Recently, a new class of dialyzers, medium cut-off membranes (MCO), designed to improve the permeability, which could provide an efficacy similar to hemodiafiltration, have been incorporated into our therapeutic possibilities. To increase the knowledge about its use, the objective of the study was to evaluate the effect of the surface and blood flow (Qb) on the depurative efficacy in the MCO membranes.
Material and methodsWe included 19 patients in the hemodialysis. Each patient received 6 sessions, in which the membrane surface was varied, 1.7 or 2.0 m2, and/or the Qb (300, 350, 400 or 450 mL/min). In each session, different solutes were determined at the beginning and end of dialysis.
ResultsThe surface change of the dialyzer did not show significant differences in the removal of small or large molecules, without changes in albumin loss. The increase in Qb was accompanied by an increase in clearance of small molecules, without showing differences in the percentage reduction of β2-microglobulin, myoglobin, prolactin, α1-microglobulin and α1-acid glycoprotein, except for some comparison with Qb 450 mL/min. There were also no differences in the loss of albumin in the dialysis fluid, less than 2.5 grams in all situations.
ConclusionThe increase of the surface area of 1.7–2.0 m2 in the MCO dialyzer has not meant a greater depurative effectiveness. In these dialyzers the increase of Qb does not seem to be as determinant as in hemodiafiltration except for the clearance of small molecules.
Recientemente, se han incorporado en nuestras posibilidades terapéuticas, una nueva clase de dializadores, membranas de medio cut-off (MCO), diseñados para mejorar la permeabilidad y podrían alcanzar una eficacia similar a la hemodiafiltración. Para aumentar el conocimiento sobre su utilización, el objetivo del estudio fue valorar en las membranas de MCO el efecto de la superficie y del flujo sanguíneo (Qb) sobre la eficacia depurativa.
Material y métodosSe incluyeron 19 pacientes en programa de hemodiálisis. Cada paciente recibió 6 sesiones, en las que se varió la superficie de membrana, 1.7 o 2.0 m2, y el Qb (300, 350, 400 o 450 mL/min). En cada sesión se determinaron diferentes solutos al inicio y al final de diálisis.
ResultadosEl cambio de superficie del dializador no mostró diferencias significativas en la depuración de pequeñas o grandes moléculas, sin cambios en la pérdida de albúmina. El aumento del Qb se acompaño de un aumento de depuración de pequeñas moléculas, sin mostrar diferencias en el porcentaje de reducción de β2-microglobulina, mioglobina, prolactina, α1-microglobulina y α1-glicoproteína ácida, a excepción de alguna comparación con Qb 450 mL/min. Tampoco se observaron diferencias en la pérdida de albúmina en el líquido de diálisis, inferior a 2.5 gramos en todas las situaciones.
ConclusiónEl incremento de la superficie de 1.7 a 2.0 m2 en el dializador de MCO no ha significado una mayor eficacia depurativa. En estos dializadores el aumento del Qb no parece ser tan determinante como en la hemodiafiltración a excepción de la depuración de pequeñas moléculas.
Online hemodiafiltration (OL-HDF) with high replacement volume has been proven to be the treatment closest to kidney clearance; the ESHOL1,2 study demonstrated that such treatment produced a reduction in mortality from any cause with respect to hemodialysis (HD) in patients prevalent in HD. A reduction in overall mortality and cardiovascular mortality has been confirmed by main randomized clinical trials3 and several meta-analyzes.4,5 Finally, the French national registries (REIN) and the registries from Australia and New Zealand have also shown an association of OL- HDF with a reduction of mortality.6,7
Recently a new class of dialyzers, medium cut-off membranes (MCO), have been incorporated to our therapeutic possibilities in HD. These are designed to improve permeability based on the increased pore size.8,9 These membranes could be an alternative to HDF-OL, since this type of HD, called expanded HD, could reach an efficiency similar to that achieved with OL-HDF .10,11
We do have limited publications on the optimal function of this type of dialyzers. It is known that they should be used exclusively in HD mode to avoid excessive albumin losses, but there are not studies comparing different surfaces and the benefits of increasing blood flow (Qb), factors that limit both diffusive and convective efficacy. The aim of the present study is to increase knowledge and to optimize the use of this new MCO membranes; the specific objective of the study is to assess the effect of the membrane surface and the Qb on clearance efficiency and albumin loss.
