history and older age. With current evidence, the risk of hypertension over the long-term in donors is higher it is compared with an equally healthy population that has not been nephrectomized. Once again, long-term donor follow-up is fundamental for early detection and treatment of hypertension if it arises.
The prevalence of obesity has increased over recent decades in the general population. This has led to an increase in the percentage of potential donors with a BMI ≥30 Kg/m2 who are assessed in LDKT schemes. Obesity is associated in the general population with an increased risk of diabetes, metabolic syndrome, hypertension, ESRD and mortality 788. Several authors have also observed an increased risk of hypertension, diabetes or ESRD when following up obese renal donors 274,789–791. The clinical guides contraindicate donation when BMI ≥35 Kg/m2, although there is no agreement on moderately obese patients with a BMI ≥ 30 Kg/m2, and the British Guide and the KDIGO Guide suggest that the decision whether to accept a donor or not should be individualized in each hospital, depending on other associated risk factors for the donor in question 2,9.
Few studies have analysed the possible implications of obesity for donor mortality over the long-term. Recently, and although the level of absolute risk was low, an increase in mortality over the long-term has been described in donors with a BMI ≥30 Kg/m2271. This datum underlines the need to insist on the importance of sufficient weight loss in potential donors, and during the subsequent follow-up, with lifestyle change programmes if necessary.
The impact of the fall in the GFR after donation on long-term mortalityThe fall in the GFR observed after nephrectomy is not associated with an increase in mortality or cardiovascular disease (B).
- •
After elective nephrectomy the GFR in donors falls by 20%-30% in comparison with the pre-donation rate, and up to 15% of donors have a GFR<60ml/min in the post-nephrectomy follow-up 792,793. It is important to underline that the gradual fall in the GFR which characterises CRD and the loss of renal function following donation are not comparable. The donor is a healthy subject whose nephron mass has fallen suddenly due to the effect of the nephrectomy. This leads to the development of compensatory hyperfiltration mechanisms in the remaining kidney, which was previously healthy, that are unconnected with glomerular hypertension, but rather are benign mechanisms such as a possible increase in the filtration surface or glomerular plasma flow 794,795. The majority of donors therefore show a compensatory rising curve of their GFR which remains during several years after donation 207,257.
In patients with CRD, falls in the GFR are associated with an increase in cardiovascular risk 796–798. The presence of albuminuria, on the other hand, amplifies this association. In any case, a patient with CRD cannot be compared with a donor.
In the Framingham cohorts or NHANES I, moderate falls in the GFR were not associated with increased risk of cardiovascular disease 799,800. In the PREVEND group 801, in the absence of proteinuria, an eGFR of 30-59ml/min/1.73m2 was also not associated with an increased risk of cardiovascular disease. However, other studies 243,802–805 found an association between eGFR <60-75ml/min and cardiovascular mortality, especially if it was also associated with the presence of albuminuria. The discrepancy between these results may be explained by differences in the risk factors for renal and cardiovascular disease in the different cohorts. Once again, due to its heterogeneous nature the general population would not be comparable with donors.
To isolate the impact of nephrectomy on cardiovascular risk in donor studies with a prolonged follow-up would be necessary, with a representative sample size and a paired control population that is comparable with the donors not only in terms of demographic factors but also in morbidity and cardiovascular risk factors. That is, equally healthy populations as the donors but without having been nephrectomized. Studies of healthy subjects who have been nephrectomized due to other causes are useful in analysing this problem. Narkun-Burgess et al. 806 compared mortality after 45 years of follow-up in two groups of soldiers after the Second World War. The first group was composed of 62 veterans who had lost a kidney as a war wound, and the second group was composed of soldiers who had not suffered nephrectomy. The authors found no differences between the survival rates in both groups after almost five decades of follow-up. Recent studies found no differences in survival or cardiovascular events between donors and healthy selected controls 120,780–782, and the discrepancy of the Norwegian study 37 may be due to the limitations described above. With the available evidence it cannot be said that the reduction in the filtration rate observed after donation is associated with an increase in mortality or cardiovascular disease.
Finally, when evaluating how a fall in the GFR influences donor cardiovascular risk and mortality, other factors should be mentioned which may determine their long-term evolution. On the one hand, the initial assessment and selection studies play a fundamental role here. It is necessary to know the exact basal GFR of the donor because among other factors, renal function after donation will be determined by this. The formulas used to estimate renal function have a margin of error of ±30% 807. When using formulas to select potential candidates, some may donate when their filtration rates are non-optimum 205,808, so that they would form a subgroup at risk of developing renal and cardiovascular disease in the future. On the other hand, it is possible that there is a group of donors with less renal capacity (due to low weight at birth or prematurity) or a genetic predisposition the additional risk of which was not analysed at the moment of selection 809. These donors may have a higher risk of hypertension and albuminuria during follow-up 810,811, especially if situations of greater demand are added, such as weight gain or metabolic syndrome after donation. Finally a very long follow-up and the correction of additional cardiovascular risk factors may influence their evolution 47. In fact, although the cases of ESRD diagnosed in donors during the first decade have been said to be due to immunological or genetic factors, in subsequent decades factors such as hypertension and diabetes are the predominant causes 812.
