There is much debate about whether idiopathic hypercalciuria (IH) affects kidney water management. For the first time in the literature, we carried out a longitudinal study of kidney water management (KWM) in patients diagnosed with IH in childhood and followed-up until adulthood (mean follow-up 17.7±1.4 years).
MethodsTwenty-nine patients (7 M, 22 F) over the age of 24 years (mean 28.2±2.9 years, range: 24.1–35.9) who were diagnosed with IH in childhood (mean 7.6±3.2 years, range: 1–14) were included. Maximum urine osmolality (UO) and/or urine volume adjusted for 100ml of glomerular filtration rate (V/GFR) in both age groups (pediatric and adult) were determined. Moreover, whenever possible, in both age groups plasma creatinine levels, plasma sodium levels, uric acid levels, the citrate/creatinine ratio and the calcium/citrate ratio were recorded and a renal and bladder ultrasound was performed.
ResultsIn the pediatric age group, KWM was altered in 9/29 cases (31%) (4 with reduced maximum UO and 5 with elevated V/GFR). In adulthood, KWM was found to be affected in 7/29 cases (24.1%) (6 with reduced UO and one with elevated V/GFR). Compared to the pediatric age group, adult patients had lower V/GFR, calcium/creatinine and citrate/creatinine values, as well as higher plasma creatinine, uric acid and calcium/citrate. There were no differences in the maximum UO in both age groups. However, UO in adulthood was significantly lower in subjects who had renal colic compared to those who did not (p=.04).
ConclusionsKWM was affected in approximately one third of patients with IH, which persisted 20 years after diagnosis. We think that these results may be due to adherence to the recommended protective diet and to the pharmacological treatment administered at the diagnosis of IH during childhood.
Existe controversia si la hipercalciuria idiopática (HI) produce alteraciones en el manejo renal del agua. Por primera vez en la literatura, llevamos a cabo un estudio longitudinal del manejo renal del agua (MRA) en pacientes diagnosticados de HI en edad pediátrica y con seguimiento hasta la edad adulta (media de seguimiento de 17,7±1,4 años).
MétodosVeintinueve pacientes (7 M, 22 F) mayores de 24 años (media 28,2±2,9 años, rango: 24,1-35,9) que fueron diagnosticados de HI en la edad pediátrica (media 7,6±3,2 años, rango: 1-14) fueron incluidos. Se determinaron la osmolaridad urinaria máxima (OsU) y/o el volumen urinario ajustado para 100ml de tasa de filtrado glomerular (V/TFG) en ambos tiempos (pediátrico y adulto). Además, siempre que fue posible, en ambas edades se recogieron los niveles plasmáticos de creatinina, sodio plasmático, ácido úrico, cociente citrato/creatinina y calcio/citrato y, además, se realizó una ecografía renovesical.
ResultadosEl MRA estuvo alterado en edad pediátrica en 9/29 casos (31%) (4 con OsU máxima reducida y 5 con V/TFG elevado). En la edad adulta, 7/29 (24,1%) presentaron alteración del MRA (6 OsU reducidos y uno con V/TFG elevado). En comparación con el grupo de edad pediátrica, los pacientes adultos mostraron valores reducidos de V/TFG, cociente calcio/creatinina y citrato/creatinina, así como aumento de creatinina plasmática, ácido úrico y del cociente calcio/citrato. No hubo diferencias en la OsU máxima en ambos tiempos. Sin embargo, la OsU en la edad adulta fue significativamente menor en aquellos que tenían cólicos renales comparado con aquellos que no los tuvieron (p=0,04).
ConclusionesLa alteración del MRA ocurrió en aproximadamente un tercio de los pacientes con HI, y no se alteró tras 20 años después de su diagnóstico. Nosotros pensamos que estos resultados pueden ser debido a un cierto cumplimiento de la dieta protectora recomendada y al tratamiento farmacológico administrado en el diagnóstico de HI en la edad pediátrica.
Idiopathic hypercalciuria (IH) is the most frequent metabolic abnormality that causes kidney stones in children and adults. The most likely cause of IH is an increase in the number of vitamin D receptors. This has been demonstrated in rats with spontaneous hypercalciuria (genetic hypercalciuric stone-forming)1,2 and it has been confirmed in humans.3 In addition, it has been observed that blood monocytes increase the production of cytokines, such as interleukin-1, granulocyte-macrophage colony stimulating factor and tumor necrosis factor4,5 which, by various mechanisms, are able to increase the elimination of urinary calcium.6
In 1960 it was discovered that adults with IH could have a defect in the ability to concentrate the urine.7 Thereafter published results on IH have not been uniform in adult patients8–12 and children.11–14 For the first time, we conduct a longitudinal study of renal water handling (RWH) in patients with IH diagnosed in pediatric age with a follow-up up to adulthood.