MethodsProspective study in one single center on stable patients on regular HD program. We included 19 patients (16 men and 3 women) with a mean age of 65.5 ± 14 years (range 41–93 years) who had been in HD program for a period of 72 ± 78 months (range 6–296 months) The etiology of chronic kidney disease was: glomerulonephritis (n = 6), diabetic nephropathy (n = 4), polycystic kidney disease (n = 2), nephropathy of vascular origen (n = 1), tubular interstitial nephropathy (n = 1), urological cause (n = 1), ischemic disease (n = 1), and unknown etiology (n = 3). All patients were dialyzed through arteriovenous fistula, except one through prosthetic fistula. For anticoagulation it was used a low molecular weight heparin (tinzaparin) in 58% of patients, sodium heparin and 21% and another 21% were dialyzed without heparin.
Each patient received 6 HD sessions with MCO dialyzers, always on the same day of the week, in which the dialyzer surface was either 1.7 m 2 (Theranova 400™, Polyarylethersulfone, Baxter) or 2.0 m2 (Theranova 500™, Polyarylethersulfone, Baxter) ; and with the 1.7 m2 the Qb varied (300, 350, 400 or 450 mL/min). The characteristics of dialyzers are shown in Table 1. The other dialysis parameters were kept constant in each of the sessions: dialysis time, 291 ± 17 min (240−300 min); dialysate flow (Qd) 400 mL/min; 5008 Cordiax monitor, HD mode. The order of the sessions was randomized. The dialysis parameters collected in each session were: programmed time, actual duration, dialyzer, Qb, Qd, Kt automatically measured by ionic dialisance, recirculation index measured by the temperature module, arterial pressure (AP), venous pressure (VP), transmembrane pressure (TMP), initial and final hematocrit, ultrafiltration and the volume of blood that had been processed.
Characteristics of the dialyzers.
| Theranova 400 | Theranova 500 | |
|---|---|---|
| Membrane | Polyarylethersulfone | Polyarylethersulfone |
| Commercial brand | Baxter | Baxter |
| UFC (mL/h/mm Hg) | 48 | 59 |
| Wall thickness (μm) | 35 | 35 |
| Inner diameter (μm) | 180 | 180 |
| B2-microglobulin SC | 1.0 | 1.0 |
| Myoglobin SC | 0.9 | 0.9 |
| Albumin SC | 0.008 | 0.008 |
| Surface (m2) | 1.7 | 2.0 |
| Sterilization | Steam | Steam |
UFC: ultrafiltration coefficient; SC: sieving coefficient.
The concentration of the following molecules were measured in serum at the beginning and end of each session to calculate the percentage reduction of these solutes: urea (60 Da), creatinine (113 Da), β2 -microglobulin (11,800 Da), myoglobin (17,200 Da), prolactin (23,000 Da), α1-microglobulin (33,000) Da), α1- acid glycoprotein (41,000 Da) and albumin (66,000 Da). The final concentration of β2 -microglobulin, myoglobin, prolactin, α1 -microglobulin, α1- acid glycoprotein and albumin were corrected for hemoconcentration and volume of distribution (approximately extracellular volume) according to Bergström and Wehle.12 Urea, creatinine and albumin concentrations were measured by molecular absorption spectrometry in the ADVIA 2400 analyzer (Chemistry System, Siemens Healthineers, Tarrytown, USA). The β2 -microglobulin and α1- acid glycoprotein were measured by immunonephelometry with the Dimension Vista analyzer (Siemens Healthineers) and the α1-microglobulin by immunonephelometry by the BNII analyzer (Siemens Healthineers). Myoglobin concentration was measured by "sandwich" enzyme immunoassay with the Dimension EXL analyzer (Siemens Healthineers). The prolactin concentration was measured by "sandwich" enzyme immunoassay with the ADVIA Centaur analyzer (Siemens Healthineers).
