Cardiovascular events are the major cause of morbidity and mortality in patients with chronic kidney disease (CKD). Inflammation and mineral-bone disorder are pathological conditions that have been associated with an increased cardiovascular risk.
ObjectiveShow paricalcitol regulation overinflammatory, fibrotic and mineral disorder parameters in CKD.
Material and methodsProspective study in 46 CKD stages III–V patients without dialysis patients with elevated parathormone in which we introduced paricalcitol. We evaluated classic and newest mineral and bone metabolism serum parameters (calcium, phosphorus, parathormone, fibroblast growth factor-23 [FGF-23], Klotho, calcidiol), inflammatory-fibrosis and anticalcifying parameters (interleukin-6 and 10, tumor necrosis factor-α [TNF-α], transforming growth factor-β [TGF-β], bone morphogenic protein-7 [BMP-7] and fetuin-A) for four months.
ResultsAt the end of study soluble Klotho increased (p=0.001), FGF-23 remained stable, calcium and phosphorus levels were not increased, calcidiol increased (p=0.010) and PTH decreased (p=0.002). Inflammation-fibrosis and calcification parameters showed positive regulation after paricalcitol treatment: interleukin-6 decreased significantly (p=0.001) and also TNF-α did (p=0.005), on the contrary, interleukin-10 and fetuin-A increased (p=0.001 for both). Anti-fibrosis marker BMP-7 increased (p=0.001) and TGF-β decreased (p=0.001). We did not find significant changes in renal function.
ConclusionsParicalcitol treatment might be profitable in regulating inflammatory and anticalcificant parameters, unmodified calcium or phosphorus seric levels and preserving kidney function in renal patients with no dialysis. Our selected parameters could indicate paricalcitol effects in mineral and endothelial disorder related to renal disease.
La principal causa de morbimortalidad en el paciente con enfermedad renal crónica (ERC) es la cardiovascular. La inflamación y las alteraciones en el metabolismo óseo-mineral en estos pacientes conllevan aumento del riesgo cardiovascular.
ObjetivosValorar el papel de paricalcitol sobre distintos parámetros séricos relacionados con inflamación, fibrosis y enfermedad óseo-mineral en la ERC.
Material y métodosEstudio prospectivo, no controlado en 46 pacientes con ERC estadios III-V sin diálisis, con niveles elevados de paratohormona, según su estadio de ERC, por lo que se introdujo tratamiento con el análogo de vitamina D paricalcitol. Durante 4 meses de tratamiento valoramos los parámetros clásicos y novedosos del metabolismo óseo-mineral en suero (calcio, fósforo, paratohormona, factor de crecimiento fibroblástico-23 [FGF-23], Klotho y calcidiol) y parámetros relacionados con el proceso de inflamación-fibrosis y anticalcificantes (interleucina-6 y 10, factor de necrosis tumoral alfa [TNF-a], factor de crecimiento transformante beta [TGF-b], proteína ósea morfogénica-7 [BMP-7], y fetuína-A).
ResultadosTras el uso de paricalcitol los niveles de Klotho aumentaron (p=0,001) y los de FGF-23 se mantuvieron estables al igual que los de calcio y fósforo; calcidiol aumentó de forma significativa (p=0,010) y paratohormona descendió (p=0,002). Los parámetros de inflamación, fibrosis y calcificación mostraron una regulación benigna con descenso significativo de interleucina-6 (p=0,001), TNF-α (p=0,005) y TGF-β (p=0,001) y aumento de BMP-7 (p=0,001), fetuína-A (p=0,001) e interleucina-10 (p=0,001). El filtrado glomerular y la proteinuria se mantuvieron estables.
ConclusionesEl tratamiento con paricalcitol en el paciente renal sin diálisis parece ser beneficioso en la regulación de los parámetros inflamatorios y anticalcificantes, preservando la función renal y el eje óseo-mineral. Los marcadores elegidos en nuestro estudio podrían indicarnos un efecto positivo de paricalcitol a nivel vascular.
