Diabetic nephropathy (DN) is one of the most frequent complications in patients with diabetes mellitus (DM) and its diagnosis is usually established on clinical grounds. However, kidney involvement in some diabetic patients can be due to other causes, and renal biopsy might be needed to exclude them. The aim of our study was to establish the clinical and analytical data that predict DN and no-diabetic renal disease (NDRD), and to develop a predictive model (score) to confirm or dismiss DN.
Material and methodsWe conducted a transversal, observational and retrospective study, including renal biopsies performed in type 2 DM patients, between 2000 and 2018.
ResultsTwo hundred seven DM patients were included in our study. The mean age was 64.5±10.6 years and 74% were male. DN was found in 126 (61%) of the biopsies and NDRD in 81 (39%). Diabetic retinopathy was presented in 58% of DN patients, but only in 6% of NDRD patients (p<0.001). Patients with NDRD were diagnosed of primary glomerulopathies (52%), nephroangiosclerosis (16%), inmunoallergic interstitial nephritis (15%) and vasculitis (8.5%). In the multivariate analysis, retinopathy (OR 26.7; 95% CI: 6.8–104.5), chronic ischemia of lower limbs (OR 4.37; 95% CI: 1.33–14.3), insulin therapy (OR 3.05; 95% CI: 1.13–8.25), time course of DM ≥10 years (OR 2.71; 95% CI: 1.1–6.62) and nephrotic range proteinuria (OR 2.91; 95% CI: 1.2–7.1) were independent predictors for DN. Microhaematuria defined as ≥10 red blood cells per high-power field (OR 0.032; 95% CI: 0.01–0.11) and overweight (OR 0.21; 95% CI: 0.08–0.5) were independent predictors of NDRD. According to the predictive model based on the multivariate analysis, all patients with a score >3 had DN and 94% of cases with a score ≤1 had NDRD (score ranked from −6 to 8 points).
ConclusionsNDRD is common in DM patients (39%), being primary glomerulonephritis the most frequent ethology. The absence of retinopathy and the presence of microhematuria are highly suggestive of NDRD. The use of our predictive model could facilitate the indication of performing a renal biopsy in DM patients.
La nefropatía diabética (ND) es una complicación frecuente de la diabetes mellitus (DM), y su diagnóstico suele ser clínico. Sin embargo, en numerosas ocasiones la enfermedad renal que presentan los pacientes diabéticos es debida a otras causas cuyo diagnóstico es histológico. El objetivo del estudio fue determinar los datos clínicos y analíticos predictores de ND y enfermedad renal no diabética (ERND), y elaborar un modelo predictivo (score) para confirmar o descartar ND.
Material y métodosEstudio observacional, transversal y retrospectivo de biopsias renales realizadas en pacientes diabéticos tipo2 entre 2000 y 2018.
ResultadosSe incluyeron 207 pacientes diabéticos con una edad media de 64.5±10,6años; el 74% eran varones. La biopsia mostró ND en 126 (61%) y en 81 ERND (39%). La retinopatía diabética estaba presente en el 58% de los pacientes con ND y en el 6% del grupo con ERND (p<0,001). Histología encontrada en la ERND: glomerulopatías primarias (52%), nefroangioesclerosis (16%), nefritis intersticial inmunoalérgica (15%) y vasculitis (8,5%). En el análisis multivariable, la retinopatía (OR26,7; IC95%: 6,8-104,5), la isquemia crónica de miembros inferiores (OR4,37; IC95%: 1,33-14,3), la insulinoterapia (OR 3,05; IC95%: 1,13-8,25), una evolución de la DM ≥10años (OR2,71; IC95%: 1,1-6,62) y la proteinuria nefrótica (OR2,91; IC95%: 1,2-7,1) fueron predictores independientes de ND. La microhematuria, definida como ≥10hematíes/campo (OR0,032; IC95%: 0,01-0,11) y el sobrepeso (OR0,21; IC95%: 0,08-0,55) lo fueron de ERND. Según el modelo predictivo resultante del estudio multivariable para ND, el rango de puntuación varió de −6 a 8 puntos. Todos los pacientes con un score >3 era tenían ND, y el 94% de los casos con score ≤1punto fueron ERND.