Patients and methodsA longitudinal study was carried out, including 29 patients (7 M, 22 F) older than 24 years (mean 28.2±2.9 years, range: 24.1–35.9) who were diagnosed with IH in the pediatric age (mean 7.6±3.2 years; 1–14) because the urinary excretion of calcium was over 4mg/kg/day13.14 (5.65±2.12; range: 4–11.8); urinary calcium/creatinine ratio: 0.30±0.09; range: 0.2–0.5. All adult patients included in the study agreed to participate in a telephone conversation conducted by one of the authors. Their names appear in an old archive, in which 104 patients diagnosed with IH during childhood were included, and had bone densitometry performed. The subsequent interview was conducted in person during clinic visit. Only one patient was followed as an outpatient in the Adult Nephrology Service and another in the Rheumatology Service. None of these 2 patients received drug treatment at the time of the study. The inclusion criteria used for the selection of patients were that, evaluation of renal water handling (RWH) had to be performed at both times, the RWH included the determination of urinary osmolarity (OsU) with a desmopressin test (children n=4, adults n=2), urinary volume adjusted to 100ml of glomerular filtration rate (V/GFR) (children n=12, adults n=3) or both (children n=13, adults n=24). In adulthood, the data collected were recorded between April and June 2016. Whenever possible, the following analytical parameters were collected at both ages: plasma values of creatinine, sodium and uric acid. The urinary citrate/creatinine and calcium/citrate ratios were also collected. These parameters correspond to the first urine sample in the morning. Initial clinical data were collected at the time of diagnosis of IH and at that time none of the children showed elevated plasma creatinine levels, hypertension or distal renal tubular acidosis. In adulthood, patients were interviewed about personal history of kidney stones and family history of urolithiasis in the first and second generation. There was no history of consanguinity. Renal and bladder ultrasound was performed in all patients at both times. In adults the estimated glomerular filtration rate (GFR) was calculated by the formula CKD-EPI. The V/GFR was calculated using values of creatinine in plasma and urine that it was obtained using the following formula: plasma creatinine×100/creatinine in urine15,16 and was considered high if it was greater than 1.03ml/100ml GFR.16
Desmopressin urine concentration testOnce bladder was evacuated the patient received 0.2mg (200μg) of oral desmopressin or 0.12mg (120μg) of sublingual lyophilized desmopressin (MELT).11,17,18 Subsequently, 3 consecutive samples were taken at intervals of 90min, and the maximum value was the UOs. A maximum value below 800mOsm/kg was indicative defect of renal concentration.11,17,18
Calcium/creatinine ratioIn adulthood, a calcium/creatinine ratio greater than 0.2mg/mg was considered elevated.4–6
Laboratory determinations and ultrasoundIn childhood the creatinine was determined by the method of creatininase (colorimetric enzymatic analysis). In adults, the creatinine was measures by kinetic colorimetric test (kinetic Jaffe method [compensated method]) using a Beckman Coulter analyzer.
Urinary calcium was measured by NM-BAPTA and the citrate by enzymatic colorimetric determination (Trinitry Biotech). These parameters were measured with a Cobas-501 Roche Diagnostics analyzer. Uric acid was determined using the Cobas-702 Analyzer Roche Diagnosis analyzer. Urinary osmolarity was determined by reduction of the freezing point in the Osmo Station OM-6050 osmometer (Menarini Diagnostics). The renal and bladder ultrasound was performed with an esaote MyLab25 Gold ultrasound and the examinations were performed by a single radiologist to avoid interobserver variability.
Statistic analysisThe Kolmogorov–Smirnov test was performed to analyze the distribution of values. Quantitative variables were expressed as mean±standard deviation. Bivariate analyzes were used for an initial evaluation of the differences. Differences between initial and follow-up values were analyzed using Student's t for paired samples. The Mann–Whitney U test was used to compare means in quantitative variables in relation to the history of urolithiasis in patient or family. The SPSS statistical software (SPSS v. 19.0, SPSS Inc., USA) was used. A value p<0.05 was considered statistically significant.
