Due to the shortage of living kidney donors and the current diabetes mellitus (DM) pandemic, studying the association of solitary kidney (SK) with DM is of paramount importance. Our aim was to assess the significance of the association between SK and DM.
Materials and methodsEighty-four patients with SK and DM (group A), with a mean age of 62.46±12.72 years, of whom 36 were males and 48 were females, were enrolled in the study.
The control group (group B) comprised 84 SK patients without DM of similar age and duration of existence of a SK. Mean age: 61.58±8.22 years, 23 males and 61 females. Serum creatinine, GFR (CKD-EPI), glycaemia, cholesterol, triglycerides, uric acid, proteinuria/24h, systolic blood pressure (SBP), diastolic blood pressure (DBP) and BMI were assessed.
ResultsThe group of patients with SK and DM (group A) had a higher BMI (p=0.0007), higher metabolic abnormalities (higher glycaemia [p<0.001], triglycerides [p=0.0004], uric acid [p=0.019] and proteinuria/24h [p=0.006]). The study group also had a higher prevalence of hypertension (p=0.003) and coronary artery disease (p=0.031).
ConclusionsWe found a higher value of proteinuria in the study group, significant metabolic abnormalities, as well as a higher prevalence of hypertension and coronary artery disease. However, no differences with respect to GFR were found, which could have significant implications for transplantation.
Dada la reducción del número de los donantes vivos de riñones y la pandemia de diabetes mellitus (DM), estudiar la asociación del riñón único (RU) con la DM es de la mayor importancia. Nuestro objetivo fue evaluar la significación de la asociación entre el RU y la DM.
Material y métodosHan sido estudiados 84 pacientes con RU y DM (grupo A), con edad media de 62,46±12,72 años; eran 36 hombres y 48 mujeres.
El grupo control (grupo B) ha estado compuesto por 84 pacientes con RU sin DM, de la misma edad y del mismo periodo de tiempo que el grupo A; la edad media de estos pacientes fue de 61,58±8,22 años; eran 23 hombres y 61 mujeres.
Hemos evaluado la creatinina sérica, el FG (CKD-EPI), la glucemia, el colesterol, los triglicéridos, el acido úrico, la proteinuria de 24 h, la tensión arterial sistólica (TAS), la tensión arterial diastólica (TAD) y el IMC.
ResultadosEl grupo de los pacientes con RU y DM (el grupo A) tuvo valores mayores del IMC (p=0,0007), anomalías metabólicas más elevadas (la glucemia [p<0,001], los triglicéridos [p=0,0004], el acido úrico [p=0,019] y una proteinuria/24 h también más elevada [p=0,006]).
El grupo de estudio tuvo también una prevalencia elevada de la TA (p=0,003) y de la enfermedad arterial coronaria (p=0,031).
ConclusionesHemos encontrado un valor más elevado de la proteinuria en el grupo estudiado, anomalías metabólicas importantes y también prevalencia más alta de la TA y de la enfermedad arterial coronaria, pero ninguna diferencia entre los FG, lo que puede tener una implicación importante en el trasplante.
Diabetes mellitus (DM) is associated during its evolution with renal function impairment. The progression of diabetic nephropathy is related to the processes of hypertrophy and hyperfiltration at the level of the kidneys. Early Glomerular Filtration Rate elevation plays a central role in the pathogenesis and progression of renal disease in diabetes.1
The solitary kidney (SK) is also associated with hypertrophy and hyperfiltration phenomena. The question is raised whether the phenomena of hypertrophy and hyperfiltration in the SK associated with DM are summed up. SK status and DM could have an additive effect on hypertrophy and hyperfiltration.
The possibility exists of a greater risk of individuals with a SK to present nephropathy in the case of coexistence of associated diabetes mellitus.2
DM can be considered to represent a risk factor in case of association with the SK.
However, it is worth remembering that only some of the diabetic patients develop diabetic nephropathy, which indicates the intervention of a genetic factor.
Due to the shortage of living kidney donors and the current DM pandemics, studying the association of the SK with DM is of paramount importance.
Many diabetic donor kidneys have been given to diabetic recipients with early graft survival being similar to that among nondiabetic recipients.3
Becker et al. suggest that diabetic kidneys can safely expand the donor pool and that diabetic kidneys can be used in transplantation without risk to patient or graft survival. Preexisting diabetic injury in the donor may increase the risk for proteinuria, compromised renal function, and posttransplant glucose intolerance.4
The aim of our study was to assess the significance of the association between the SK and the presence or absence of DM.
