Combined homocystinuria and methylmalonic aciduria cbl-C type is an inborn error of metabolism of vitamin B12 that leads to increased levels of homocysteine and methylmalonic acid. The spectrum of clinical manifestations and disease severity is broad. Atypical hemolytic uremic syndrome (aHUS) and pulmonary hypertension can be the first manifestations of the disease.
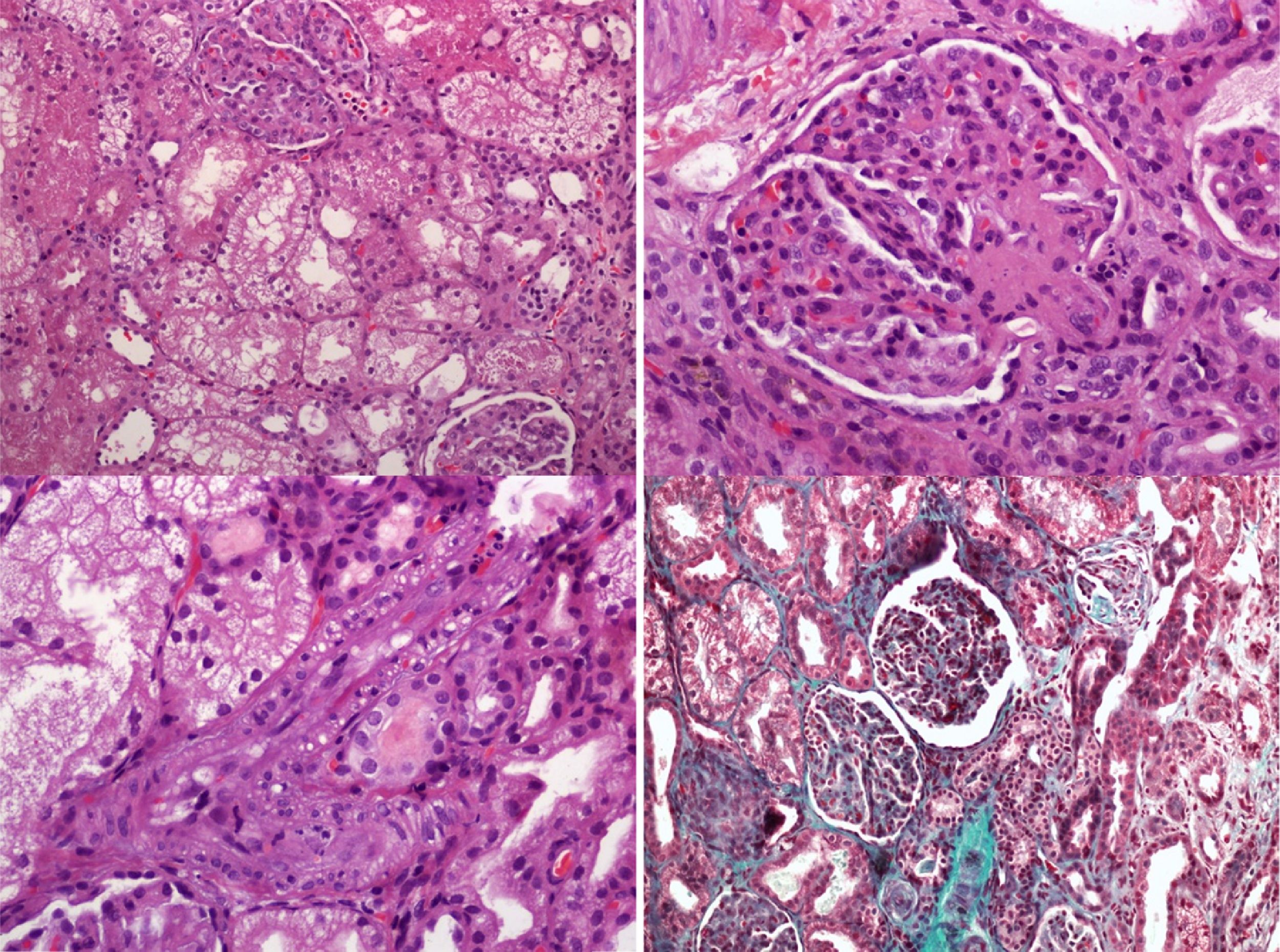
We present a rare case of cbl-C, with aHUS, recurrent pulmonary edema and severe pulmonary hypertension (PAH): A 2-year-old boy was admitted to the Pediatric Intensive Care Unit (PICU) with severe hypertension and pulmonary edema, both attributed to renal failure due to aHUS. He had a history of chronic hemolytic anemia of unknown origin since the age of 15 months and aHUS of unknown etiology had been diagnosed a month earlier. Treatment with eculizumab (anti-C5 monoclonal antibody) had been administered, as primary aHUS had been suspected. Parents were a healthy couple from north Africa with a low level of consanguinity and had two other healthy children. During PICU admission, our patient presented with arterial hypertension requiring multiple drugs, recurrent episodes of pulmonary edema and progressive renal failure ensued requiring continuous veno-venous hemofiltration on day 7. Serial