All patients signed the informed consent. The procedures and protocols carried out in this study complied with the ethical, administrative and data protection requirements established by the Nuestra Señora de Candelaria University Hospital, which are established in accordance with the law of Spain and the Helsinki declaration.
ResultsThe clinical symptoms at the time of diagnosis of IH were variable: urinary tract infection without associated symptoms (n=2) or with other symptoms (n=7), urinary symptoms (dysuria, frequency, nocturnal enuresis, n=4), nonspecific abdominal pain (n=6) or presumably associated to renal colic (n=3) and macroscopic hematuria (n=1). In the remaining 6 cases the assessment of urinary calcium excretion was performed by family history of IH.
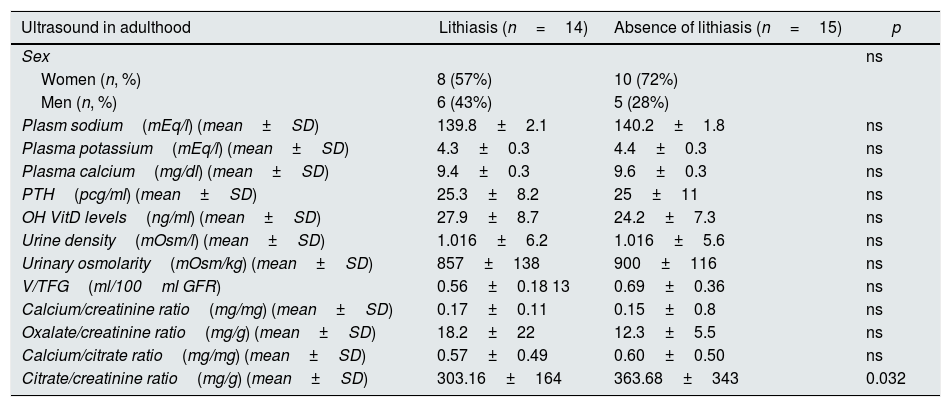
The review of medical records showed that, in the pediatric age, 23 patients followed a preventive diet, 2 of them were treated with thiazides, 2 received treatment with potassium citrate and the other 2 received both medications at different times of their life. In all cases, the prescription of these medications was maintained for at least one year. In adulthood, 9 patients (29%) had kidney stones throughout their lives and 18 of the 29 families (62.1%) had a family history of urolithiasis. Nineteen patients (65.5%) had a normal calcium/creatinine ratio in adulthood. In the renal ultrasound performed in the pediatric age, 8 children (26%) presented nephrolithiasis, predominantly in the female gender (n=5; 62%) and in adulthood the presence of nephrolithiasis was observed in 14 of the 29 patients (48%). Table 1 shows the main biochemical and urinary parameters of patients with and without lithiasis in adulthood.
Biochemical and urinary parameters of patients with and without lithiasis in adulthood.
| Ultrasound in adulthood | Lithiasis (n=14) | Absence of lithiasis (n=15) | p |
|---|---|---|---|
| Sex | ns | ||
| Women (n, %) | 8 (57%) | 10 (72%) | |
| Men (n, %) | 6 (43%) | 5 (28%) | |
| Plasm sodium(mEq/l) (mean±SD) | 139.8±2.1 | 140.2±1.8 | ns |
| Plasma potassium(mEq/l) (mean±SD) | 4.3±0.3 | 4.4±0.3 | ns |
| Plasma calcium(mg/dl) (mean±SD) | 9.4±0.3 | 9.6±0.3 | ns |
| PTH(pcg/ml) (mean±SD) | 25.3±8.2 | 25±11 | ns |
| OH VitD levels(ng/ml) (mean±SD) | 27.9±8.7 | 24.2±7.3 | ns |
| Urine density(mOsm/l) (mean±SD) | 1.016±6.2 | 1.016±5.6 | ns |
| Urinary osmolarity(mOsm/kg) (mean±SD) | 857±138 | 900±116 | ns |
| V/TFG(ml/100ml GFR) | 0.56±0.18 13 | 0.69±0.36 | ns |
| Calcium/creatinine ratio(mg/mg) (mean±SD) | 0.17±0.11 | 0.15±0.8 | ns |
| Oxalate/creatinine ratio(mg/g) (mean±SD) | 18.2±22 | 12.3±5.5 | ns |
| Calcium/citrate ratio(mg/mg) (mean±SD) | 0.57±0.49 | 0.60±0.50 | ns |
| Citrate/creatinine ratio(mg/g) (mean±SD) | 303.16±164 | 363.68±343 | 0.032 |
The RWH was altered in pediatric age in 9/29 cases (31%) (4 with reduced maximum UOs and 5 with high V/TFG), while in adults this alteration was observed in 7/29 cases (24.1%) (6 with reduced maximum UOs and one with high V/TFG).