Bone-mineral disease (BMD) related to chronic kidney disease (CKD) (CKD-MBD) – includes abnormalities in mineral metabolism parameters: a tendency to a reduction serum calcium (Ca), increased serum phosphorus (P), elevation of parathyroid hormone (PTH), reduction of calcitriol, increased fibroblastic growth factor 23 (FGF-23) and reduction of Klotho1,2; these alterations contribute to vascular calcification through different mechanisms: hyperphosphatemia, the decrease in Klotho promote the entry of P through the Pit1/2 channels into vascular smooth muscle cells with its subsequent osteogenic transformation; the decrease in active vitamin D, and the increase in FGF-23 may be also involved.3–5 Other factors that influence the development of vascular calcification in CKD would be the alteration of parameters related to inflammation and fibrosis, such as the decrease in fetuin A (Ft-A) and bone morphogenic protein 7 (BMP-7) or the increase in tumor necrosis factor α (TNF-α) and transforming growth factor β (TGF-β).3–9. This procalcifying environment of the patient with CKD justifies their high cardiovascular risk.3
Calcitriol and the analogs or vitamin D receptor activators (VDRA), such as paricalcitol (PCT), regulate the levels of PTH, Calcium (Ca) and phosphate (P); in addition they have pleiotropic effects at a systemic level8 such as endothelial protection.9,10 Different studies have shown that calcitriol and VDRA protect the endothelium from calcification by inhibiting calcifying proteins and reducing proinflammatory cytokines.11,12 However, the effect of calcitriol may differ from that of PCT.13,14 Calcitriol, at the vascular level, could have two antagonistic consequences that are dose dose-dependent: with doses that produce hypercalcaemic calcitriol has a procalcifying effect on CMLV mediated in part by the actions of P and procalcifying molecules at the cellular level; PCT does not seem to present this dose-dependent effect.13,14
For this reason we study the effect of PCT on CKD-MBD and molecules associated with vascular calcification, inflammation and fibrosis in patients with CKD (stages III–V not on dialysis) in search of a modulating effect on them.
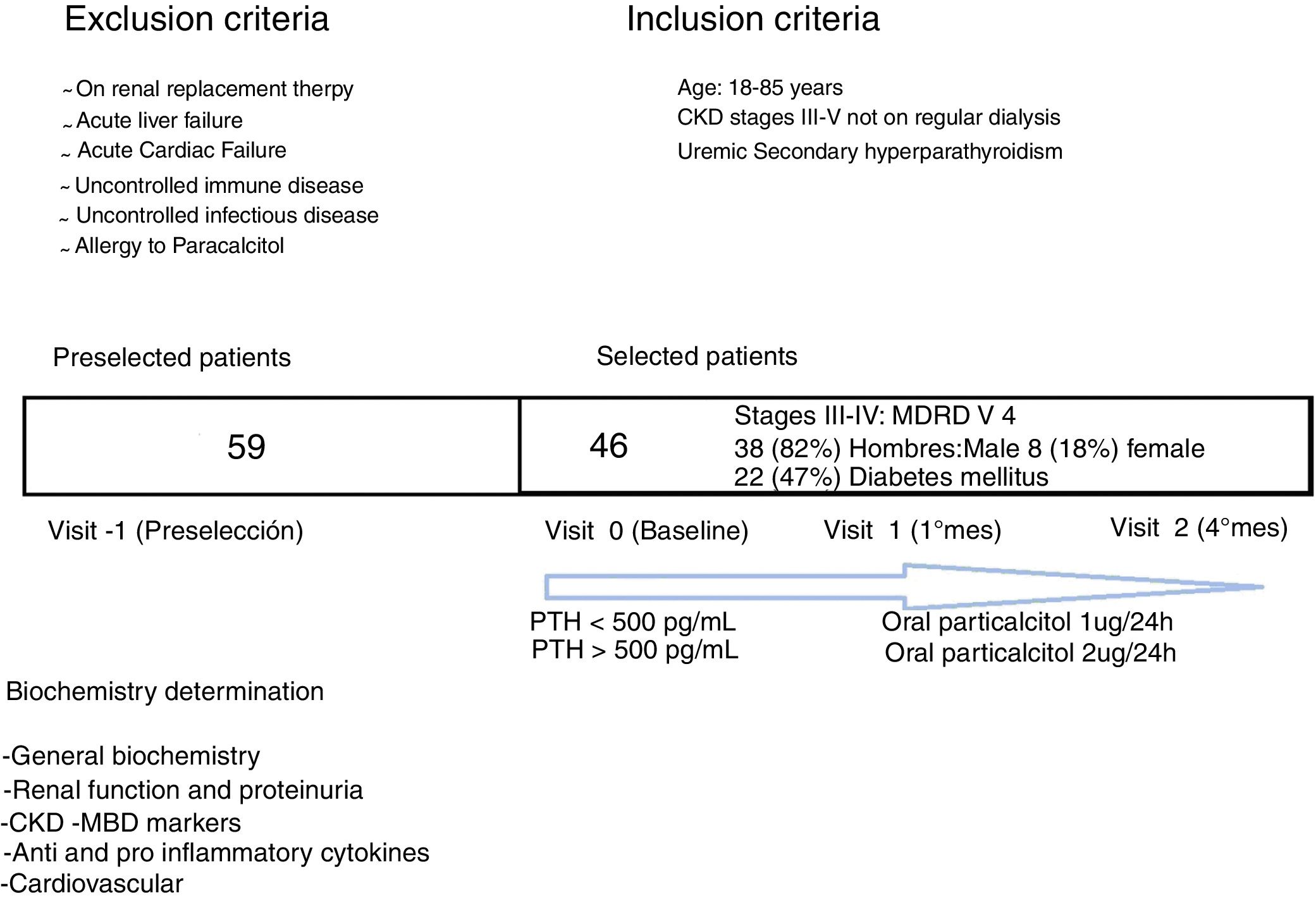
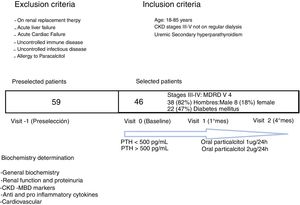
MethodsPatientsThis is a prospective uncontrolled study conducted at the University Hospital La Princesa (Madrid). There were 59 CKD stage III–V not in dialysis patients pre-selected. They were of legal age and susceptible of using PCT due to PTH increase according to CKD staging their (1): stage III >70pg/mL, stage IV >110pg/mL, stage V >150pg/mL (SEN guides). The exclusion criteria were: inclusion in renal replacement therapy, acute cardiac and/or hepatic failure, active infectious or immunological disease and allergy to PCT (Fig. 1). Finally, 46 patients were selected that had a four-month follow-up and were on treatment with PCT: 1mcg daily if PTH<500pg/mL, and 2mcg daily if PTH>500pg/mL. No patient exceeded 500pg/mL of PTH. PRCT doses would be suspended if PTH<70pg/mL. Seven patients were on calcidiol; there were no other treatments related to vitamin D. Patients underwent a washing period of one month prior to the initiation of the study. There were no patient losses during the four month period of follow-up.