In all cases reagents were adequate. The reference values of our laboratory are: urea: 10−50 mg/dL; creatinine: 0.3–1.3 mg/dL; β2- macroglobulin: 0.1–2.3 mg/L; myoglobin: 0−100 ng/mL ; prolactin: 2.8−15 ng/mL; α1 -microglobulin: 5−25 mg/L; α1- acid glycoprotein : 0.38–1.18 g/L ; and albumin: 34−48 g/L.
Likewise, a proportional part of the dialysis fluid was collected by means of a reverse infusion pump (RAZEL™, Vermont, USA) to quantify the loss of solutes including albumin. The concentration obtained was multiplied by the total volume of the dialysis fluid. Urea, creatinine and prolactin were measured as in serum. However, since the concentrations of β2-microglobulin and albumin were very low, it was necessary to analyze them with the same technology, but using methods applicable in urine samples in order to increase analytical sensitivity. The β2-microglobulin was measured with a detection index of 0.004625 mg/L.
Finally, a global assessment of the purification capacity (Global removal score) was calculated using the recently published formula13 : (UreaRR + β2-microglobulinRR + myoglobinRR + prolactinRR + α1-microglobulinRR + α1- acid glycoproteinRR - albuminRR)/6.
The results are expressed as the arithmetic mean ± standard deviation. Statistical significance of quantitative parameters was analyzed by the Student t test for paired data and ANOVA for repeated data (Bonferroni test). A p < 0.05 has been considered statistically significant. The analysis was performed using the SPSS version 23 program (SPSS, Chicago, IL, USA).
ResultsAll dialysis sessions were conducted without significant clinical incidents. None of the sessions had coagulation events in the lines or dialyzer. Comparison of the dialysis parameters is shown in Table 2. There were no differences in the total amount of blood processed, Qd, real duration of the sessions, initial weight, final weight, weight gain, measurements of initial or final hematocrit by the dialysis monitor, recirculation of the vascular access, AP or VP between the 2 surfaces studied (Table 2); the TMP was slightly higher in the membranes with smaller surface. Only 14 patients achieved a Qb of 450 mL/min for the assessment of the different blood flows. As expected, differences in total blood processed by the monitor, recirculation of vascular access, AP, VP and TMP were observed when the sessions were compared with different Qb (Table 2).
Comparison of dialysis parameters in the 6 study situations.
| Theranova 400 | Theranova 500 | p | Theranova 400 Qb 300 mL/min | Theranova 400 Qb 350 mL/min | Theranova 400 Qb 400 mL/min | Theranova 400 Qb 450 mL/min | |
|---|---|---|---|---|---|---|---|
| Processed blood (L) | 124.37 ± 13.3 | 123.9 ± 14.2 | 0.548 | 85.7 ± 5.6 | 99.5 ± 6.3 * | 114.4 ± 8.0 * a | 127.8 ± 8.1 * ab |
| Recirculation (%) | 14.5 ± 2.6 | 13.8 ± 2.5 | 0.163 | 10.1 ± 2.1 | 11.6 ± 2.4 c | 13.3 ± 2.1 * d | 13.9 ± 2.4 * d |
| Real time (min) | 286.8 ± 15 | 286.2 ± 14 | 0.468 | 286.1 ± 19 | 284.6 ± 18 | 285.6 ± 20 | 285.4 ± 19 |
| Initial Weight (kg) | 71.0 ± 16.7 | 71.2 ± 17.1 | 0.695 | 72.5 ± 19.4 | 72.6 ± 19.2 | 72.7 ± 19.6 | 72.6 ± 19.6 |
| Final Weight (kg) | 68.4 ± 16.6 | 68.9 ± 16.8 | 0.131 | 69.8 ± 18.8 | 69.9 ± 18.9 | 70.1 ± 19.0 | 69.7 ± 19.0 |
| Weight gain (kg) | 2.66 ± 0.89 | 2.35 ± 1.25 | 0.318 | 2.70 ± 0.98 | 2.68 ± 0.99 | 2.58 ± 0.94 | 2.89 ± 1.15 |
| Initial hematocrit (%) | 28.4 ± 4.1 | 29.3 ± 3.4 | 0,343 | 29.3 ± 4.1 | 29.9 ± 3.4 | 27.9 ± 3.7 | 27.9 ± 3.9 |
| Final hematocrit (%) | 34.0 ± 5.2 | 34.0 ± 4.2 | 0.966 | 34.7 ± 5.2 | 35.0 ± 3.7 | 33.8 ± 4.4 | 33.3 ± 5.3 |
| Arterial pressure (mm Hg) | −214 ± 18 | −219 ± 22 | 0.396 | −130 ± 35 | −164 ± 30 * | −196 ± 29 * a | −214 ± 24 * a |
| Venous pressure (mm Hg) | 206 ± 23 | 204 ± 31 | 0.698 | 146 ± 17 | 170 ± 23 * | 196 ± 28 * a | 205 ± 21 * a |
| TMP (mm Hg) | 31.7 ± 6.3 | 27.3 ± 4.2 | 0.010 | 26.4 ± 4.2 | 25.7 ± 3.9 | 27.4 ± 5.2 | 31.1 ± 6.8 |
TMP: transmembrane pressure.