In adults, the GFR estimated by CKD-EPI was 113.9±13.9ml/min/1.73m2 and the urine albumin/creatinine ratio was 8.3±10.6mg/g. Three patients showed an estimated GFR by CKD-EPI between 81 and 90ml/min/1.73m2. In 2 of them (67%) it was observed an alteration in the RWH (p=n/s).
The results of the RWH study were not always the same at both times of life. Therefore, only 3 of the 9 children with alteration of RWH showed the same defect in adulthood.
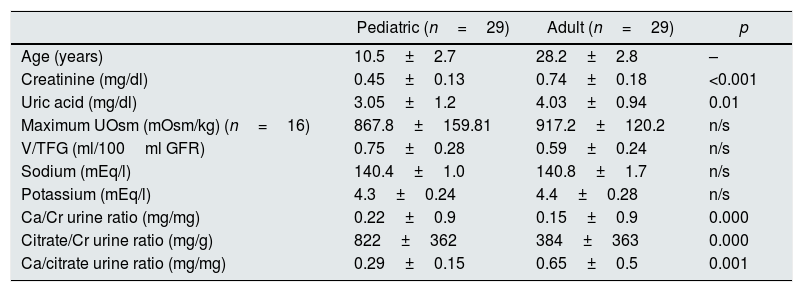
Compared to pediatric age, adult patients showed reduced value of V/GFR (0.59±0.24 vs. 0.75±0.28 in childhood, p=n/s), calcium/creatinine (0.15±0.9 vs. 0.22±0.9 in the pediatric age, p=0.000) and citrate/creatinine ratio (384±363 vs. 822±362 in the pediatric age, p=0.000).
In addition, a significant increase in the calcium/citrate ratio was observed in adulthood (0.65±0.5 vs. 0.29±0.15 in pediatric age, p<0.001), creatinine levels (0.74±0.18 vs. 0.45±0.13 in pediatric age, p<0.001) and uric acid (4.03±0.94 vs. 3.05±1.2 in the pediatric age, p<0.001). There were no significant differences in the values of the maximum UOs when comparing both ages (Table 2).
Characteristics of patients at both ages: pediatric and adult.
| Pediatric (n=29) | Adult (n=29) | p | |
|---|---|---|---|
| Age (years) | 10.5±2.7 | 28.2±2.8 | – |
| Creatinine (mg/dl) | 0.45±0.13 | 0.74±0.18 | <0.001 |
| Uric acid (mg/dl) | 3.05±1.2 | 4.03±0.94 | 0.01 |
| Maximum UOsm (mOsm/kg) (n=16) | 867.8±159.81 | 917.2±120.2 | n/s |
| V/TFG (ml/100ml GFR) | 0.75±0.28 | 0.59±0.24 | n/s |
| Sodium (mEq/l) | 140.4±1.0 | 140.8±1.7 | n/s |
| Potassium (mEq/l) | 4.3±0.24 | 4.4±0.28 | n/s |
| Ca/Cr urine ratio (mg/mg) | 0.22±0.9 | 0.15±0.9 | 0.000 |
| Citrate/Cr urine ratio (mg/g) | 822±362 | 384±363 | 0.000 |
| Ca/citrate urine ratio (mg/mg) | 0.29±0.15 | 0.65±0.5 | 0.001 |
No correlation was found between the Ca/Cr ratio and the RWH in pediatric and adult ages.
By separating the sample according to whether or not there was a personal history of renal stones, it was found no differences in the initial biochemical parameters studied. By contrast, the UOs in adults with renal calculi throughout their lives was significantly lower than in those without calculi (773.5 [245] [n=9]) vs. 931 [142]mOsm/kg [n=17]; p=0.04). These differences were not demonstrated in relation to the presence or absence of kidney stones on ultrasound performed in adulthood. A negative correlation was observed between age and maximum urinary osmolarity in adulthood (r=−0.4; p=0.04).