echocardiographic studies demonstrated progressive pulmonary hypertension causing acute cor pulmonale. Pulmonary thromboembolism was ruled out by a thoracic CT scan. The diagnostic workup (Table 1) included a kidney biopsy that showed a thrombotic microangiopathy (TMA) (Fig. 1). A panel for autoimmune diseases was negative. The diagnostic workup for metabolic inborn errors of metabolism was compatible with homocystinuria and methylmalonic aciduria type cbl-C. Treatment with hydroxycobalamin, betaine and carnitine was started upon diagnosis (second week after admission). A genetic test confirmed the diagnosis, with homozygous mutation in the gene MMACHC of chromosome 1 (276G>A+276 G>A). The clinical course of pulmonary hypertension was unfavorable, unresponsive to multiple pulmonary vasodilators (nitric oxide, bosentan, sildenafil and iloprost) and continuous infusion of heparin. In the catheterization laboratory pulmonary pressure was suprasystemic. An atrial septostomy was performed as the patient had two episodes of cardiac arrest due to pulmonary hypertensive crisis. He died due to pulmonary hemorrhage a month after PICU admission in spite of receiving specific metabolic treatment.
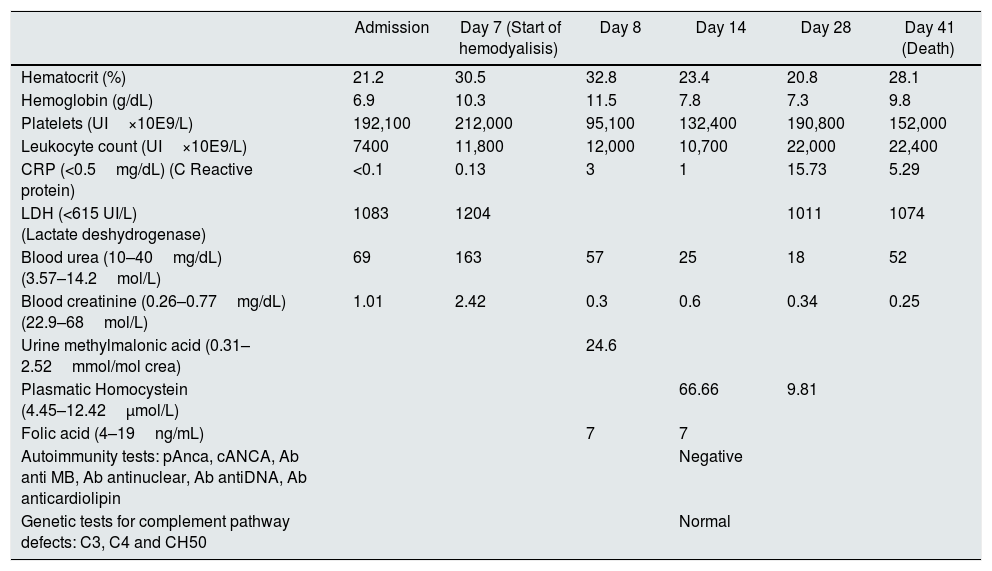
Blood tests and laboratory findings during hospitalization.