The risk of preeclampsia in donorsWe suggest informing female donors of fertile age of the increased risk of gestational hypertension and preeclampsia in pregnancies after donation, and that here is no evidence of other adverse results for mother or foetus (C).
It is important to inform potential female donors of fertile age about the risks associated with a future pregnancy. Eight relevant studies have been published to date which include almost one thousand pregnancies after renal donation in 800 donors (the majority of whom were Caucasian).
Three single centre studies 813–815 in the 1980s and 1990s included a small number of cases, without controls and based on surveys. None of these studies found a higher risk of deterioration of the GFR, proteinuria or maintained hypertension associated with donor pregnancy. In 2009, a Norwegian study compared the complications of pregnant women after donation with two unpaired control groups: pregnant women prior to donation and pregnant women selected at random from a registry of births in the general population 816. The percentage of preeclampsia in the post-donation pregnant women was significantly higher than it was in the donors prior to nephrectomy and the registry control group (5.7% vs 2.6% vs 3.1%; p=0.026). The small number of events made multivariate analysis impossible. A North American study found similar results, although without a non-donor control group 124. A Canadian study of female renal donors who had been pregnant compared their evolution with healthy non-donor women who were similar in age and access to social and health services 123. There were higher rates of both preeclampsia and gestational hypertension in the donors than they were in the control group (11% (15/131) vs. 5% (38/788), OR 2.4 p<0.01). There were no differences in the incidence of Caesarean births, prematurity or low birth weight and perinatal or maternal mortality. Once again the low number of events restricted multivariate analysis. Finally, two more recent studies, one in an Asian population 817 and the other based on a North American registry 818 compare post-donation pregnancies with control group of the general population, and they found no significant differences in terms of adverse maternal-foetal results.
Further studies are needed to establish definitive recommendations. The studies published to date show a small number of events, and they lack a control group that was paired according to donor characteristics. Although we are able to inform potential female donors of fertile age about the risk of preeclampsia and gestational hypertension, the absolute risk of this is low and it is not associated with an increase in preterm births.
KT in renal donorsAccording to American registry data more than 150,000 individuals had made a renal donation while alive. Their incidence of ESRD was 10-30 events per 10,000 donors in 15 years 35,229, and their risk varied depending on their age, sex, race and time post-donation 224.
Many transplantation schemes have established policies which give priority in the waiting list to previous renal donors with ESRD who do not wish or cannot select an anticipated LDKT. In the U.S.A. from 1996 and in the Netherlands from 2011 they have been assigned priority to minimise their time in dialysis 127,819. Other countries which give priority to these cases are Sweden, Norway, Belgium, Luxembourg, Austria, Croatia and Slovenia. In Spain each transplanting hospital is responsible for following up its living donors; if necessary they are able to establish policies which give priority to certain cases.
Given the scarcity of ESRD cases in each LDKT scheme, there is very little bibliography on how these patients progress before and after transplantation. Multicentre registries are essential to gain an understanding of the problem as a whole. All efforts are directed towards ensuring that affected donors progress well, but if they develop ESRD it is the responsibility of the transplantation hospital to return them the therapeutic benefit which they had created for the scheme in the past.
Mortality due to any causeEarly mortality deriving from renal donation is very low, and it is lower than the level corresponding to similar elective procedures (B)
- •
The long-term mortality rate due to neoplasia in donors is no different from the rate in a comparable control population (B).
- •
According to American registry data mortality in the first three months after nephrectomy stands at 0.03% 118,120,820. This is lower than the rate corresponding to other surgical procedures, such as laparoscopic cholecystectomy or nephrectomy for other reasons, although it is higher than the estimated risk of peri-partum mortality 820. The most frequent causes of death are haemorrhage, cardiovascular events and pulmonary thromboembolism. After this time, mortality in the first year is comparable to that of healthy controls 120. Apart from the studies described above on survival and cardiovascular risk, two recent publications found no differences in terms of mortality between donors and healthy controls after average follow-ups of 8 years821 and 11 years822. Neoplasias were the most common cause of death in the follow-up of each of the groups in both of these recent studies. Several registry studies confirm this finding 784,823,824. However, the lack of a control population makes it impossible to draw conclusions regarding risks. None of these publications permits the conclusion that donors are at higher risk of mortality due to neoplasia than is the case for the general population. Nevertheless, the importance of extensively ruling out neoplasia before donation has to be underlined, and this should include recent urological and gynaecological examinations, while also ruling out malignity in suspicious skin lesions before continuing with the process.
The recipients of LDKT have longer survival than those who receive a kidney transplant from deceased donors. This may be explained by many factors; it avoids the renal lesion associated with brain death, the donors are in good health and have little comorbidity, cold ischemia times are very short and the surgical conditions for the recipient are optimum because it is a planned elective procedure. Moreover, with this type of donation early transplantation is often attempted, or the time spent by recipients in dialysis is minimised.