BiochemistryBlood and urinary analytical determinations were performed at baseline, one month and four months after initiation on PCT.
Blood samples obtained for specific tests were centrifuged at 1200rpm for 10min, the serum was separated, aliquoted and stored at −80°C for further analysis. ELISA determinations were made according to the protocol described by the manufacturer. The concentrations of the parameters were determined by the standard curve and the respective dilutions. The biochemical parameters assessed in each of the visits were: creatinine (Cr), glomerular filtration rate (GFR) MDRD 4, proteinuria in mg/24h urine collection, Ca, P, calcidiol (Roche/Hitachi Cobas), PTHi (Roche/Hitachi Cobas®: fragments 1-84), soluble Klotho (ELISA Cusabio®, 7.8–500pg/mL), iFGF-23 (Millipore®; 9.9-–2400pg/mL). Parameters related to inflammation: interleukin (IL), IL 6 (R&D®, HS600B; 0.156–10pg/mL), TNF-α (ELISA R&D, HSTA00D; 0.52–32pg/mL), IL10 (R&D, 0.78–50pg/mL), C-reactive protein (CRP) (Roche/Hitachi Cobas)®. Parameters related to calcification and fibrosis: Fetuin-A (Bio Vendor; 0.688–2.330g/L), BMP-7 (R&D; 31.20–2000pg/mL)®, TGF-β1 (R&D systems Quantikine ELISA®; 31.20–2000pg/mL).
Statistical analysisWe conducted an uncontrolled prospective before vs. after study. The variables collected were analyzed by the statistical program SPSS-21. The analysis was done considering the three biochemical evaluations of the patients (baseline, one and four months). Numerical variables are shown as mean±standard deviation (SD). Nominal variables are shown as numbers or percentages. Intragroup differences were analyzed using one way ANOVA for repeated measurements. A p<0.05 value was considered significant.
Security measures and ethical considerationsThe present study is considered category II, with minimal risk. In each visit patients were inquired about the presence of adverse events and appropriate measures were adopted. Informed consent was obtained from all patients. The data collection was obtained preserving the identity the patient according to the Law of Personal Data Protection and the Spanish ethical aspects of research in humans were strictly followed.
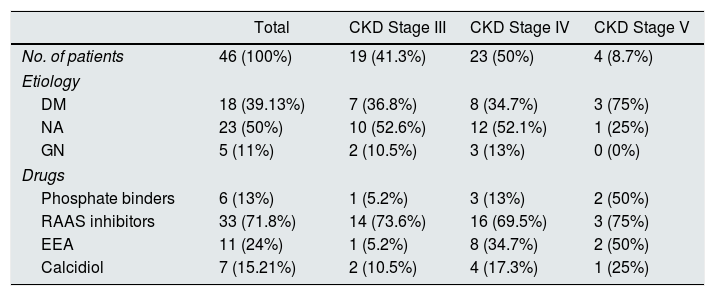
ResultsCohort descriptionWe selected 46 patients: 38 men (82%) and 8 (18%) women, with an average age of 73 years. The characteristics according to CKD staging and etiology are shown in Table 1. In relation with pharmacological treatments, 7 patients (15%) were previously on calcidiol (they underwent 1month washing period before the study), 6 patients (13%) were treated with phosphate binders and 33 patients (71.8%) were on renin angiotensin aldosterone (RAA) system inhibitors. None of them was on cinacalcet.