ConclusionesLa ERND es frecuente en pacientes con DM (39%). La etiología más frecuente son las glomerulonefritis primarias. La ausencia de retinopatía y la presencia de microhematuria son altamente sugestivas de ERND. La utilización de un sistema de puntuación facilita la indicación de biopsia renal en pacientes diabéticos.
Diabetes mellitus (DM) is a major health problem worldwide, due to its high prevalence and the increase experienced in recent years. Worldwide, it is estimated that in 2012 around 347 million people were diabetic, in 2015 the figure increased to 415 million and it is estimated that by 2040 there will be 642 million affected.1,2
Renal involvement secondary to DM is the most frequent cause of chronic kidney disease in patients on replacement therapy.3 About 30–40% of patients with type 2 DM 2 (DM2) have renal involvement after 10 years of evolution. Tervaert et al.4 classify diabetic nephropathy (DN) in five stages of evolution, based on the involvement of the basal membrane, mesangial proliferation, nodular sclerosis or advanced glomerulosclerosis. The diagnosis of DN is mainly clinical, based on the duration of diabetes, the presence of neuropathy, retinopathy or other complications, as well as the manifestation of a slow and progressive proteinuria,5 although it is increasingly more frequent an atypical course of DN in patients without significant proteinuria and with progressive deterioration of renal function.6
However, diabetic patients may develop another type of renal disease that is not attributable to diabetes, known as non-diabetic kidney disease (NDKD).7 According to a recent meta-analysis8 the prevalence of NDKD varies between 6.5% and 94%. Among the most common causes of NDKD is IgA and membranous GN.8,9 It is important to detect the presence of NDKD in diabetic patients, because it generally involves a better prognosis, in many cases it has treatment and it is potentially reversible.9–11
There have been described certain factors that are clinical predictors of NDKD such as severe proteinuria of rapid establishment, the absence of retinopathy, a short duration of diabetes, the presence of hematuria and acute deterioration of renal function.12,13 However, the variability of the clinical course and the cardiovascular disease associated with diabetes make it difficult to differentiate between DN and NDKD. It is accepted that microhematuria is not a common finding in DN, and its presence suggests an NDKD; so the American Diabetes Association believes that microhematuria is a criterion for the indication of renal biopsy in patients with DM.14 There is no consensus on what are the indications of biopsy in patients with DM,10,15,16 since its main purpose would be to detect cases of NDKD,10,12,13,15–17 but it is an invasive technique and has certain risks (hematuria, perirenal hematoma or the need for arterial embolization).15,18
The objective of this study was to analyze the risk factors associated with diabetic nephropathy in patients with DM2 and develop a predictive model by creating a scoring scale in order to facilitate the clinician's decision to perform, or not, a renal biopsy in these patients.
MethodsObservational, cross-sectional and retrospective study in patients diagnosed with DM219 who underwent a kidney biopsy (KBx) at the University Hospital October 12 in Madrid between January 2000 and December 2018. During this period, 2205 biopsies were performed in native kidneys, and in 207 cases they corresponded to diabetic patients (9.4%). Patients were asked for a written informed consent to perform the biopsy, according to our Nephrology Department protocol.
Indication of KBx in diabetic patients is established in the following situations: acute deterioration of kidney function (increase >0.3mg/dl from baseline creatinine in a period of less than three months); acute increase in proteinuria (increase >0.5g/24h of basal proteinuria in the last 3 month period); nephrotic proteinuria (proteinuria ≥3.5g/24h); nephrotic syndrome (nephrotic proteinuria accompanied by edema, hypoalbuminemia and dyslipidemia); kidney disease under study (chronic renal failure under study without apparent cause), and microhematuria (≥10 red blood cells/campo).
The KBx were processed for study by optical microscopy, immunofluorescence and electron microscopy. The samples were assessed independently by two pathologists. Patients were diagnosed with DN according to the classification proposed by Tervaert et al.,4 according involvement is the thickening of the basement membrane (class I), mesangial proliferation, small (class IIa) or severe (class IIb), nodular sclerosis (class III) or advanced glomerulosclerosis (class IV). The NDKD was diagnosed based on classical pathological criteria according to the results of optical, electronic and immunofluorescence microscopy. When nephroangiosclerosis and diabetes lesions coexisted, they were included as DN. Only one patient had lesions of DN and acute tubulointerstitial nephritis, which was included in the NDKD group.