United Kingdom data show that 10 years after transplantation, 75% of the adults who received their first KT from a brain dead donor from 1998-2000 are alive. This compares with the survival of 90% of patients who received their first LDKT during the same period. There was therefore a 20% improvement in patient survival at 10 years after living donation versus deceased donation for those who were transplanted during this period 825.
Transplantation versus dialysis- •
We recommend KT due to its lower mortality rate in comparison with overall patient survival in the waiting list, regardless of the type of transplantation. These differences are detectable after the third month post -RT (Quality of evidence: B).
- •
We recommend shortening the time spent in dialysis prior to transplantation, as this is a factor that can be modified, and it strongly influences long-term survival after transplantation (B).
- •
We suggest that LDKT should always be considered as the first option for patients with CRD, and above all in unfavourable clinical situations (young patients, ones with a high comorbidity, a high level of surgical complexity or highly sensitized patients) who have fewer possibilities of accessing a deceased donor renal transplant (C).
Patients with ESRD have significantly higher mortality than the general population and those with a functioning renal graft 826. To centre on patients who are KT candidates, longitudinal studies have shown that the overall mortality of patients in the waiting list is significantly higher than mortality in transplanted patients, independently of the type of transplantation. These differences may be detected after the third month post -KT 827. The most frequent causes of mortality during the first year of replacement therapy are cardiovascular disease, followed by infectious complications 828. There is not only a clear benefit in comparison with dialysis in terms of recipient morbimortality, as this benefit also includes the economic repercussions for healthcare systems 829.
Rarely do we have to choose between a LDKT that is “suboptimum” or “ideal” for a potential recipient. The alternative to a LDKT from a donor of any age is to remain in dialysis, waiting for a suitable organ from a deceased donor. The annual mortality rate for patients in dialysis in Spain stands at 15%-16%, and it is far higher in elderly patients, a significant percentage of whom die while they remain in the waiting list 830. To complicate this situation even more, comorbidity may increase over time in patients who are receiving dialysis 831, and the higher their degree of comorbidity, the more likely it is that they will be excluded from the KT list 832.
Moreover, a long time spent in the waiting list may increase the possibilities of receiving blood transfusions due to the appearance of additional complications. This may give rise to a higher risk of sensitization against a possible graft due to the formation of anti-HLA antibodies, thereby prolonging the waiting time even more and permitting the emergence of comorbidities 833,834.
The duration of dialysis prior to transplantation is still an important and potentially modifiable factor in long-term survival after transplantation. Unfortunately, young recipients may take some time before accessing a KT due to the scarcity of younger donors. 56% of the patients included in the waiting list in the United Kingdom in 2011-12 received a transplant after 3 years, 6% had been eliminated from the waiting list and 5% had died while awaiting a donor 825. This situation often arises, with long waits before transplantation. Mortality while waiting is another important matter when considering a potential living donor among transplantation options.
Expanded living donor KT criteriaWe recommend LDKT even when donors are over the age of 69 years, as it is a beneficial alternative to remaining in dialysis or to spending more time in the deceased donor waiting list (B).
- •
Graft survival is shorter in recipients with living donors older than 70 years than it is in recipients with standard donors, although patient survival is similar (B).
- •
We suggest that donor renal function may be more important than age in the results of LDKT, although this is not confirmed by all studies (C).
- •
We suggest that donors should not be excluded solely on the grounds of age. Older donors should be accepted or rejected after an exhaustive medical assessment (NG).
- •
The persistent disparity between the number of patients waiting for a RT and the available organs in the group of deceased donors, together with increasing knowledge and familiarity with the results of LDKT for donors and recipients, has led the transplantation community to consider candidate donors who would have been ruled out in the past due to their demographic characteristics or intrinsic medical problems.
The areas of uncertainty associated with the short- and long-term results for medically complex living donors should be considered depending on the cause of this clinical situation (age, hypertension, obesity, DM, lithiasis or oncological problems) as described in other chapters in these recommendations.
In terms of results for the recipient, several alternative options should be considered: the probability of receiving a transplantation from a deceased donor using standard or expanded criteria, the result of the said transplantation, the morbidity and potential risk of mortality while waiting for a suitable organ from a deceased donor, the suitability/risks associated with emergency rather than elective surgery, the result that can be expected with such an organ from a living donor in comparison with the foreseeable life expectancy of the same, etc.
The literature is sufficiently conclusive in supporting LDKT from older donors for at least one cohort of patients. Renal function gradually falls with age, and the renal function of an older living donor may be reduced 835. Several single centre or registry studies show poorer results in terms of renal survival after LDKT as donor age increases 560,836,837. In comparison with the recipients of grafts from standard donors, graft survival falls in recipients whose living donors were ≥70 years old, although patient survival was similar. Renal transplantation recipients from older donors had better graft and patient survival than did recipients with expanded criteria donors 560 (Table 17). In general, it seems to be beneficial to use older LDKT donors, equivalent to waiting for a deceased renal donor 560. Estimation of the GFR has been shown to be an important factor in transplanted renal function 216, and it has been suggested that donor renal function may be more decisive than their age for the final results, although this has not been confirmed by all studies 838.