MethodsEighty-four patients with SK and DM (group A), mean age: 62.46±12.72 years, 36M and 48F, with a mean duration of a SK of 15.7±15.15 years were enrolled into the study. Six patients (7.14%) had a congenital SK.
The control group (group B) comprised 84 SK patients without DM of similar age and duration of existence of a SK: mean age: 61.58±8.22 years, 23M and 61F, and mean duration of existence of a SK: 15.26±13.76 years. Four patients (4.76%) had a congenital SK.
The inclusion criteria were history of unilateral nephrectomy in patients with surgically acquired SK and presence of a SK confirmed by at least two imaging methods in patients with congenital SK.
The study was approved by the Ethics Committee of the Emergency Clinical County Hospital Timisoara, Romania.
All patients were assessed for serum Creatinine, GFR(CKD-EPI), glycemia, cholesterol, triglycerides, serum uric acid, proteinuria/24h, systolic blood pressure (SBP), diastolic blood pressure (DBP), BMI. Patients from group A were also assessed for HbA1c.
Statistical analysisData is presented as mean value±standard deviation. Mean values were compared using the t-student test (parametric variables) or the Mann–Whitney U-test (non-parametric variables). Percentages were compared using the chi-square test.
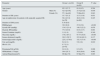
ResultsThe comparative assessment of SK patients with DM (group A) vs. SK patients without DM (group B) is presented in Table 1.
Comparative assessment of solitary kidney patients with (group A) and without Diabetes Mellitus (Group b).
| Parameter | Group A (n=84) | Group B (n=84) | P value | |
|---|---|---|---|---|
| Age (years) | 62.5 (12.7) | 61.6 (8.2) | 0.594 | |
| Gender | Male (%) | 42.9 [n=36] | 27.4 [n=23] | 0.036 |
| Female (%) | 57.1 [n=48] | 72.6[n=61] | 0.043 | |
| Duration of SK (years) | 15.7 (15.2) | 15.3 (13.8) | 0.931 | |
| Age at nephrectomy for patients with surgically acquired SK | 50.4 (13.9) [n=78] | 48.6 (13.0) [n=80] | 0.425 | |
| Duration of DM (years) | 8.78 (8.6) | - | ||
| BMI(kg/sqm) | 30.9 (6.9) | 27.6 (5.0) | <0.001 | |
| Systolic BP (mmHg) | 134.5 (21.4) | 140.5 (22.2) | 0.078 | |
| Diastolic BP(mmHg) | 77.0 (10.9) | 83.6 (13.7) | 0.001 | |
| Serum Creatinine (mg/dL) | 1.8 (1.4) | 1.5 (0.8) | 0.340 | |
| Glycemia (mg/dL) | 170.2 (87.1) | 97.75 (16.0) | <0.001 | |
| Serum cholesterol (mg/dL) | 201.5 (59.7) | 215.94 (62.3) | 0.137 | |
| Serum triglycerides (mg/dL) | 225.2 (179.8) | 152.3 (73.4) | <0.001 | |
| Uric acid (mg/dL) | 6.6 (2.1) | 5.9 (1.8) | 0.019 | |
| HbA1c (%) | 8.7 (2.1) [n=41] | - | - | |
| Proteinuria/24h (g/24h) | 0.6 (1.0) | 0.3 (0.5) | 0.006 | |
| GFR(ml/min.1.73sqm) | 50.6 (25.7) | 50.8 (20.6) | 0.962 | |
| Arterial Hypertension(%) | 86.9[n=73] | 67.9[n=57] | 0.003 |
Values expressed as mean (DS).
DM refers to patients with Type 2 DM.
The group of patients with SK and DM (group A) had a significantly higher BMI (p=0.0007), significantly higher metabolic abnormalities: glycemia (p<0.001), triglycerides (p=0.0004), uric acid (p=0.019), and significantly higher proteinuria/24h (p=0.006).
The study group also had a higher prevalence of arterial hypertension (p=0.003) and coronary artery disease (p=0.031).
The group of patients with SK without DM (group B) presented higher DBP than the study group A (p=0.001).
There was no difference in the Glomerular Filtration Rate (GFR) between the two groups (p=0.962).
DiscussionA reduced renal mass such as occurs in SK patients may augment the renal glomerular hyperfiltration associated with diabetes mellitus and increase the risk of later developing nephropathy.5
Experimentally, glomerular hyperfiltration has been shown to result from elevations in the glomerular capillary blood flow and the glomerular capillary hydraulic pressure.6
Experimental studies conducted by Whiteside et al. in dogs with DM induced by pancreatectomy found that after nephrectomy an additive effect on kidney hypertrophy was identified.7 Likewise, experimental studies in rats with streptozocin-induced DM showed that nephrectomy of a kidney is associated after 3 months with worsening of diabetes-induced glomerular injury.8
Our study assessed 84 SK patients with DM as compared to 84 SK patients without DM.