The risk factors analyzed were the following: age, sex, time of evolution and type of diabetes treatment, vascular disease (heart, brain and lower limbs), diabetic retinopathy, hypertension (HTN), overweight, renal function and urinary sediment.
The diagnosis of chronic lower limb ischemia (CLLI) was established according to the clinical intermittent claudication of >2 weeks, along with consistent physical examination and objective demonstration by imaging (doppler ultrasound, angiography). The history of coronary heart disease (acute myocardial infarction or coronary revascularization) and acute stroke (clinical diagnosis or evidence by imaging test) were reviewed. The presence of one or more events (ischemic heart disease, stroke and/or CLLI) was considered cardiovascular disease.
Diabetic retinopathy (DR) was diagnosed according to consensus criteria20 via evaluation of the eye fundi by expert ophthalmologist, both non-proliferative and proliferative forms were included. HTN was defined as a systolic blood pressure ≥140mmHg or the diastolic blood pressure ≥90mmHg, or if the patient was on antihypertensive treatment. Body mass index (BMI) was used as anthropometric measure; overweight patients were considered those with a BMI≥27kg/m2 (degree 2 overweight) according to the criteria of the Spanish Society of Endocrinology, Diabetes and Obesity (SEEDO).21
Other variables evaluated at the time of performing the KBx were serum creatinine and glomerular filtration rate (GFR), which was estimated according to the equation Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI),22 the proteinuria in 24h urine collection, the urinary sediment and the quantification of microhematuria. For the study of risk factors, the presence of ≥10 red blood cells/field was considered as microhematuria.
Statistic analysisContinuous variables with a normal distribution are presented as mean and standard deviation, and as a median (25–75 percentile) those that do not meet this characteristic.
For the analysis of the association of type of nephropathy all studied variables were entered as categorical, including continuous: age (older or younger than 65 years, corresponding to the median) and the time evolution of diabetes (greater or less than 10 years). The chi-square test was used to assess this association.
Variables whose p value was less than or equal to 0.25 in the univariate analysis were included in a binary logistic regression analysis. The dependent variable was to present diabetic versus non-diabetic nephropathy. Retinopathy was considered as the main variable. Before discarding any of the risk factors, it was assessed the confusion and interaction with the main variable.
The calibration of the final model was done by applying the Hosmer Lemeshow goodness of fit test. Its discrimination was analyzed with the receiver operator curve (ROC) of the probabilities predicted by this model.
The regression coefficients of the final model were used to generate the score of point, assigning to each variable a score proportional to the same that was rounded to the nearest integer value or decimal (0.5) according to Sullivan et al.23 The agreement between the probability of DN calculated by the logistic regression model and the calculated by the point system was analyzed using the kappa index.
Statistical analysis was performed using the SPSS software (IBM SPSS® Statistics20).
ResultsA total of 207 DM2 patients with kidney biopsy performed during the study period were included. Of these, 154 were male (74%). The age was 64.5±10.6 years (range 42–87 years). The average duration of diabetes was 10 years, ranging between 1 and 34 years. Microscopic evaluation revealed that DN was observed in 126 patients (61%); class III was the most common (40.5%), followed by the class IIb (39.5%), less frequently was class IV (16.8%) and class IIa (3.2%). The main clinical and analytical findings are summarized in Table 1.
Clinical and biochemical characteristics of diabetes mellitus (DM) patients with kidney biopsy.