Survival of renal graft and patient adjusted for donor sex and race, and the age, sex, race, renal disease aetiology and HLA of the recipient (Ref 560). LDKT: living donor kidney transplantation. eGFR: estimated glomerular filtration rate.
| Graft survival at 10 years | Patient survival at 10 years | |
|---|---|---|
| Donor group (reference=standard donor) | ||
| LDKT <60 yearsLDKT 60-64 yearsLDKT 65-69 yearsLDKT >70 yearsExpanded criteria donor | 0.73 (0.71-0.74), p<0.0011 (0.91-1.11), p=0.991.2 (1.02-1.41), p=0.031.73 (1.29-2.31), p<0.0011.83 (1.78-1.88), p<0.001 | 0.70 (0.69-0.72), p<0.0010.82 (0.76-0.89), p<0.0010.98 (0.86-1.11), p=0.741.08 (0.86-1.36), p=0.521.32 (1.29-1.35), p<0.001 |
| Donor group (reference=LDKT<60 years) | ||
| LDKT 60-64 yearsLDKT 65-69 yearsLDKT >70 years | 1.41 (1.25-1.59), p<0.0011.67 (1.36-2.05), p<0.0012.18 (1.50-3.17), p<0.001 | 1.15 (1.03-1.28), p=0.011.39 (1.18-1.64), p<0.0011.54 (1.14-2.08), p=0.01 |
| Donor group (reference=eGFR >150) | ||
| eGFR: 120-149ml/min/1,73 m2eGFR: 90-119ml/min/1,73 m2eGFR: 60-89ml/min/1,73 m2eGFR: <60ml/min/1,73 m2 | 0.99 (0.76-1.29), p=0.961.08 (0.84-1.39), p=0.521.16 (0.9-1.48), p=0.251.32 (1.01-1.71), p=0.04 | 1.12 (0.85-1.47), p=0.411.08 (0.84-1.4), p=0.551.07 (0.83-1.38), p=0.611.09 (0.84-1.43), p=0.51 |
Older donors are more likely than younger ones to be excluded from donation due to problems discovered during their medical assessment. Nevertheless, each case should be considered individually, and if an older donor is considered suitable after a rigorous medical assessment, and if their renal function is normal after correction for age and sex, there is no evidence that they should be excluded from donation solely due to their age 839,840. In a similar way to age, the advisability of assuming that a live donor kidney will have “non-ideal” survival in the recipient (due to comorbidities, renal function or HLA compatibility …) must also be considered on an individual basis for each particular recipient.
High risk recipientsWe suggest that “high risk” recipients should not be automatically excluded, to prevent unfairness in access to KT. Although the results in terms of survival may be statistically “worse” due to their associated comorbidities, each case should be assessed individually after the relevant studies (NG).
- •
We suggest that, with some exceptions, patients accepted for deceased donor KT are also suitable for a LDKT (C).
- •
We suggest that the LDKT option should not be considered when it is estimated that post-transplantation survival will be not reach two years, or when there is a high probability of renal disease relapse in the graft (C).
- •
LDKT may offer opportunities to patients whose perioperative risks for an emergency procedure are considered to be unacceptably high, although they may be acceptable for an elective KT, optimizing harvesting from an optimum donor, with a team to manage any special difficulties, commencing immunosuppression before transplantation and minimising any delay in graft functioning (NG).
- •
We suggest that the acceptance or rejection of LDKT in high-risk patients should be based on the opinions of expert multidisciplinary teams. A lack of data makes it impossible to make clear recommendations for highly complex recipients (NG).
- •
The success of a KT scheme cannot be judged solely on the basis of recipient and graft survival in patients with ESRD. There may be errors in data interpretation, and it is more probable that a scheme which takes no risks will give results corresponding to excellent recipient survival. This policy may potentially be associated with unfair lack of access to transplantation for patients who are highly complicated in surgical terms, or at immunological risk and subject to a higher risk of death in the waiting list. It is not easy to measure the difficulty certain populations have in accessing transplantation 841.
To ensure terminological uniformity, a high risk recipient is one with a significantly higher risk of death, complication or graft failure due to pre-existing comorbidity or their immunological status. Statistically, this is equivalent to poorer results for the graft and patient survival. There are no robust scoring systems for this risk evaluation. Although it is probable that models will soon be developed 842, it is known that with the uncertainty of current predictive tools there is still dependence on the clinical judgement of the transplantation professionals who are involved. The recent British guidelines, and solely as a recommendation with a low level of evidence (C2), state that in cases where the probability of post-transplantation survival is less than two years, the option of LDKT should not be considered LDKT (2).