* p < 0.001 with respect to Qb 300;
a p < 0.001 with respect to Qb 350;
b p < 0.001 with respect to Qb 400;
c p < 0.05 with respect to Qb 300;
d p < 0.05 with respect to Qb 350; (ANOVA for repeated data).
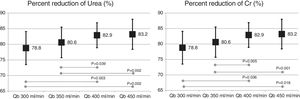
The change in the surface of dialyzer did not have a significant influence in the dose of dialysis or clearance of small molecules, expressed as Kt, 69.6 ± 5.1 L with 1.7 m2. and 69.7 ± 6.1 L with 2.0 m2 (p = 0.935); urea reduction ratio (RR) was 83.6 ± 4.6% with 1.7 m2 vs 83.6 ± 4.0% with 2.0 m2 ; p = 0.957; and creatinine RR was 77.3 ± 4.9% with 1.7 m 2 and 77.1 ± 4.5% with 2.0 m2 ; p = 0.791. However, the increase in Qb produced an increase in the clearance of small molecules (Fig. 1).
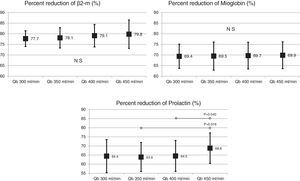
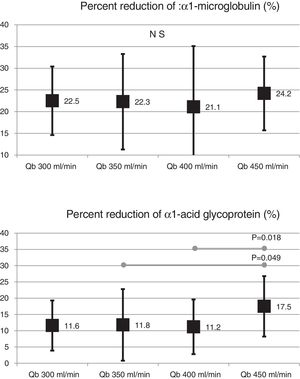
Also, the change in the surface of the dialyzer showed no significant differences in the removal of large molecules: of β2-microglobulin RR (80.4 ± 5.8% with 1.7 m2 vs. 80.3 ± 3.6% with 2.0 m2 ; p = 0.914); myoglobin RR (70.7 ± 6.3% with 1.7 m2 vs. 70.4 ± 6.8% with 2.0 m2 ; p = 0.780); prolactin RR (68.8 ± 8.0% with 1.7 m2 vs. 67.0 ± 7.8% with 2.0 m2 ; p = 0.275); α1-microglobulin RR (22.3 ± 14% with 1.7 m 2 vs. 19.4 ± 11% with 2.0 m2 ; p = 0.347) and of α1- acid glycoprotein RR (14.2 ± 8.3% 1.7 m2 vs. 11.6 ± 10.1% with 2.0 m2 ; p = 0, 246). With respect to the increase in Qb, no significant differences were observed in of β2-microglobulin, myoglobin or α1-microglobulin RRs. Only Qb of 450 mL/min produced a moderate increase in prolactin and α1- acid glycoprotein RRs (Figs. 2 and 3).
No significant differences were observed in albumin RR (10.9 ± 6.8% with 1.7 m2 vs. 11.8 ± 7.6% with 2.0 m2 ; p = 0.579); nor in the amount of albumin eliminated in the dialysate in the 2 surfaces evaluated (2.35 ± 1.03 g with 1.7 m2 vs. 1.89 ± 0.97 g with 2.0 m2 ; p = 0.104). Changes in Qb did not produce changes in blood albumin RR or in dialysate albumin loss (Fig. 4).