| DM patients with kidney biopsy (n=207) | Min–Max | |
|---|---|---|
| Age years, mean±SD | 64.5±10.6 | 42–87 |
| Male gender | 154 (74.4%) | |
| Duration DM in years, median (p25–p75) | 10 (6–14) | 1–34 |
| Evolution of diabetes ≥10 years | 110 (53.1%) | |
| Retinopathy | 79 (38.2%) | |
| Insulin therapy | 69 (33.3%) | |
| BMI in kg/m2, mean±SD | 29.1±5.6 | 19–54 |
| Overweight grade II | 129 (62.3%) | |
| Hypertension | 183 (88.4%) | |
| Cardiovascular disease | 76 (36.7%) | |
| Acute stroke | 23 (11.1%) | |
| Coronary heart disease | 37 (18%) | |
| Chronic ischemia of distal lower limbs | 41 (19.8%) | |
| Serum creatinine mg/dl, mean (p25–p75) | 2.3 (1.6–3.5) | 0.7–11.3 |
| FGR ml/min/1.73m2, mean (p25–p75) | 29 (17.4–45) | 4.2–99.5 |
| Proteinuria in g/24h, mean (p25–p75) | 3.8 (1–6.1) | 0–20 |
| Nephrotic proteinuria | 111 (53.6%) | |
| Microhematuria (≥10 red blood cells/field) | 55 (26.6%) | |
| Acute impairment of kidney function | 112 (54.1%) | |
| Acute increase in proteinuria | 87 (42%) | |
| Nephrotic syndrome | 55 (26.6%) | |
| Kidney disease under study | 38 (18.4%) | |
| Biopsy proven Diabetic nephropathy | 126 (60.9%) |
SD: standard deviation; GFR: glomerular filtration rate according to CKD-EPI; BMI: body mass index.
Analysis of the renal lesions other than the renal involvement of diabetes observed in 81 patients (39%), revealed that the most frequent histological finding was primary glomerulopathies (52%), followed by hypertensive nephroangiosclerosis (16%) and acute immunoallergic interstitial nephritis (15%). IgA nephropathy was the most frequent primary glomerulonephritis in our series (17/42), followed by membranoproliferative glomerulonephritis (9/42) (Table 2).
Histological findings in diabetic patients presenting non-diabetic kidney disease (NDKD).
| NDKD (n=81) | |
|---|---|
| Glomerulopathies | 42 (52%) |
| IgA nephropathy | 17 |
| Membranoproliferative | 9 |
| Focal segmental Hyalinosis | 8 |
| Membranous nephropathy | 6 |
| Minimum changes | 2 |
| Nephroangiosclerosis | 13 (16%) |
| Acute interstitial nephritis | 12 (15%) |
| Vasculitis | 7 (8.5%) |
| Other | 7 (8.5%) |
Only 8 patients (5 from the DN group and 3 from the NDKD) presented complications after KBx: perirenal hematoma, with anemia and need for blood transfusion.
Comparison of patients with ND or NDKD show significant differences in the univariate analysis. In the ND group males were more frequent, the time of evolution of the diabetes was greater, as well as the need for treatment with insulin and the presence of diabetic retinopathy (DR) (the proliferative form was present in 15/126 patients with DN and none with NDKD). DR was observed more frequently in patients with nodular sclerosis and global glomerulosclerosis (classes III and IV) than in patients with mild DN with mild or severe mesangial proliferation (67% vs. 33%, p=0.007).
Cardiovascular disease was more frequent in patients with DN, at the expense of CLLI, since there were no differences between the presence of acute strokes or coronary heart disease. Patients with DN presented more frequently nephrotic proteinuria, although the presence of nephrotic syndrome was similar in both groups. Patients with NDKD had more age, more overweight and they had significant microhematuria at the time of the biopsy (Table 3).
Analysis of univariate risk factors in patients with diabetic nephropathy (DN) or non-diabetic kidney disease (NDKD).