LDKT may offer opportunities to individuals whose perioperative risks for an emergency procedure are considered to be unacceptably high, as they may be suitable for elective transplantation. The advantages include: recipient optimization, resolving and controlling their active health problems beforehand, the ad integrum availability of all of the personnel in the multidisciplinary team (surgeons, anaesthetists, intensive care specialists, nephrologists, immunologists and nursing staff, etc.), with the administration of immunosuppressant drugs prior to transplantation and a high quality organ with very low risk of retarded graft functioning.
The key question is whether, although these patients can expect a relatively worse transplantation outcome in comparison with standard risk patients, their result may be better than it would be if they remain in dialysis. The persistence of cardiovascular disease, pulmonary disease, obesity, diabetes o hypertension will affect the survival of renal patients whether they receive a transplantation or are dependent on dialysis 843.
Another group of patients who may be considered complex is composed of those at high immunological risk and sensitized. LDKT recipients with HLA incompatibility are often excluded from survival analysis in the results of LDKT as their rates of survival are not as good as those for compatible transplantations without antibodies. However, for some patients transplantation in these circumstances is the only way they will achieve independence from dialysis. The recommendation is to establish a specific scheme to select a donor against whom the recipient does not produce antibodies. This objective can be achieved in LDKT by paired exchange strategies. Patients with DSA should only receive a transplantation if these measures cannot be applied and after desensitization programmes. The presence of DSA that can be detected using techniques other than complement-dependent cytotoxicity is considered to be a risk factor in itself but not a contraindication 101. In the case of HLA incompatibility, the use of desensitization techniques in ABOi KT offers similar long-term results to ABOc KT 53.
The donor and the recipients should be informed of the possible risks and results. Although this is also the case for living donation transplantation in general, it is particularly important for highly complex recipients, where the risk of an adverse result is higher than it is in cases of standard transplantation. It is proves impossible to reach an agreement within a particular transplantation hospital due to differences of opinion on the degree of risk, the option of referral to another hospital for a second opinion should be discussed with the potential recipient and donor.
Although we unify the inclusion criteria of our protocols, clinical practice will surely eventually lead us to differences in who we include. Nevertheless, this would not be a problem and it would be corrected by the patient's freedom to request second or third opinions about the advisability of a LDKT. It is important that the decision to transplant into high-risk recipients should not be influenced by the sometimes unwarranted concern about the possible results. It is very difficult to set standards for results in this LDKT group, given the heterogeneous nature of the patients and the differences between the definitions of what constitutes high risk between different units.
19Coordination of the living kidney donation processAll of the successive phases of the LDKT process have to be perfectly coordinated to ensure its success, while always guaranteeing the complete protection of the living donor, the basic element of all LDKT Schemes 2,3,8,27,42,64,844,845. The different international standards agree in guaranteeing the principle of non-commercialization and the absence of practices which would be compatible with human or organ trafficking. The process should emphasise the need for free, informed and express consent to be obtained. It is also necessary to evaluate a range of medical, family, social, professional and economic aspects to ensure the well-being of the donor over the short- and long-term, minimizing the risks associated with the donation.
The LDKT process is regulated in Spain by Law 30/1979, of 27 October, on the donation and transplantation of organs 846 and Royal Decree 1723/2012, of 28 December, which governs the activities of obtaining organs, clinically using them and the territorial coordination of the human organs for transplantation, establishing quality and safety requisites 41. Our regulatory framework emphasises respect for the basic principles described above, and it standardises several aspects of the process, including; i) the medical and psychosocial assessment of the potential donor by a doctor other than the ones who will harvest and transplant the organ; ii) evaluation of the procedure by an Ethics Committee; and iii) the donor has to appear before the Court appearance. Compliance with these legal requirements and managing the different logistical aspects of the process requires coordination work which may be undertaken by one or more professionals with different profiles and origins.
According to the document TR de donante vivo en España. Análisis de Situación y Hoja de Ruta (“Living Donor KT in Spain. Situation Analysis and Route Map”), prepared by the ONT, the SEN and the SET, of the 33 Spanish hospitals with a LDKT scheme, the Transplantation Coordinator (TC) is in charge of coordinating the process in 10 of them, chiefly in transplanting hospitals with little activity 847,848. In 15 of the 19 hospitals that have no specifically designated professional for this coordination work, the TC supports different stages of the process. More specifically, they work together with the transplantation teams in the logistic aspects of the process, support the healthcare Ethics Committee (EC) and take part as a guarantor of the protection of the donor who is independent of the transplantation team, as required by Spanish law and as recommended internationally–the “donor advocate” 88.
The professional in charge of coordinating LDKT has to coordinate their work with the KT team, and this justifies their inclusion in the multidisciplinary LDKT group, on condition that their independence is ensured, together with their role of guarantor of the rights of the donor 3,27.