| Admission | Day 7 (Start of hemodyalisis) | Day 8 | Day 14 | Day 28 | Day 41 (Death) | |
|---|---|---|---|---|---|---|
| Hematocrit (%) | 21.2 | 30.5 | 32.8 | 23.4 | 20.8 | 28.1 |
| Hemoglobin (g/dL) | 6.9 | 10.3 | 11.5 | 7.8 | 7.3 | 9.8 |
| Platelets (UI×10E9/L) | 192,100 | 212,000 | 95,100 | 132,400 | 190,800 | 152,000 |
| Leukocyte count (UI×10E9/L) | 7400 | 11,800 | 12,000 | 10,700 | 22,000 | 22,400 |
| CRP (<0.5mg/dL) (C Reactive protein) | <0.1 | 0.13 | 3 | 1 | 15.73 | 5.29 |
| LDH (<615 UI/L) (Lactate deshydrogenase) | 1083 | 1204 | 1011 | 1074 | ||
| Blood urea (10–40mg/dL) (3.57–14.2mol/L) | 69 | 163 | 57 | 25 | 18 | 52 |
| Blood creatinine (0.26–0.77mg/dL) (22.9–68mol/L) | 1.01 | 2.42 | 0.3 | 0.6 | 0.34 | 0.25 |
| Urine methylmalonic acid (0.31–2.52mmol/mol crea) | 24.6 | |||||
| Plasmatic Homocystein (4.45–12.42μmol/L) | 66.66 | 9.81 | ||||
| Folic acid (4–19ng/mL) | 7 | 7 | ||||
| Autoimmunity tests: pAnca, cANCA, Ab anti MB, Ab antinuclear, Ab antiDNA, Ab anticardiolipin | Negative | |||||
| Genetic tests for complement pathway defects: C3, C4 and CH50 | Normal |
This case is an atypical presentation of combined homocystinuria and methylmalonic aciduria type cbl-C, with aHUS, severe pulmonary hypertension and recurrent pulmonary hemorrhage at 2 years of age, outcome was poor despite specific treatment provided. Combined homocystinuria and methylmalonic aciduria type cbl-C is the most common form of inborn errors of the metabolism of vitamin B12. There is a deficient transformation of cobalamine to adenosylcobalamin (AdoCbl) and metilcobalamine (MeCbl). Results in an accumulation of methylmalonic acid and homocysteine.1
There is great variability in the clinical presentation of Cbl-C: growth retardation, microcephaly or dilated cardiomyopathy can be the first manifestations in the prenatal period. During the first years of life lethargy, hypotonia, neurological symptoms, ophthalmological disorders and aHUS can appear. During adulthood encephalopathy, neurological degeneration and thromboembolic events can also be found.2,3
Homocysteine may cause endothelial injury in rats and pulmonary thromboembolism in mice.4 In humans, hyperhomocysteinemia is an established independent risk factor for myocardial infarction and stroke in adults,5 due to thromboembolic events.
In this case, a thrombotic microangiopathy was found in renal biopsy, which may have been caused by the endothelial damage induced by hyperhomocysteinemia. Throughout PICU admission, the patient presented overt pulmonary hypertension unresponsive to pulmonary vasodilators. Different degrees of PAH have been described in cblG and cblC with aHUS.4,6,7 In two cases a good outcome is described after receiving specific treatment (cblG and cblC), but in another case (cblC), the patient died and renal biopsy showed thrombi in arteriolar vessels. There is a published case series of five patients8 with cblC and combined PAH/r-TMA: two are diagnosed postmortem and another two, despite aggressive treatment with PAH-targeted drugs and hydroxicobalamin, died of right ventricular failure and progressive pulmonary vasculopathy. Another case report9 presented reversible pulmonary arterial hypertension with treatment. There is a case report10 with cblC and isolated PAH with complete mormalization of metabolic abnormalities with treatment. Despite this he died of fatal pulmonary hypertensive crisis.
We hypothesize that in our patient, suprasystemic pulmonary hypertension may have been caused by pulmonary microagiothrombotic events secondary to hyperhomocistinemia and it remains speculative if the microvascular damage may have taken place at the postcapillary level justifying the recurrent pulmonary edema and pulmonary hemorrhage that our patient suffered. The outcome is poor when cblC is associated with HUS, with a high rate of mortality,3 especially if any type of dialysis is needed.
Our case is an unusual presentation of cblC with secondary aHUS, PHT, pulmonary edema and pulmonary hemorrhage. In this case rapid clinical worsening was observed in spite of the specific treatment, probably due to the advanced state of the disease and the severity of the pulmonary hypertension.
Homocysteine and methylmalonic acid measurement should be included in the workup of renal TMA and PAH in children. An early diagnosis and treatment may be useful to reverse PAH despite is not still demonstrated.
Funding sourceNo funding was secured for this study.
Financial disclosureNo financial relationships relevant to this article to disclose.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Contributors’ statementsZuriñe Martínez de Compañón, Miriam Poblet-Puig and Griselda Vallès: We drafted the initial manuscript, changed some aspects after the review by the other authors and approved the final manuscript as submitted.
Mireia Del Toro, Ramón Vilalta and Antonio Moreno: They reviewed the manuscript each one the aspects of his speciality and approved the final manuscript as submitted.
Joan Balcells: He did the last review and approved the final manuscript as submitted.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.