| DN (n=126) | NDKD (n=81) | Raw odds ratio (95% CI) | χ2 | |
|---|---|---|---|---|
| Age >65 years | 52 (32.5%) | 48 (59%) | 0.951 (0.925–0.979) | 0.011 |
| Male gender | 100 (79.4%) | 54 (66.7%) | 1.923 (1.022–3.618) | 0.041 |
| DM evolution ≥10 years | 82 (65.1%) | 28 (34.6%) | 3.528 (1.963–6.341) | 0.000 |
| Retinopathy | 73 (58%) | 6 (6.2%) | 17.217 (6.97–42.50) | 0.000 |
| Insulin therapy | 55 (43.7) | 14 (17.3%) | 3.707 (1.887–7.281) | 0.000 |
| Hypertension | 114 (89%) | 69 (86.3%) | 1514 (0.634–3.619) | 0,348 |
| Overweight grade II | 66 (52.4%) | 63 (77.8%) | 0.314 (0.167–0.590) | 0.000 |
| Cardiovascular disease | 54 (43.2%) | 22 (27.5%) | 2.005 (1.095–3.672) | 0.023 |
| Coronary heart disease | 24 (19.2%) | 13 (16.5%) | 1.206 (0.57–2.54) | 0.620 |
| Chronic ischemia of distal lower limbs | 31 (25.2%) | 10 (12.7%) | 2.325 (1.068–5.062) | 0.031 |
| Acute stroke | 13 (10.7%) | 10 (12.7%) | 0.823 (0.342–1.980) | 0.663 |
| Nephrotic proteinuria | 78 (61.9%) | 33 (40.7%) | 2.364 (1.336–4.183) | 0.003 |
| Nephrotic syndrome | 37 (29.4%) | 18 (29.4%) | 1.455 (0.76–2.785) | 0.256 |
| Microhematuria (≥10 red blood cells/field) | 12 (9.5%) | 43 (53.1%) | 0.093 (0.044–0.195) | 0.000 |
| Increase in proteinuria | 64 (50.8%) | 23 (28.4%) | 2.603 (1.434–4.724) | 0.001 |
| Impaired renal function | 71 (56.8%) | 41 (50.6%) | 1.283 (0.73–2.25) | 0.384 |
| Under evaluation renal insufficiency | 18 (14.3%) | 20 (24.7%) | 0.508 (0.250–1.034) | 0.059 |
DM: diabetes mellitus.
In multivariate analysis there were identified seven independent predictors of diabetic nephropathy (Table 4), five positive and two negative: presence of DR, insulin therapy, duration of diabetes ≥10 years, CLLI, nephrotic proteinuria, overweight and microhematuria (≥10 red blood cells/field).
Multivariate logistic regression analysis in patients with diabetic nephropathy vs. non-diabetic kidney disease.
| Odds adjusted ratio | 95% CI | p | |
|---|---|---|---|
| Diabetic retinopathy | 26.68 | 6.81–104.51 | 0.000 |
| Chronic ischemia of lower limbs | 4.37 | 1.33–14.28 | 0.010 |
| Nephrotic proteinuria | 2.91 | 1.20–7.09 | 0.016 |
| Insulin therapy | 3.05 | 1.13–8.2 5 | 0.024 |
| Evolution diabetes ≥10 years | 2.71 | 1.10–6.62 | 0.027 |
| Overweight grade II | 0.21 | 0.08–0.55 | 0.001 |
| Microhematuria (≥10 red blood cells/field) | 0.032 | 0.009–0.114 | 0.000 |
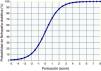
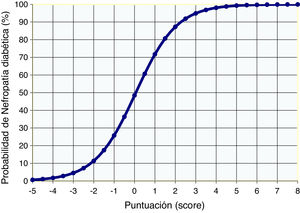
The system of score with points to predict DN was calculated using the coefficients of the logistic model of risk for DN; the score ranged from −5 to +8 (Table 5). Fig. 1 shows the probability of presenting DN according to the logistics results and the value obtained using the points system.
Score of points according to risk factor of diabetic nephropathy.
| Risk factor | To add score |
|---|---|
| Diabetic retinopathy | |
| No | 0 |
| Yes | 3.5 |
| Chronic ischemia of lower limbs | |
| No | 0 |
| Yes | 1.5 |
| Insulinotherapy | |
| No | 0 |
| Yes | 1 |
| Nephrotic proteinuria (≥3.5g/d) | |
| No | 0 |
| Yes | 1 |
| Evolution diabetes ≥10 years | |
| No | 0 |
| Yes | 1 |
| Overweight grade II (BMI≥27kg/m2) | |
| No | 0 |
| Yes | −1.5 |
| Hematuria ≥10 red blood cells/field | |
| No | 0 |
| Yes | −3.5 |
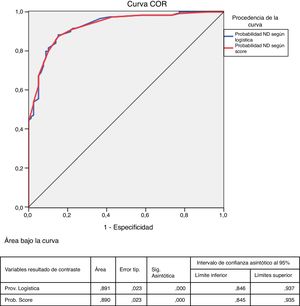
The comparison between the probability of presenting DN calculated by the logistic function and by the points system (score) showed a high concordance (kappa index of 0.944, p<0.0001). In addition, the ROC curves of these probabilities showed an area under the 0.92 curve in both cases, indicating and excellent fit (Fig. 2).