Coordination of living donor KTHealthcare coverage by the National Health System or private insurers must be guaranteed for the direct costs of pre-transplantation assessment and the medical and surgical interventions in the LDKT process. It must be ensured that the donation will be economically neutral for the donor, facilitating reimbursement of the direct and indirect costs of the same. (Quality of evidence: B)
For foreign living donors, the relationship between the donor and recipient must be verified, and the former must comply with the legal requisites to enter and remain in the country during the LDKT process. (B)
The logistics of the process should be organised in such a way that evaluation of the viability and compatibility of the donor and recipient takes place in the most effective and fluid way possible, always offering complete information, particularly to the donor, about the risks they are accepting and the expected benefits of the LDKT.
As well as collaborating with the nephrologists of the LDKT scheme by informing patients, professionals in the dialysis units and ESRD units of the hospital and others for which the transplanting hospital is the referral hospital, the individual in charge of coordinating the LDKT process may also collaborate in the following 10 phases:
Initial assessment by the nephrologist who is responsible for the living donorThe process commences with the interview by the responsible nephrologist and the potential donor. This permits an initial evaluation of the feasibility of the process and factors in connection with the protection of the donor. According to Royal Decree 1723/2012, of 28 December41, it is necessary to verify that the principles of voluntary donation, altruism, confidentiality, the absence of a desire for profit and freedom from charge have been fulfilled. The laboratory tests should then be requested, HLA and the imaging tests described in other sections of this document. Visits to the other professionals involved in the scheme also have to be arranged, to gather all of the opinions that make it possible to discuss the case in a multidisciplinary way before it is approved or rejected: urology, anaesthesia, psychology or psychiatry and other specialities (vascular surgery, cardiology, oncology and haematology, etc.).
Offer the donor a free space where they can express their doubts and resolve questionsIt is advisable to offer a potential donor an interview without the presence of the recipient, to give them a space where they can express themselves freely, revealing their worries and doubts. In this interview the donation process has to be explained once again, clarifying and resolving any questions in a way that will allow them to give their valid informed consent. The importance of the EC should also be explained, together with the legal obligation to appearance in Court, to ratify that the organ is donated freely, without economic or any other form of coercion, and that they have been duly informed.
As this interview with the donor is in private, it is important to verify that the donor fulfils the recommended requisites in the Council of Europe Guide to safety and quality assurance for organs, tissues and cells27 and the ones in these Recommendations, including the evaluation of risk factors and behaviours to minimise the possibility of transmitting infectious diseases–and diseases of other types - to the recipient. Although this task should be carried out by the doctor in charge of assessing the state of health of the donor, it is important that an independent professional should also do so. The latter should, in a coordinated way with the transplantation team, indicate precautionary measures to the donor, informing them of the risks which certain forms of behaviour involve for the recipient.
Review of the documentation and the relationship of the living donor with the potential recipient (Table 1)Documentation has to be requested which accredits the identity of the potential donor and recipients, and which guarantees the relationship between them, although it is the responsibility of the Examining Magistrate to verify the veracity of this documentation.
This fact is particularly important in the cases of live donors who are not genetically or emotionally related, as well as when the donor (and/or the recipient) are foreigners, either individuals from another country who have travelled specifically for the transplantation, or foreign residents in Spain. In such cases, some groups propose that, prior to commencing the tests in the studies of the health of the donor and recipient, an interview should be held to exhaustively review the documentation which proves the tie between them and ensure that both the donor and the recipient are authorised to legally stay in Spain. It is also suggested that the EC should be consulted first to evaluate the advisability of interviewing the donor before continuing with the assessment process.
Foreign donors (and/or recipients) who live outside Spain are recommended to count on a known and trusted medical team in a referral hospital, so that they are able to perform the basic tests in their country of origin which are necessary to assess the suitability of the donor, thereby avoiding unnecessary travel and expense for the donor if the donation is contraindicated. The tests involved may include at least blood group, viral serology and renal function and ultrasound scan. In this case only donors genetically or emotionally related to the recipient should be accepted, for direct transplantation as well as for participation in the Paired KT Scheme of the ONT 543. A legal stipulation is that living donors must be supplied with medical treatment for their recovery, as well as life-long follow-up. It is therefore indispensable to ensure medical coverage in their country of origin, to guarantee medical care for the donor after the donation process. They must also be followed-up encouraging healthy lifestyle habits to help them preserve their health. The above recommendations are contained in a Government Resolution on the principles for the selection, assessment, donation and follow-up of non-resident living organ donors85.
Table 18 describes the documentation used to prove the relationship between the donor and recipient, and we suggest reviewing this based on the origin of the donor and/or recipient.
