Despite its significant impact on public health, chronic kidney disease (CKD) is often referred as a silent epidemic due to its low diagnosis rate and the lack of awareness among the global population. The estimated prevalence of CKD in adults in Spain is 15.1%.1 Several modifiable factors influence the development of CKD, including hypertension, diabetes, obesity, dyslipidemia, smoking, hyperuricemia, and cardiovascular disease.2 The increasing prevalence of these major risk factors, along with the progressive aging of the population, are contributing to a significant rise in the CKD burden that will surely continue to grow in the next years.3 According to data from The Global Kidney Health Atlas 2019, 759 patients per million population receive kidney replacement therapies (KRT) due to the progressive deterioration of renal function over the course of disease.4 Indeed, in the last decade the prevalence of advanced CKD requiring KRT has increased by 30%.5 In addition to impairing health-related quality of life, CKD imposes a significant economic burden, accounting for more than 3% of all healthcare costs.6
The best strategy to reduce mortality and sanitary costs is through effective, standardized and coordinated clinical management of factors potentially related to CKD, with the specific aim of preventing disease progression and achieving early diagnosis and treatment.
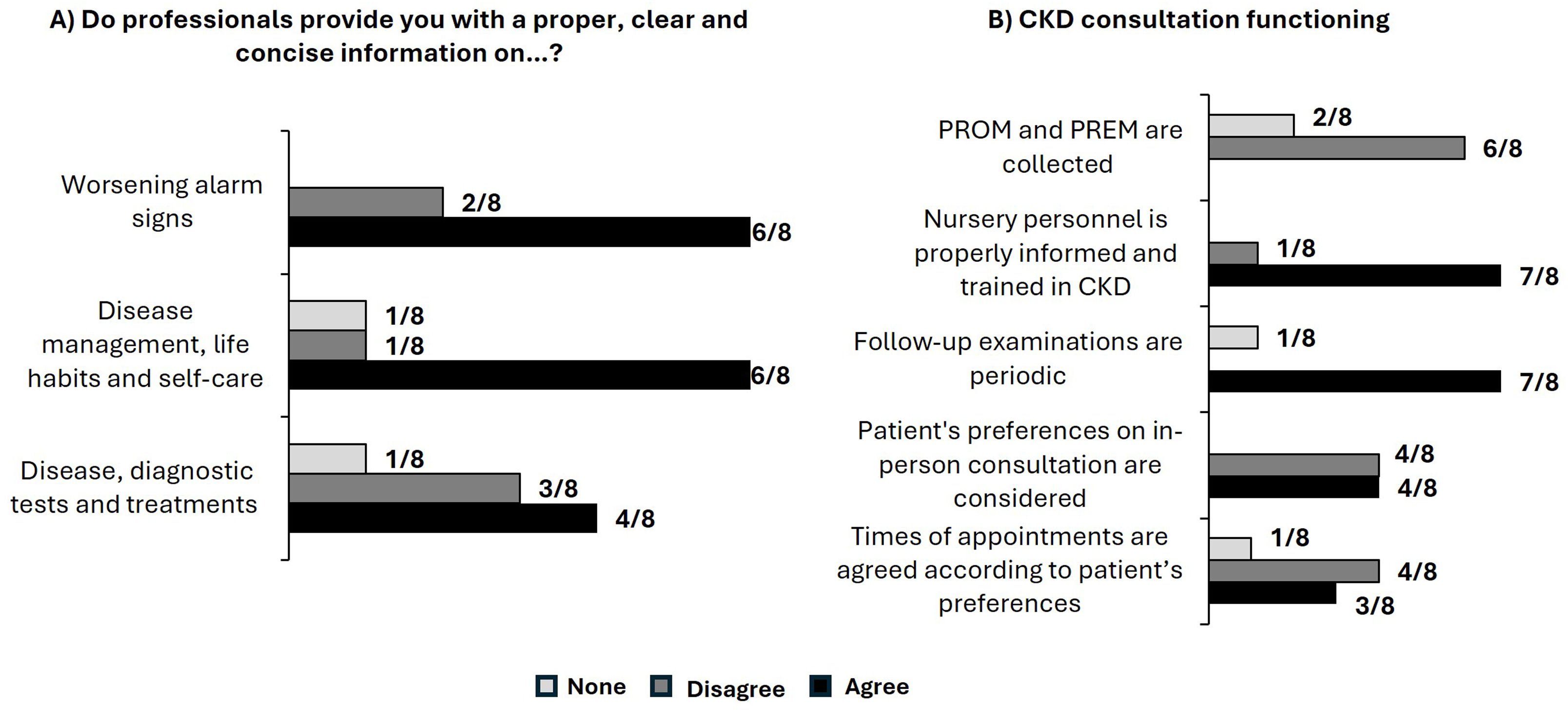
Patient-reported outcomes measures (PROM) and patient-reported experience measures (PREM) are increasingly recognized as essential components of comprehensive patient-centered CKD care. These tools allow patients to describe the impact of their disease on their health status, share their experiences, and provide feedback on the quality of care received. These measures can enhance the effectiveness of medical interventions and improve communication between CKD patients and healthcare professionals.7–9 In this respect, a series of face-to-face interviews with CKD patients conducted by specialized nurses revealed that while efforts are being made to provide adequate care, there is still significant room for improvement in the current CKD care pathway (Fig. 1).