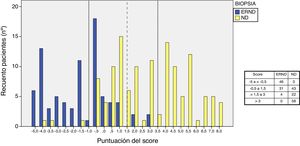
Analysis of the distribution of scores and the type of nephropathy shows that with <−0.5 points, only 3 patients had ND, in the range of 1.5–3 points there were 4 patients with NDKD, and with a score >3 points all patients had ND. In the interval between −0.5 and 1.5 points, patients with DN or with NDKD are distributed equally (Fig. 3).
DiscussionThe prevalence of NDKD described in the literature is very variable, from 6.5% to 94%8 including mixed forms; the cases of NDKD without diabetic lesions represent between 13% and 83%.15 In our study we found that 39% of DM patients with a kidney biopsy had isolated NDKD. These results are similar to those found in series in which a high number of patients with a kidney biopsy,9,11,12,17,24–27 whose prevalence is between 36% and 54%. This great variability depends on the different criteria used for kidney biopsy and the characteristics of the populations studied.
Among lesions other than DN, the most frequent glomerulopathy in our study was nephropathy with IgA deposits (21%), followed by membranoproliferative glomerulonephritis (11%) and focal segmental hyalinosis (10%). IgA nephropathy is described in the literature with a variable frequency, about 15%,8,28 increasing up to 43.5% in the Asian population.8,29 Membranous nephropathy accounts for 11%–23% of cases of NDKD,16,25,29,30 and focal segmental hyalinosis is estimated at 10–19%.8,12,25,31 Immunoallergic interstitial nephritis was observed in 15% of our patients, similar to that reported in other studies where the average prevalence was estimated at 23%.12,24,25,29,31,32
A comparison of patients with DN with diabetics with NDKD reveals that male sex was associated with increased risk of DN, although this difference was not found in the multivariate analysis. Gender is not described as a risk factor; only Wilfred et al.,32 reported that the female gender is associated with an increased risk of NDKD.
As in most of the series the time of evolution of diabetes was longer in patients with DN,8,11,12,28,30,32–34 and it is considered as an independent risk factor for DN in the meta-analysis of Liang et al.13 In our series, a time of evolution of diabetes ≥10 years was an independent factor of DN. We also found that insulin therapy was independently associated with the presence of DN, similar to what has been described in other studies.6,30,35 For Horvatic et al.,33 the absence of insulin treatment is a strong predictor of NDKD.
Patients with NDKD frequently presented overweight, and multivariate analysis demonstrated a BMI≥27kg/m2 was an independent factor for NDKD. Studies that analyze this issue have observed that obesity and a high BMI are more frequent in the NDKD13,34 other have reported no differences between the groups.6,11,28,30,35 We do not find an reason that justifies why in diabetics patients overweight is associated more frequently with the presence of renal involvement other than DN.
Retinopathy was present in 58% of patients with DN. In the multivariate analysis the diabetic retinopathy is the most significant factor associated with DN. Similar results are described in most studies in which DN has been diagnosed by KBx.12,18,25,32–36 Liang et al.,13 in the meta-analysis performed on 2322 biopsied diabetic patients, showed that retinopathy was the independent risk factor with the greatest significance for presenting DN. He et al.,37 observe similar results in their meta-analysis, and they found that if retinopathy is proliferative, it is a highly specific predictive of DN. These results support the idea that the absence of retinopathy predicts NDKD in diabetic patients,16,35,36,38 which should be considered as a possible indication of KBx.3 Diabetic rethynopathy is most frequently observed in the most severe forms of DN and is an independent factor of disease progression,39 which is similar to the results obtained in our study where retinopathy was significantly associated with the DN class III and IV.