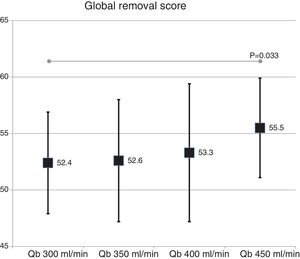
The global removal score was not different in the two dialyzer surface (54.9 ± 5.2% with 1.7 m2 vs. 53.3 ± 4.8% with 2.0 m2 ; p = 0.151). A slight increase was observed with the increase in Qb that reach significance only with the Qb of 450 mL/min as compared with 300 mL/min (Fig. 5).
DiscussionThe present study shows that the change in surface area from 1.7 to 2.0 m 2 of the MCO dialyzer showed no significant differences in clearance or albumin loss. The increase in Qb was accompanied by an increase in clearance of small molecules, with no significant differences in the percentage reduction of β2 -microglobulin, myoglobin, prolactin, α1 -microglobulin and α1- acid glycoprotein, except for some effects with the use of Qb of 450 mL/min. There were no differences observed in dialysate albumin loss which was less than 2 g in all situations. After a review of the literature, the present study is the first one that evaluates the effect of the surface and Qb in MCO dialyzers.
Dialyzers with MCO membranes in HD have been postulated as an alternative to HDF-OL, since they achieve a very similar removal efficacy and is safe, and dialysate albumin loss is less than 3 g.8–10 Some articles have shown that with MCO the removal of myoglobin, prolactin, complement factor D, α1 -microglobulin and free light chains are somewhat greater than with HDF-OL11,14; similar results are shown in another study15 and, finally, additional study shows a somewhat lower clearance of β2-microglobulin, myoglobin and prolactin.16 Most of these studies have been performed using dialyzers of 2.0 m2. The global removal score obtained in the present study with MCO membrane which was 52–55%, would be between the 47% obtained with HDF-OL in 2002 using the first generation of polysulfone17 and the 58% obtained in 2014 with latest generation of helixone.18
The present study has shown similar results with surfaces of 1.7 m2 and 2.0 m2, except for a 14% higher TMP in the smaller surface as the only differential characteristic, which did not mean any technical or alarm problems. The change in surface area was not very decisive in the post-dilution HDF-OL.19
In a previous study,20 in the HDF-OL modality, it was demonstrated the importance of Qb in the achievement of convective volume, which consequently increased the purification capacity of small molecules and β2-microglobulin, myoglobin and α1-microglobulin. In the present study, MCO dialyzers were also associated with better clearance of small molecules with the increase in Qb; however, the results of solute reduction ratios between 12 and 40 kDa were very similar, and reflected that the increase in Qb was not associated with a greater convective effect by backfiltration. Therefore, these results would indicate that HD with MCO dialyzers could be an alternative to HDF-OL, especially in those patients with low Qb.
We conclude that the increase in the surface area from 1.7 to 2.0 m2 in the MCO dialyzer is not associated with a greater purification efficiency. With these dialyzers, the increase in Qb does not seem to be as decisive as in post-dilution hemodiafiltration, except for the purification of small molecules. Considering the logistical, technical and economic reasons of each dialysis unit, HD with MCO membranes, could be an alternative to OL-HDF, especially in those patients with low blood flows. Of course will have to wait for clinical trials to show results on morbidity and mortality.
Conflict of interestsThe authors declare that there has been no financial support for this project. FM has received fees from Amgen, Baxter, Bellco, Fresenius Medical Care and Nipro. The rest of the authors declare no conflict of interest.
We want to express our gratitude to all the patients who have participated in the study, as well as to all the staff of the Dialysis Section of the Hospital Clínic de Barcelona for their collaboration and enthusiasm in this study.
Please cite this article as: Maduell F, Rodas L, Broseta JJ, Gómez M, Montagud-Marrahi E, Guillén E, et al. Valoración de la influencia de la superficie de la membrana y el flujo sanguíneo en dializadores de medio cut-off. Nefrologia. 2019;39:623–628.