Documentation to prove the tie between donor and recipient.
| Spanish nationality |
| DNI (National Identity Card) |
| Medical insurance (social security, private, etc.) |
| Proof of the tie with the recipient (libro de familia (official family record booklet), civil registry, historic municipal registration, photographs, shared bank accounts, testimonies by friends or family members) |
| If the donor has children who are minors or their children's family lives abroad or does not live with the donor, some groups suggest that the partner or family should be aware of the donation process |
| Foreigners: As well as the four documents listed above, we suggest |
| The documents which prove the tie should be translated into Spanish, English or a comprehensible Latin-based language. Any translation must be by an official translator. It is recommendable to consult the respective Embassy or Consulate, to verify the validity of the documents which prove the tie. |
| Valid passport, preferably one with future validity of at least 6 months |
| Visa or legal residence permit which covers the donation and transplantation process period |
Appropriately coordinating all of the studies, depending on the needs of the patient, makes it possible to perform them in a short period of time. It also permits monitoring the studies of a living donor, verifying that nothing relevant is lacking and speeding them up. As the Living Donor Route Map and Situation Analysis Document (Documento de análisis de situación y hoja de ruta del Donante Vivo)847 states, the lack of an individual in charge of this aspect (except in hospitals where a clinical route or similar pathway has been implemented) slows down procedures because they have to be done over and above the normal daily work for the professionals involved.
Collaboration in decision-making and the socioeconomic, familial, social and professional evaluation of a donorTo ensure that donors are completely protected, the interview seeks to discover the nature of their decision-making process and their family and social context, as well as their economic and professional situation:
The decision to donate:
- •
How they obtained the information on how to become a donor
- •
Motivations to be a donor
- •
Feelings they have experienced
- •
Whether they have asked people they know about their decision
- •
Pressures that may have influenced their decision
- •
Implications and consequences at every level
Family situation:
- •
The individuals in their close family
- •
Family conflicts
- •
Individuals who would care for them after post-donation discharge from hospital
- •
How they have planned for the well-being of those who are dependent on them in case of any complication arising, particularly if there are children or family members who live abroad who are economically dependent on the donor
- •
Professional and economic situation
Profession and employment:
- •
type of contract, the effects of their decision on their work, the possibility of time off work.
Economic repercussions:
- •
Measures they have adopted
- •
Their social situation and family and social support.
The multidisciplinary team will therefore be able to check with the donor and guide them regarding possible reimbursement for the direct and indirect costs deriving from their donation, such as travelling expenses, allowances, lodgings, childcare and time off work, to prevent the donation having a negative economic impact. These aspects are especially important when the donor lives abroad. The National Health System in Spain or the recipient's private insurance policy covers the costs deriving from pre-transplantation assessment and medical and surgical procedures. In the case of private donations, care must be taken to ensure that these costs are paid for by the recipient. Foreign living donors in particular, and most especially if they require treatment that was unconnected to the donation process (such as if a previously unknown neoplasia is detected), then it important to inform the donor that such costs are not covered by the healthcare coverage of the recipient (2,85,848). It is advisable to check that the donor has someone who will be able to care for them, at least during the first days after discharge from hospital (2).
Participation in the Informed Consent procedureThe nature of a valid consent process (free, informed and express) was described in the living donation Ethics Chapter in this document. The LDKT coordinator has to verify that the donor understands the information given and that all of their questions have been answered. Acting as the guarantor of the donor's rights, the coordinator has to verify that their consent is granted expressly, freely, consciously and selflessly. They have to clarify that this decision is reversible, so that at any time the potential donor may withdraw from the process without giving any explanation and without any repercussion whatsoever. In all cases, and in particular for unrelated and altruistic donors, there must be verification that they are under no external pressure and that there no other circumstance which suggests that there is an economic, social, psychological or any other type of conditioning factor.
Arranging the multidisciplinary meeting to accept a living donorDepending on the level of LDKT activity, it is recommendable to schedule a regular meeting to discuss all of the cases once they have been assessed as possible living donors and before they are presented to the EC for evaluation. This meeting should be multidisciplinary, with all of the professionals who are assessing the suitability of the living donor candidates in terms of the physical and mental health. This assessment will include nephrology, urology, anaesthesiology, nursing, radiology, immunology, haematology, social work, psychology and psychiatry. The LDKT coordinator will be able to take part in this meeting by offering their opinion as the donor advocate, offering information on the relationship between the donor and recipient, and explaining their social, familial and professional situation. An overall clinical evaluation of the donor and recipient will determine whether they fulfil the criteria set by each transplantation group, and the decision is taken if to approve the case as a living donor for a specific recipient, or if they will enter the National Paired Renal Transplantation Scheme. It is advisable to prepare the Minutes of this meeting to be appended to the clinical history of the donor.
Coordination of the legal process: theEC and appearing before the CourtLDKT is facilitated by a specific professional who coordinates the ethical-legal process. In turn they have to guarantee that the hospital where the process of donation, harvesting and transplantation will take place has been expressly authorised for the harvesting of living donor organs by the corresponding Autonomous Community health authority.
We recommend that the EC should meet regularly, especially in hospitals with a high level of transplantation activity. The EC should receive the medical reports on the donor and recipient, as prepared by the nephrologist in charge of the process, as well as the mental state report prepared by the psychologist or psychiatrist and the social, familial and professional evaluation of the donor, which may be supplied by the LDKT coordinator. The nephrologist, the coordinator and other professionals who are considered relevant will attend the EC meeting. After deliberation, the EC will prepare the report which authorises the donation.