Despite the strong association between retinopathy and DN, 42% of the patients in the DN group did not present retinopathy and it was observed in 6% of the patients in the NDKD group. This prevalence is similar to that described in the review article by Prakash et al.,7 which concludes that 20–40% of patients with biopsied ND do not have retinopathy. Therefore, the absence of retinopathy does not exclude histological lesions of diabetes, and we consider that the presence or absence of retinopathy does not rule out the need for a RBx.7,9,16
The association between cardiovascular disease and presence of DN has not been adequately investigated. In our series we found a higher prevalence of cardiovascular disease in patients with DN, similar results were observed by Liu et al.34 However, it was the CLLI what was independently associated with the presence of ND.
In our study, the presence of nephrotic range proteinuria was more frequent in ND than NDKD patients, a difference that we also found by the multivariate analysis. There is much disparity in the literature regarding the value of proteinuria as a predictor of DN vs. NDKD. Most studies have found an association between a nephrotic proteinuria and an increased risk of DN compared to NDKD27,28,38,40,41; however in the meta-analysis of Liang et al.,13 as well as in other series,24,26,32,33,36 the amount of proteinuria was not able to differentiate DN from NDKD. By contrast, Bi et al.26 found that a greater amount of proteinuria is associated with NDKD.
In our series, microhematuria was the main independent factor associated with the presence of NDKD: it was observed in 9.5% of the patients with ND and in 53% of the cases with NDKD. The meta-analysis by Fiorentino et al.,8 includes 48 studies of KBx patients with DM, 25 of them analyze the prevalence of microhematuria that varied from 6% and 78%. Similarly, Jiang et al.,42 in a recent meta-analysis, found microhematuria in 23–76% of patients with NDKD. In both meta-analyzes8,42 the definition of microhematuria is very variable, from 2 to 20 red blood cells/field, or the simple presence of hematuria in the test strip, which explains this great dispersion.
Diabetic patients with isolated ND may present microhematuria of glomerular origin. We have analyzed t series that evaluate hematuria which include more than 50 patients with KBx9,12,24–27,30,34,36,38,40,41,43–46; the presence of microhematuria in DN is between 5% and 75%, and this variation is related to the estimation of hematuria (≥3 or >10 red blood cells/field) (Table 6). Okada et al.,47 analyzed 84 patients with KBx and pure diabetic nephropathies, microhematuria was found in 43% of cases, although only 10% had sediments with >10 red blood cells/field. In other studies hematuria varied between 3.3%40 and 14.7%,34 if microhematuria is consider as >15 red blood cells/field. The presence of red blood cells of glomerular origin in DN is due to alterations in the glomerular basement membrane or to microaneurysms that can rupture.48 It also appears to be associated with the presence of a greater degree of glomerular sclerosis and markers of long-time with DM.35
Studies of prevalence of microhematuria in patients with diabetic nephropathy and non-diabetic kidney disease.
| Author | Year | Diabetic nephropathy | Non-diabetic kidney disease | Hematuria definition | Significance | ||
|---|---|---|---|---|---|---|---|
| n | Hematuria, n (%) | n | Hematuria, n (%) | ||||
| Mak et al.40 | 1997 | 34 | 10 (29%) | 17 | 10 (59%) | >10 H/field | p<0.005 |
| Tone et al.43 | 2005 | 35 | 11 (31%) | 46 | 25 (54%) | >10 H/field | ns |
| Pham et al.25 | 2007 | 64 | 48 (75%) | 124 | 98 (79%) | ≥2 H/field | ns |
| Zhou et al.41 | 2008 | 60 | 10 (10.7%) | 50 | 34 (68%) | >10 H/field | OR 4.45 (p<0.002) |
| Akimoto et al.44 | 2008 | 34 | 14 (41.2%) | 16 | 12 (75%) | >10 H/field | p<0.05 |
| Chang et al.24 | 2011 | 43 | 24 (56%) | 64 | 31 (48%) | ≥3 H/field 3 | ns |
| Bi et al.26 | 2011 | 120 | 20 (16.7%) | 100 | 68 (68%) | ≥3 H/field | p<0.05 |
| Oh et al.46 | 2011 | 50 | 37 (75) | 65 | 51 (78%) | >5 H/field | ns |
| Chong et al.36 | 2012 | 69 | 28 (40.6%) | 21 | 16 (76%) | Undefined | p=0.002 |