Etiology and pharmacological characteristics of the patients included in the study according to the degree of CKD at the beginning of the study.
| Total | CKD Stage III | CKD Stage IV | CKD Stage V | |
|---|---|---|---|---|
| No. of patients | 46 (100%) | 19 (41.3%) | 23 (50%) | 4 (8.7%) |
| Etiology | ||||
| DM | 18 (39.13%) | 7 (36.8%) | 8 (34.7%) | 3 (75%) |
| NA | 23 (50%) | 10 (52.6%) | 12 (52.1%) | 1 (25%) |
| GN | 5 (11%) | 2 (10.5%) | 3 (13%) | 0 (0%) |
| Drugs | ||||
| Phosphate binders | 6 (13%) | 1 (5.2%) | 3 (13%) | 2 (50%) |
| RAAS inhibitors | 33 (71.8%) | 14 (73.6%) | 16 (69.5%) | 3 (75%) |
| EEA | 11 (24%) | 1 (5.2%) | 8 (34.7%) | 2 (50%) |
| Calcidiol | 7 (15.21%) | 2 (10.5%) | 4 (17.3%) | 1 (25%) |
The percentage refers to the number of patients in each stage.
EEA: erythropoiesis stimulating agent; DM: diabetes mellitus; GN: glomerulonephritis; NA: nephroangiosclerosis; RAAS: renin–angiotensin aldosterone system.
After administration of PCT no significant changes were observed in hemoglobin, iron, transferrin saturation, total CO2, albumin, prealbumin, liver and lipid profile.
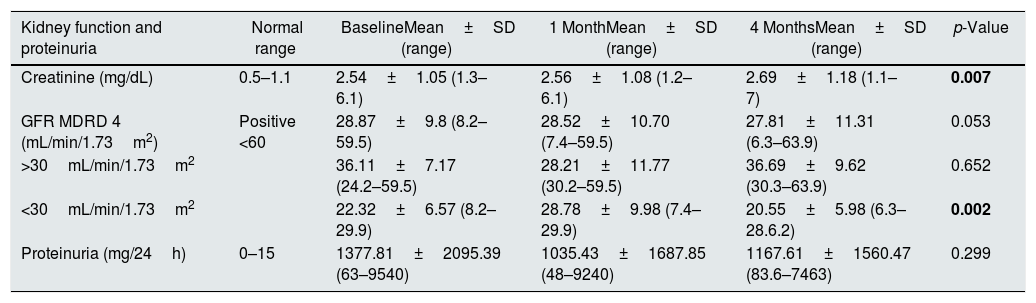
Proteinuria did not decrease significantly (p=0.299); not even in the 28% of patients that did not receive treatment with SRAA inhibitors (p=0.766). Serum creatinine levels increased significantly increase in creatinine (p=0.007) and the GFR decreased almost significantly (p=0.053). With these results we considered to divide patients according to the degree of CKD, categorizing as severe if GFR<30mL/min/1.73m2 (n=25) or moderate if GFR>30mL/min/1.73m2 (n=21). In patients with severe CKD there was a significant reduction in GFR (p=0.002); however, among patients with moderate CKD, there was no statistical change in GFR (p=0.652). The data is shown in Table 2.
Changes in variables of kidney function and proteinuria during the study.