In this context where the transformation and optimization of the CKD healthcare pathway could benefit from a roadmap, the CARABELA-CKD initiative was launched. Its objective was to establish a framework that serves as a catalyst for improving and innovating the current system. This collaborative effort involved the Spanish Society of Nephrology (S.E.N.), the Spanish Society of Endocrinology and Nutrition (SEEN) and the Spanish Society for Healthcare Quality (SECA), in partnership with AstraZeneca. The CARABELA-CKD initiative is part of a “fleet” of CARABELA initiatives aimed at catalyzing a mindset shift to enhance the effectiveness and sustainability of current care models for chronic diseases that generate a significant burden for the public national healthcare system.10 These initiatives are led and powered by scientific societies and are co-organized and supported by AstraZeneca.
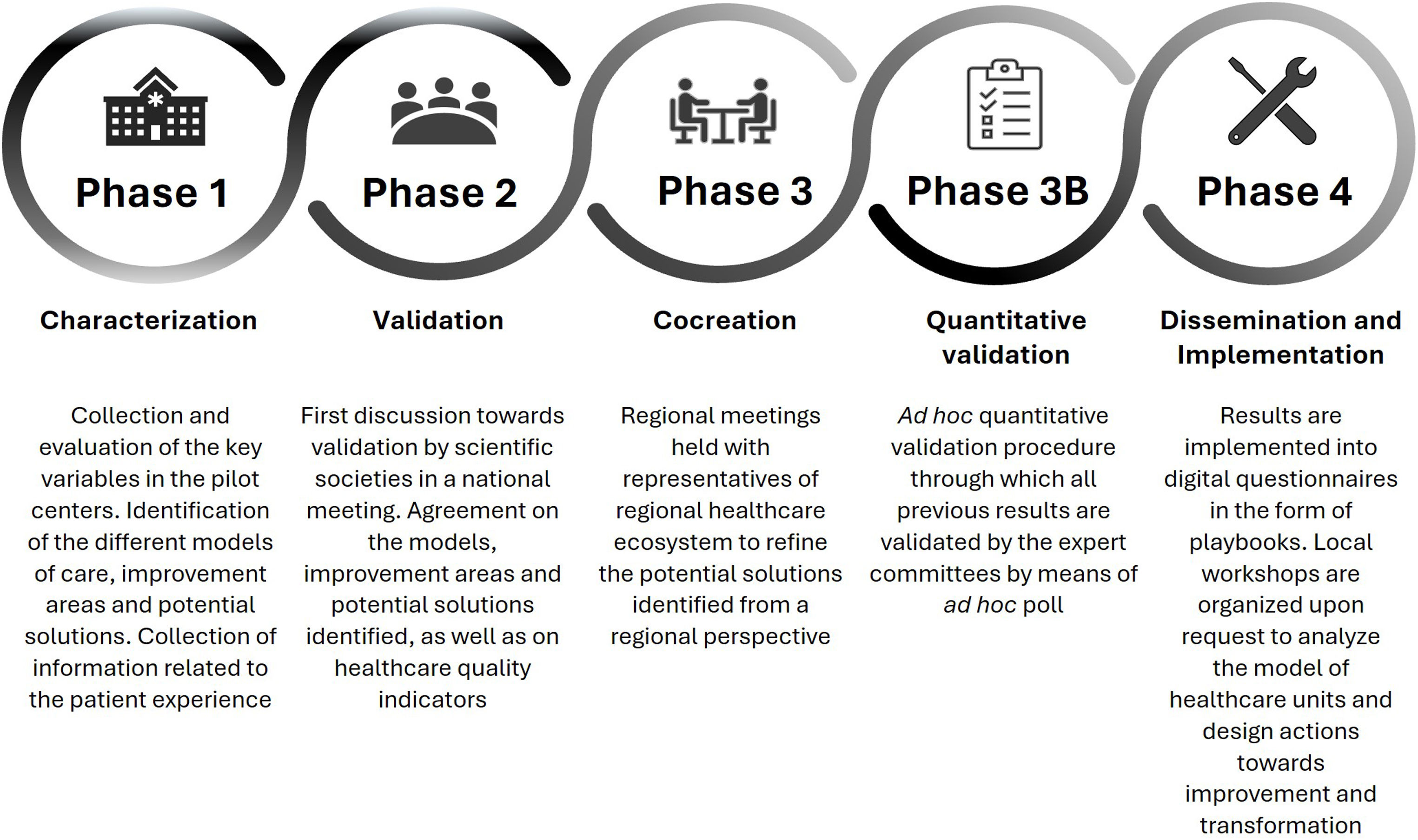
The main objective of CARABELA-CKD is to achieve the standardization and excellence of CKD care nationwide, ensuring all patients have equal access to early high-quality care and innovative therapies. We outline the general workflow of the CARABELA methodology in Fig. 2.
Flowchart of the CARABELA methodology. Adapted from Escalada J et al.10.