One objective of the study was to evaluate whether the adaptive phenomena of hypertrophy and hyperfiltration which accompany the SK are summed up with the ones encountered in DM, where the kidney also suffers phenomena of hypertrophy and hyperfiltration.
There was no difference in the Glomerular Filtration Rate (GFR) between the two groups, as such the supposition of the summation of the phenomena of hypertrophy and hyperfiltration of the solitary kidney and of diabetes mellitus could not be proven.
In order to interprete these data we took into consideration the duration of SK existence in the two groups of patients. We observed a similar duration of SK existence. As such, if the adaptive phenomena of hypertrophy and hyperfiltration of the SK are amplified by the presence of DM, the patients with DM should have shown greater values of the GFR, which proved not to be the case in our study.
It should be noted that in persons in whom nephrectomy was performed for a unilateral disease, the GFR will go up in time, reaching 75% of the normal value.9
The congenital SK, due to its particular intrauterine development, presents about 75% of the nephrons of a person with 2 functional kidneys.
The reduced number of persons with congenital SK did not allow for a statistical interpretation of the GFR in patients with congenital SK in our study.
The duration of DM in our study group was 8.78±8.55 years, a time frame in which the phenomena of hypertrophy and hyperfiltration are manifest, however these are not reflected in an increase of the GFR in our study.
Our study could argue in favor of the usage of living kidney donors with diabetes mellitus.
We found however in the SK patients with DM significantly higher metabolic abnormalities: glycemia, triglycerides, uric acid, and significantly higher proteinuria/24h. The study group also had a higher prevalence of arterial hypertension and coronary artery disease.
It is known that patients with diabetes have an increased incidence of atherosclerotic cardiovascular, peripheral arterial, and cerebrovascular disease. Hypertension and abnormalities of lipoprotein metabolism are often found in people with diabetes.10
As the metabolic abnormalities and co-morbidities encountered in DM represent important risk factors and are amenable to therapeutic intervention, our study proves valuable in highlighting them.
Proteinuria is a risk marker for renal disease progression.11 One cause for the increased proteinuria could be the phenomena of hypertrophy and hyperfiltration at the level of the kidneys. The significantly higher proteinuria (more than double) found in the SK patients with DM suggests that these patients present a higher degree of renal injury than those without DM, although this is not reflected in the GFR.
The association of the SK with DM is thus important due to the presence of significantly higher metabolic abnormalities, of renal injury as reflected by the increased proteinuria and the increased frequency of arterial hypertension and coronary artery disease, which represent important risk factors and should be addressed therapeutically.
Given the role of the kidney in primary hypertension, as has been demonstrated by transplants from hypertensive animal donors to normotensive animal recipients, some centers deny hypertension which is also a pandemics in pre-existing donors whether living or cadaveric. As Becker has posited that diabetic kidneys can safely expand the donor pool, this has inspired the present paper.
ConclusionsOur study showed a higher value of proteinuria due to the phenomena of hypertrophy and hyperfiltration in the study group, significantly higher metabolic abnormalities: glycemia, triglycerides, uric acid, as well as a higher prevalence of arterial hypertension and coronary artery disease.
There was no difference in the Glomerular Filtration Rate (GFR) between the two groups, as such the supposition of the summation of the phenomena of hypertrophy and hyperfiltration of the solitary kidney and of diabetes mellitus could not be proven.
Patients with SK and DM should be carefully monitored from a nephrological viewpoint for proteinuria, GFR and arterial hypertension and for cardiovascular diseases.
Authors contributionCristina Gluhovschi designed the study and wrote the paper. Gheorghe Gluhovschi, Florica Gadalean, Silvia Velciov, Ligia Petrica were responsible for the acquisition of data. Adriana Kaycsa performed the required laboratory determinations, while Romulus Timar critically read the manuscript.
Ethical approvalInformed consent was obtained from all patients included in the study.
FundingThis research received funding from an Internal Grant of “Victor Babes” University of Medicine and Pharmacy Timisoara, PIII-C1-PCFI-2014/2015. The supporting source had no involvement in study design, in collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the manuscript for publication.
Conflict of interestNone.
The summary of this paper was presented as a poster at the 51st European Renal Association-European Dialysis Transplant Association Congress, May 31–June 3, 2014, Amsterdam, Netherlands.