| Kidney function and proteinuria | Normal range | BaselineMean±SD (range) | 1 MonthMean±SD (range) | 4 MonthsMean±SD (range) | p-Value |
|---|---|---|---|---|---|
| Creatinine (mg/dL) | 0.5–1.1 | 2.54±1.05 (1.3–6.1) | 2.56±1.08 (1.2–6.1) | 2.69±1.18 (1.1–7) | 0.007 |
| GFR MDRD 4 (mL/min/1.73m2) | Positive <60 | 28.87±9.8 (8.2–59.5) | 28.52±10.70 (7.4–59.5) | 27.81±11.31 (6.3–63.9) | 0.053 |
| >30mL/min/1.73m2 | 36.11±7.17 (24.2–59.5) | 28.21±11.77 (30.2–59.5) | 36.69±9.62 (30.3–63.9) | 0.652 | |
| <30mL/min/1.73m2 | 22.32±6.57 (8.2–29.9) | 28.78±9.98 (7.4–29.9) | 20.55±5.98 (6.3–28.6.2) | 0.002 | |
| Proteinuria (mg/24h) | 0–15 | 1377.81±2095.39 (63–9540) | 1035.43±1687.85 (48–9240) | 1167.61±1560.47 (83.6–7463) | 0.299 |
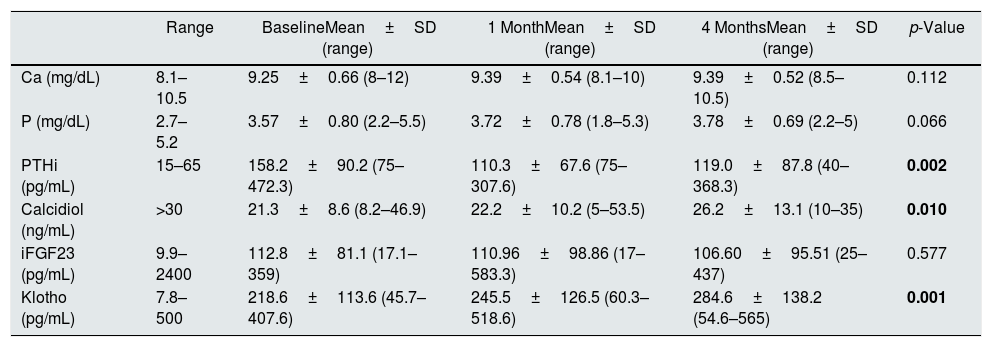
The classic markers of the CKD-MBD such as Ca and P were not modified by the administration of PCT (p=0.121, p=0.066, respectively). There were no increases in Ca concentration above >10.5mg/dL or P>5.5mg/dL, so there was no need to change the treatment of P binders or dose of PCT. IT was observed significant reduction in iPTH levels (p=0.02); however, iPTH values remained in the range recommended by the SEN Guidelines, so there was no need to discontinue PCT in any of the patients. We also observed a significant increase in calcidiol (p=0.01). Data is shown in Table 3.
Evolution of the biochemical variables of CKD-MBD during the study.
| Range | BaselineMean±SD (range) | 1 MonthMean±SD (range) | 4 MonthsMean±SD (range) | p-Value | |
|---|---|---|---|---|---|
| Ca (mg/dL) | 8.1–10.5 | 9.25±0.66 (8–12) | 9.39±0.54 (8.1–10) | 9.39±0.52 (8.5–10.5) | 0.112 |
| P (mg/dL) | 2.7–5.2 | 3.57±0.80 (2.2–5.5) | 3.72±0.78 (1.8–5.3) | 3.78±0.69 (2.2–5) | 0.066 |