Phase 1 focused on the initial characterization of current CKD care models in Spain. This investigative phase 1 was overseen by a scientific committee comprising specialists in CKD management appointed by their respective scientific societies including S.E.N., SEEN and SECA. During this phase, current CKD management models were characterized in five participating pilot hospital centers and key healthcare quality indicators of CKD management, improvement areas (grouped into four challenges), and potential solutions (grouped into eight axes of change and 17 lines of actions) were identified.
Phase 2 primarily aimed to validate the findings from phase 1. To this end, a group of professionals with expertise in CKD nominated by their respective scientific societies convened in a national meeting to validate the improvement areas and potential solutions applicable to all identified care models and key CKD healthcare quality indicators that were identified in the previous phase.
In phase 3, a series of four regional meetings were organized in which additional experts in CKD management refined potential solutions from a regional perspective.
During phase 3B, an ad hoc quantitative validation procedure was conducted and reviewed by the steering committee as the final step.
Finally, in phase 4, the analysis and potential solutions were disseminated to as many Spanish healthcare centers as possible, to facilitate an in-depth transformation of the CKD healthcare process for the optimization of clinical pathway and patient management. Results were implemented in a digital questionnaire in the form of playbook that provided a detailed analysis of CKD healthcare units at each center and the design of improvement actions.
The results from the CARABELA-CKD initiative will be published shortly after this communication and will offer a framework for the development of an improved future CKD care model, based on a comprehensive and integrated approach aimed at addressing the improvement areas associated with the transversal potential solutions identified. The goal is to provide attending specialists and patients with standardized, excellent care aimed at optimizing the early detection and management of the disease and delaying its progression to more advanced stages.
FundingAll support for the present manuscript was provided by AstraZeneca Farmacéutica Spain.
Conflicts of interestBQ is secretary of Sociedad Española de Nefrología, and has received consulting fees, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, and support for attending meetings and/or travel from Vifor-Pharma, Astellas, Amgen, Ferrer, Novartis, AstraZeneca, Sandoz, Laboratorios Bial, Esteve, Sanofi-Genzyme, and Otsuka. JJGM has the following financial relationships: advisor on scientific boards for AstraZeneca, Bayer, Janssen Pharmaceuticals, Eli Lilly and Company, Menarini and Novo Nordisk; lectures for Abbott, Amarin, AstraZeneca, Boehringer Ingelheim Pharmaceuticals Inc, Janssen Pharmaceuticals, Eli Lilly and Company, Menarini, Mundipharma Pharmaceuticals, Novo-Nordisk and Roche Pharma, and research activities for AstraZeneca, Eli Lilly and Company, Mundipharma Pharmaceuticals and Novo Nordisk. JID is an employee at the Medical Department of Departamento médico, AstraZeneca Farmacéutica Spain. PR declares no conflict of interest.
The authors acknowledge the participation of all the professionals attending the regional meetings of the CARABELA-CKD initiative, and of all those involved in the pilot phase, from Hospital Clínico Universitario de Valencia (Valencia, Spain), Hospital Universitario de Cruces (Vizcaya, Spain), Hospital Universitario 12 de Octubre (Madrid, Spain), Hospital Universitario Reina Sofía (Córdoba, Spain), Hospital Universitario Vall d’Hebron (Barcelona, Spain), Hospital Universitario Lucus Augusti (Lugo, Spain), Complejo Hospitalario Universitario de A Coruña (A Coruña, Spain), Hospital Universitario Marqués de Valdecilla (Santander, Spain), Hospital Universitario de Bellvitge (Barcelona, Spain), Hospital Universitario Juan XXIII (Tarragona, Spain), Hospital Universitario Doctor Josep Trueta (Gerona, Spain), Hospital Universitario Fundación Jiménez Díaz (Madrid, Spain), Centro de Salud Rafael Alberti (Madrid, Spain), Hospital Universitario Virgen de las Nieves (Granada, Spain), Hospital Universitario Virgen Macarena (Sevilla, Spain), Hospital Universitario de Badajoz (Badajoz, Spain).
Medical writing support under the guidance of the authors was provided by Susana Cañón, PhD, Blanca Piedrafita, PhD, and Javier Arranz-Nicolás, PhD, from Medical Statistics Consulting (MSC), Valencia, Spain, in accordance with Good Publication Practice guidelines (DeTora, L. M. et al. Ann Intern Med. 2022).
The CARABELA-CKD Scientific Committee consists of the following members: Borja Quiroga (Sociedad Española de Nefrología, S.E.N.), Javier Escalada (Sociedad Española de Endocrinología y Nutrición, SEEN), Juan José Gorgojo (SEEN), Manuel Pérez Maraver (SEEN), Mercedes Salgueira (S.E.N.), Patricia de Sequera (S.E.N.), Pedro Ruiz (Sociedad Española de Calidad Asistencial), Alberto Prado Dominguez (Departamento médico, AstraZeneca Farmacéutica Spain), Jesús Ignacio Diago (Departamento médico, AstraZeneca Farmacéutica Spain), and Lucía Regadera (Departamento médico, AstraZeneca Farmacéutica Spain).