| Sharma et al.12 | 2013 | 227 | 63 (27.8%) | 220 | 74 (33.6%) | >5 H/field | ns |
| Liu et al.34 | 2014 | 93 | 9 (8.4%) | 107 | 23 (21.5%) | >10 H/field | OR 2.664 (p<0.004) |
| Dong et al.38 | 2016 | 61 | 4 (6.6%) | 137 | 23 (17%) | >10 H/field | OR 3.64 (p=0.034) |
| Liu et al.30 | 2016 | 68 | 21 (30.9%) | 175 | 61 (35%) | >10 H/field | ns |
| Bermejo et al.27 | 2016 | 38 | 9 (24%) | 68 | 32 (47%) | Undefined | p<0.05 |
| Mami et al.45 | 2017 | 18 | 1 (5%) | 33 | 12 (36%) | >3 H/field | OR 7.20 (p<0.036) |
| Liu et al.9 | 2018 | 717 | 98 (13.6%) | 788 | 161 (20.5%) | Not defined | p<0.05 |
| García-Martín et al. | 2019 | 126 | 10 (9.5%) | 81 | 40 (53%) | ≥10 H/field | OR 31.25 (p<0.0001) |
H/field: red blood cells per field; ns: not significant.
However, the presence of microhematuria in a diabetic patient suggests NDKD, and is one of the most frequent indications for RBx.8,16 The fundamental aspect is how to define microhematuria so it can be considered predictive factor of non-diabetic nephropathy, and what value should be consider as a differentiator. In our series we found that from 10 red blood cells/field the microhematuria was discriminative and had an independent predictive value of NDKD. The presence of dysmorphic red blood cell in >80% is a better predictor of NDKD than the amount of microhematuria.47Table 6 describes different series that have been analyzed; it is observed that values >10 red blood cells/field is an independent predictor of NDKD with OR between 2.66 and 4.45,34,38,40,41,44 with a similar power to that diabetic retinopathy and the duration of diabetes have for prediction of ND.12,13,31,43
In our predictive model (score by points) we found that the factor of greatest weight for ND was the presence of DR, followed by CLLI. As predictors of NDKD the presence of microhematuria was the most important factor, followed by being overweight. Most patients with scores below to −0.5 points have NDKD. There is a “gray” zone between −0.5 and 3 points, and within this range we can consider an area between −0.5 and 1 points in which the majority are NDKD and another that goes from 1 to 3 points in which predominates ND, but NDKD cannot be excluded exclude completely. Therefore, we believe that the kidney biopsy would be indicated in scores ≤1, and should be individualized when the score is between 1 and 3 points. Kidney biopsy would not be indicated if the score is higher than 3 (Fig. 3).
Although KBx is an invasive procedure, we have observed a low complication rate (3.8%), and in no case the complication was severe, this is similar to what has been described in other series.16 Our study has the limitations inherent to any retrospective study. In addition, being a biopsy-based study, it has the subjectivity of histopathological studies; there is selection bias, since biopsies have only been performed in diabetic patients with a high suspicion of NDKD and the presence of microhematuria was one of the indications to perform the biopsy, so our results could be overestimating the true prevalence of NDKD in the diabetic population. However, when we analyze other studies (Table 6), our series is third in the number of patients with DN shown in the biopsy, so we believe that our criteria for indicating KBx should not be considered restrictive.
In summary, in diabetic patients isolated NDKD is observed frequently, and glomerular diseases are the most frequent cause. In diabetic patients, the presence of retinopathy is highly suggestive of DN, and microhematuria of ≥10 red blood cells/field, is the independent factor with the greatest weight in the prediction NDKD in diabetic patients. The proposed predictive model, based on data from the multivariate study, discriminates the presence of NDKD and it had to be considered when deciding KBx in diabetic patients, although the final decision must always be individualized.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: García-Martín F, González Monte E, Hernández Martínez E, Bada Boch T, Bustamante Jiménez NE, Praga Terente M. ¿Cuándo realizar biopsia renal en pacientes con diabetes mellitus tipo 2? Modelo predictivo de enfermedad renal no diabética. Nefrologia. 2020;40:180–189.