| PTHi (pg/mL) | 15–65 | 158.2±90.2 (75–472.3) | 110.3±67.6 (75–307.6) | 119.0±87.8 (40–368.3) | 0.002 |
| Calcidiol (ng/mL) | >30 | 21.3±8.6 (8.2–46.9) | 22.2±10.2 (5–53.5) | 26.2±13.1 (10–35) | 0.010 |
| iFGF23 (pg/mL) | 9.9–2400 | 112.8±81.1 (17.1–359) | 110.96±98.86 (17–583.3) | 106.60±95.51 (25–437) | 0.577 |
| Klotho (pg/mL) | 7.8–500 | 218.6±113.6 (45.7–407.6) | 245.5±126.5 (60.3–518.6) | 284.6±138.2 (54.6–565) | 0.001 |
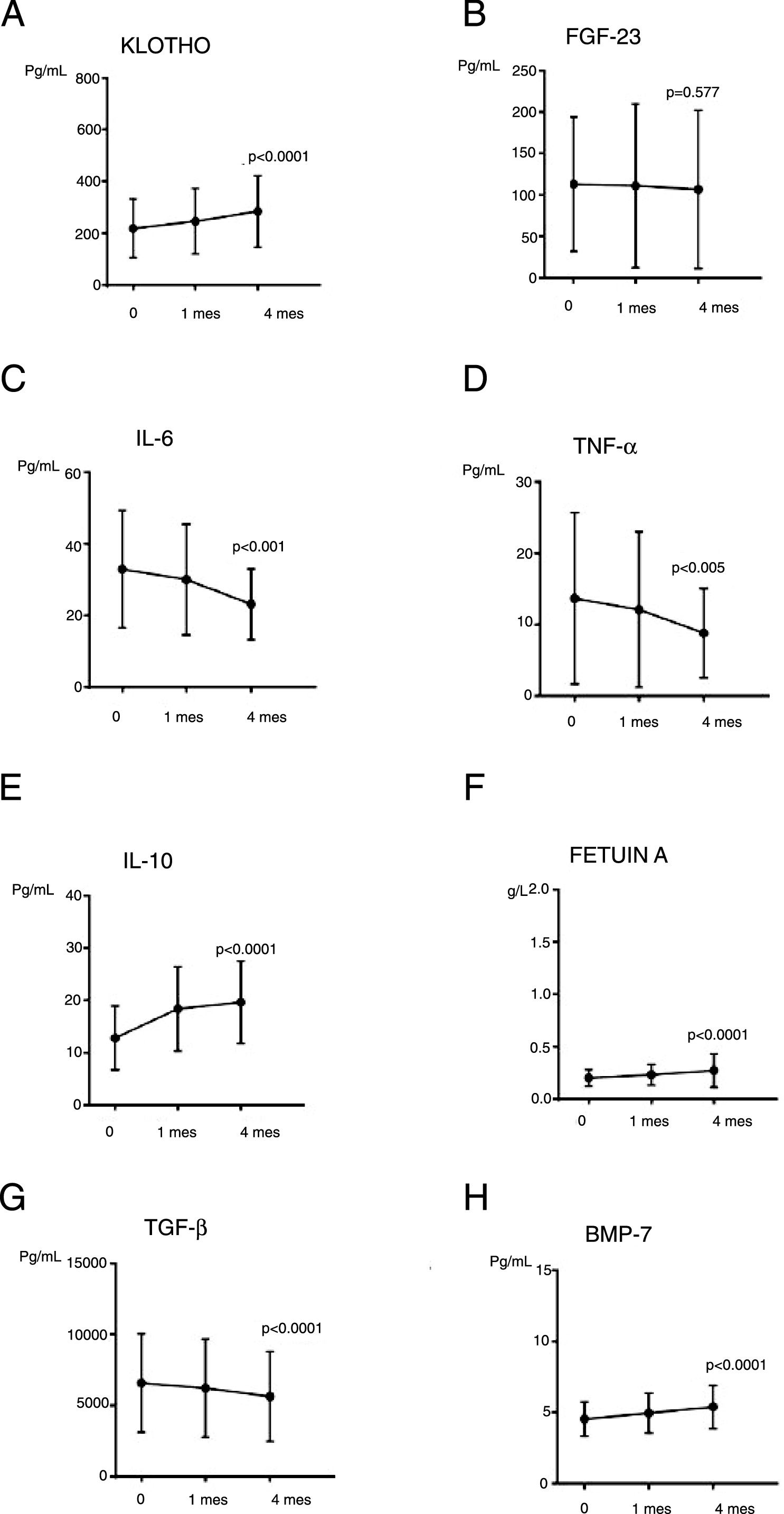
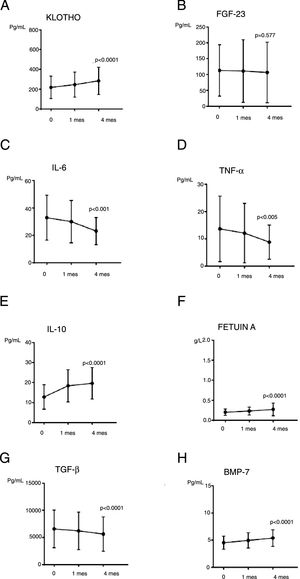
Regarding more recent parameters of CKD-MBD (Fig. 2), the levels of iFGF-23 were not significantly modified throughout the study (p=0.577); and iFGF-23 was not changed in patients on P binders (n=6; p=0.125). The difference of means of iFGF-23 at the end of the study (Student's t for independent samples) in patients with and without treatment P binders was calculated, the result did not reach statistical significance (p=0.953, with a mean difference of 2.48). Interestingly, a significant increase in Klotho levels (p=0.001) was observed.
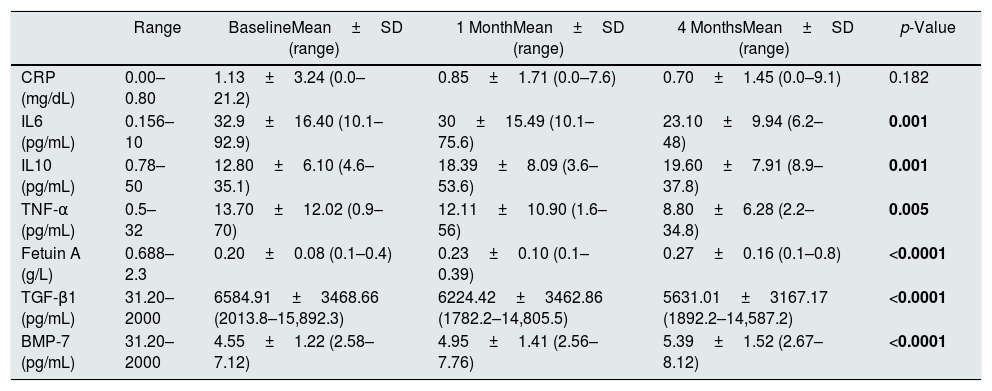
The effect of PCT on parameters of inflammation, fibrosis and vascular calcificationThe levels of CRP did not change although it was observed a downward trend. The concentration of IL6 and TNF-α decreased significantly (p=0.001 and p=0.005, respectively). IL10, an anti-inflammatory parameter, increased significantly (p<0.001). Regarding parameters of fibrosis and vascular calcification, it was observed a significant increase in Fetuin-A (p<0.001) and BMP-7 (p<0.001) and a decrease in TGF-β1 (p<0.001). These results are shown in Table 4 and Fig. 2.