The LDKT process coordinator will be able to manage the appearance in Court, agreeing the day and time for this with the Judge, together with the presentation of the documentation and the ratification of the consent. The donor, the doctor in charge of the process and the surgeon in charge of the harvesting and transplantation must all go to the Court. All of them will have to show their identification national card or passport (if the donor is foreign). In the case of a living donor who does not speak any of the official languages of Spain, they must be accompanied in the Court by an independent interpreter who has no ties with the donor, the process coordinator or the transplantation team, after approval by the Judge.
The following relevant documents must be given to the court:
- •
The application to appear before the Court, signed by the donor.
- •
Certification of the donor's state of health, signed by a doctor who is independent of the living donor study process.
- •
Copy of the consent document or the authorization for living donor renal donation
- •
The EC Report
In the case of a donor in the National Paired KT Scheme, the Judge will be given a copy of the codes generated by the scheme. The Judge will also be given a copy of the Paired KT Authorization Document - generated by the ONT, and which contains the information on the pairs taking part - signed by the donor.
In the Court, the Judge will verify the donor's free decision, and before the Judge those attending will sign the Certificate of Consent for the Donation of human organs between living individuals. The Court will give a copy for the donor's history and another copy for the donor him/herself.
LDKT report for the competent authorities and the Registry.In the case of a foreign live donor, prior to donation the Autonomous Community Transplantation Coordination authority and the ONT must be informed, sending information which verifies the tie, passports and medical coverage of the donor and recipient. In the case of a living donor for paired KT, the KT unit has to inform the ONT that the donor and recipient have accepted to participate in the program. The Codes of the National Paired KT Scheme are obtained from the ONT web portal 543, together with a table showing the hospitals and donors involved in each paired donation and the ONT Paired Donation Scheme Authorization Document, which the donor has to sign. In turn, on the day after the donation, the Autonomous Community Transplantation Coordination authority will be informed and it will be registered in the National and Autonomous systems of the Living Donor Registry and, in the case of a foreign donor, it will be possible to also inform the respective Registry in the donor's country of origin.
Renal transport management in the paired living donor schemeThe transport and/or reception of the kidney to and from the hospitals involved has to be organised by the National Paired KT Scheme 543. The packaging, preservation and transportation have to be verified according to ONT specifications 849,850 and Royal Decree 1723/2012, of 28 December 41. An internal document of the hospital of origin will be appended, making it possible to identify the donor (ONT Paired Code, hospital clinical history, etc.), the side of the kidney, the macroscopic appearance of the organ, the type of harvesting procedure used, warm ischemia times and the time of renal perfusion and the solution in which the organ is preserved. The report on donor and organ characteristics also has to be included (including blood group and recent serologies, from at least 7 days before donation), in a similar way to the procedure with a kidney from a deceased donor, and according to legal stipulations.
Good communication and coordination is necessary between the different teams taking part in the National Paired KT Scheme, together with the ONT and the Coordinating authorities of the Autonomous Communities involved. This will guarantee that the terrestrial and air transport systems used function at the highest level of efficacy, and that everyone involved is constantly informed in real time about the situation in each hospital. This will make it possible to reduce cold ischemia time to a minimum. Some recent publications in the U.S.A. and Australia, where distances are very long, have described that a cold ischemia time of 16hours or less has no significant affect on renal function 745,851,852. Once the chain of paired transplantations has ended, it is advisable to report any incident that may have occurred during the process so that the relevant corrective measures can be applied, acting to improve the National Scheme. All of the information corresponding to the donation and engraftation should be shown in the clinical history of the donor, either on paper or in a computerized clinical history.
O summarise, LDKT schemes have to scrupulously comply with the legal regulations that are in force. The complete protection of the living donor has to be a priority in all schemes. The work of coordinating the LDKT described here should be carried out by one or several professionals in each authorised hospital, and in Spain this work is done by the TC, wholly or partially. The final aim is to facilitate and coordinate the different stages of the process, ensuring its ethical and legal correctness while guaranteeing the protection of the donor and the transparency of the process, promoting measures that will improve the whole system.
Conflict of interestsThe authors have no conflict of interests to declare.
This study was partially supported by grants from the Instituto de Salud Carlos III, co-financed by the European Regional Development Fund–ERDF (Redinren RD16/0009) and grants ICI14/00016 and PI17/02043) of the Spanish Government Ministry of the Economy and Competitiveness. These institutions did not take part in designing the study or the gathering, analysis and interpretation of data or writing the manuscript. We would like to thank Silvia Musquera for the drawings in Figure 12.
Marta Crespo RD16/0009/0013 (ISCIII FEDER RedinRen) is the beneficiary of an intensification scholarship for research (INT21/00003, Spanish Ministry of Health ISCIII FIS-FEDER).