Evolution of parameters of inflammation, fibrosis and vascular calcification.
| Range | BaselineMean±SD (range) | 1 MonthMean±SD (range) | 4 MonthsMean±SD (range) | p-Value | |
|---|---|---|---|---|---|
| CRP (mg/dL) | 0.00–0.80 | 1.13±3.24 (0.0–21.2) | 0.85±1.71 (0.0–7.6) | 0.70±1.45 (0.0–9.1) | 0.182 |
| IL6 (pg/mL) | 0.156–10 | 32.9±16.40 (10.1–92.9) | 30±15.49 (10.1–75.6) | 23.10±9.94 (6.2–48) | 0.001 |
| IL10 (pg/mL) | 0.78–50 | 12.80±6.10 (4.6–35.1) | 18.39±8.09 (3.6–53.6) | 19.60±7.91 (8.9–37.8) | 0.001 |
| TNF-α (pg/mL) | 0.5–32 | 13.70±12.02 (0.9–70) | 12.11±10.90 (1.6–56) | 8.80±6.28 (2.2–34.8) | 0.005 |
| Fetuin A (g/L) | 0.688–2.3 | 0.20±0.08 (0.1–0.4) | 0.23±0.10 (0.1–0.39) | 0.27±0.16 (0.1–0.8) | <0.0001 |
| TGF-β1 (pg/mL) | 31.20–2000 | 6584.91±3468.66 (2013.8–15,892.3) | 6224.42±3462.86 (1782.2–14,805.5) | 5631.01±3167.17 (1892.2–14,587.2) | <0.0001 |
| BMP-7 (pg/mL) | 31.20–2000 | 4.55±1.22 (2.58–7.12) | 4.95±1.41 (2.56–7.76) | 5.39±1.52 (2.67–8.12) | <0.0001 |
During the study there were no adverse reactions to the medication, the treatment was not changed. There were no hospital admissions or cardiovascular events.
DiscussionIn our study, patients presented an improvement in parameters related to inflammation, fibrosis and vascular calcification. The CKD-MBD markers were stable: Ca, P, FGF-23 or they show improvement as Klotho or PTH. CKD-MBD and inflammation are determinants in the onset and progression of CKD and cardiovascular risk (CVR) in these patients.15,16 PCT has demonstrated, in multiple experimental studies, that it is safe and beneficial in the regulating CKD-MBD and inflammatory markers in CKD without aggravating the renal disease.17,18
The effect of PCT on renal function and proteinuria has been evaluated in different studies. The VITAL study19 demonstrated, in type 2 diabetic patients, a significant reduction in proteinuria at a dose of 2μg/day. Similar results were reported in the study by Agarwal et al.20 in patients with CKD stages III–IV. The antiproteinuric effect of PCT, and active vitamin D analog, is due to the regulation of the proliferative and fibrotic process21,22 and the blockade of the RAAS19,23; pharmacological inhibition of this axis may require an increase in the dose of PCT to achieve a decrease in proteinuria. In our study we did not accomplish a significant change in proteinuria, perhaps due to the wide use of SRAA inhibitors (71.8%). Regarding the GFR, we did not observed a significant change, although there was a tendency to decrease, perhaps due to the significant deterioration in GFR observed in patients with GFR<30mL/min/1.73m2. Regarding the significant increase in Cr, it could be related to the influence of external variables such as sex, age, muscle mass and nutritional status on this variable. It can be concluded that the activation of the vitamin D receptor with PCT maintain the GFR stable, which is in agreement with other studies.24,25 This effect of PCT result in attenuation of glomerular, tubulointerstitial and endothelial damage that has been demonstrated in experimental studies by anti-inflammatory effects26,27, antifibrotic28 and antiproteinúricos effects19,20 that will be commented later.
As expected, PCT reduced PTHi significantly coinciding with the findings by other authors.29,30 We have shown that the levels of Ca and P have been stable, although in the case of P the tendency was upward. As compared with calcitriol PCT has less hypercalcemic and hyperphosphatemic which is due to less production of intestinal calbindin and a lower affinity for intestinal vitamin D receptors31; this effects may influence the genesis of vascular calcification. There is no clear mechanism to explain the increase in calcidiol in our study. It may be due to the stability of FGF-23 (which stimulate 24-hydroxylase that inactivates calcidiol). In this sense, and following the MBD, the effect of PCT on markers such as FGF-23 and Klotho would be relevant since these molecules may participate in vascular health calcification, inflammatory status and renal function. After PCT treatment Klotho was significant increased which has beneficial effects at myocardial level and left ventricular hypertrophy33,34, PCT had anti-inflammatory effects by reducing the levels of IL6 and 1832,36 antifibrotic35 by decreasing TGF-β and antiapoptoic effects37 that could reduce vascular calcification.32,36 PCT has been shown to up-regulate the levels of Klotho in various studies such as the one by Lau et al.11 where the treatment with PCT and calcitriol, in uremic mice with vascular calcification, significantly increased urinary and serum Klotho levels. Regarding FGF-23 levels, our study showed different results than IMPACT38 or PARADIGM39 studies showing that the use of PRCT or calcitriol, in patients on hemodialysis, produced an increased in FGF-23; the difference in results could be explained by the inclusion of dialysis patients. The increase in FGF-23 in CKD does not induce vascular calcification directly40 but without doubt it is associated with increased vascular morbidity and mortality.41–43 FGF-23 suppresses the levels of calcitriol3,6 and Ft-A (a protein acting against vascular calcification)41 causes endothelial dysfunction, left ventricular hypertrophy and proteinuria.41,42 Many of these FGF-23 procalcifying mechanisms aggravate the inflammatory environment of CKD3,44 which is stimulated by FGF-23 inducing the increase of IL6 and TNF-α.45 The inflammatory state in turn upregulates upward FGF-23 production;: TNF-α and NF-κB may inhibit bone matrix and increase the production of FGF-23 by the osteocyte.45 In addition, the proinflammatory and fibrotic state of CKD downregulate Klotho production.36
Inflammation itself is a therapeutic target in CKD and PCT has demonstrated its beneficial anti-inflammatory effects.13,14 After treatment with PCT, our patients had a favorable anti-inflammatory biochemical effect by significant reduction of IL6 and TNF-α levels and an increase in IL10, perhaps mediated by the increase in Klotho and stability of FGF-23 or by a direct effect of drug. Our results are in agreement with the data by Donate et al.22; these authors demonstrated in patients with CKD and elevated PTH (n=8; IIIB–IV staging) that treatment with PCT caused a significant decrease in IL6 and TNF-α. The antiinflammatory effect of PCT may have be conditioned by a reduction in cytokine production by T cells.26,27 These anti-inflammatory effects of PCT could influence anti-calcifying parameters such as Fetuin-A or BMP-7 (that are reduced in patients with CKD),8,46 and profibrotic molecules such as TGF-β (increased in CKD). The increase in Fetuin-A after the use of PCT could reduce the procalcifying profile, as well as the results we obtained on BMP-7 which, in addition to its anti-calcifying effects47,48 inhibits the Na/Pi cotransporter of vascular smooth muscle cells (VSMC), promotes bone formation and exhibits anti-inflammatory effects.47,49 The effect of TGF-β is the opposite of BMP-7; its significant decrease observed in our study could result in nephroprotection and attenuation of vascular damage50 by decreasing the migration of osteoprogenitor mesenchymal stem cells to vascular areas51 promoted by TGF-β and by decreasing cell apoptosis, proliferation and differentiation.52
Our study has limitations; the patients are controls of themselves, we do not have a placebo group, the observation time is short and our cohort is small; all this could lead to alterations in the interpretation of results. In addition, we do not differentiate the time of the year of inclusion of patients that could influence calcidiol levels which vary seasonally. We base our results on blood markers and not on experimental models of vascular injury; likewise have provided no data on other molecules as RANKL (ligand receptor activator nuclear factor κ beta), osteopontin or osteoprotegerin. As a counterpoint, the parameters analyzed cover an important influence on vascular calcification, inflammation and fibrosis with possible favorable regulation after treatment with PCT.
ConclusionsThe treatment of renal patient not on dialysis with PCT, seems to have beneficial effects on the regulation of inflammatory, fibrotic and anti-calcifying parameters; as well as on classic and non-classic markers of the CKD-MBD and it may help to preserve renal function. There is not one marker that assesses the pleiotropic effects of PCT in kidney disease. The markers chosen in our study reflect the PCT effect on bone-mineral kidney disease and, specifically, in the process of vascular calcification.
The data obtained could indicate a positive modulating effect of PCT on markers related to vascular calcification, and the inflammatory and fibrotic state of CKD.
The therapeutic use of the VDRA in patients with CKD remains unexplored, being relegated as an exclusive treatment of secondary hyperparathyroidism without conducting clinical trials with broader expectations in clinical practice in recent years. This leaves a field still to be explored especially in relation to vascular calcification in patients with chronic kidney disease.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Salanova Villanueva L, Gil Giraldo Y, Santos Sánchez-Rey B, Aguilera Peralta A. Efecto regulador de paricalcitol sobre parámetros inflamatorios, fibróticos y anticalcificantes en el paciente con enfermedad renal crónica. Más allá de la regulación de la enfermedad óseo-mineral. Nefrologia. 2020;40:171–179.