DiscussionIn this study, renal water management was analyzed by measuring the capacity to concentrate the urine and V/TFG in urine. Urine concentration is the result of a complex glomerulo-tubular mechanism that culminates with the stimulation of water reabsorption by arginine-vasopressin (ADH) which is mediated by action of aquaporins designed to reabsorb water in the renal collecting tubule. The renal concentrating capacity depends on an adequate supply of the glomerular ultrafiltrate to the tubules, a hypertonic medullary interstitium, a structurally intact countercurrent mechanism and the normal response of water permeability to ADH in the collecting tubules.19 The ability to concentrate is then highly dependent on the renal medulla.20 Therefore, it is not surprising to observe that if there is a defect in any of the many factors involved in this very complex mechanism, the ability to concentrate urine deteriorates early.
In our recently published we observed that the maximum UOs is altered if there is a loss of renal parenchyma (one or more scars, single kidney, hypodysplasia, chronic kidney disease), in cases where there is an increase in renal parenchymal pressure (vesicoureteral reflux, obstruction of the ureteropelvic junction) and when there is acute inflammation in the renal parenchyma (acute pyelonephritis).21 Urine volume is closely related to glomerulotubular function and the ability to concentrate urine. It has been established that 99% of the fluid content in the glomerular ultrafiltrate is reabsorbed along the renal tubules. If any of the different mechanisms involved in concentrating urine is altered, there is an increase in urinary volume that, may not be clinically detectable in mild cases. It has been known for many years that the urinary volume increases progressively and the concentration capacity progressively decreases as GFR deteriorates.22 Urine volume corrected by 100ml FG is rarely used in daily practice. The results obtained by our group in a previous study showed that urine volume corrected by 100ml FG is a parameter easy to calculate that can be used as marker to detect the loss of renal parenchyma and it is almost as sensitive as maximal urine osmolality.23 In our study, uric acid levels were collected as an indirect expression of contraction and volume expansion.24,25
The alteration of the RWHin adult patients was first described by Gill and Bartter in 1961. These authors verified that this defect could be related to excess calcium and that it was not dependent on a serious sodium transport failure.7 Gill and Bartter observed an alteration of the maximum reabsorption of free water (Tc H2O) during osmotic diuresis in 3 individuals with hypercalciuria. The maximum negative water clearance was normal in these same patients when hypercalciuria was corrected.
In 1976, Backman studied 41 adult patients with idiopathic recurrent kidney stones and investigated electrolyte excretion during water deprivation and aqueous diuresis. All patients had a normal concentration capacity according to the determination of Tc H2O.8 In a subsequent work in adult patients, Yumiya et al. confirmed that high urinary calcium does not interfere with urinary concentration.9 On the contrary, Suki et al. observed a reduction of the maximum UOs in adults with IH (701±138mOsm/kg) in relation to the controls (1108±72mOsm/kg). These authors postulated that this concentration defect is not a consequence of hypercalciuria since the reduction of calcium excretion by oral phosphate administration failed to correct defect.10
In pediatric patients with IH studied by Stapleton and Miller, urine osmolality after 18h fluid deprivation ranged from 700 to 1018mOsm/kg with an average value of 864±34mOsm/kg was significantly reduced as compared with sm controls (1059±31.2mOsm/kg). Three of the 10 children studied (30%) showed values below 800mOsm/kg.13 In another pediatric study, 10.2% (5/49) of patients with IH had a reduced maximum UOs.14
The existence of a possible defect in the ability to concentrate in patients with IH has been related to the same hypercalciuria through its possible stimulating effect on the calcium-sensitive receptor (CaSR)26–28 or deterioration in the concentration mechanism due ad deposits of calcium salts in the renal parenchyma.11,12
In this sense, it has been described that the activation of the CaSR of the cell surface in the apical membranes of the main cells of the inner medullary connecting duct (IMCD)26,27 reduces the aquaporin na-2 and therefore, the rate of water resorption.28 It has even been postulated that modulation of urinary volume through activation of IMCD CaSR could be an important mechanism of protection against stone formation.29
Although the mechanisms that relate the concentration of calcium ions with the abundance of aquaporins in the membrane are well supported by experiments in cells and animals,28,30 however, the relevance of this mechanism in humans with calcium kidney stones does not seem be significant.31.32 This effect would be much more noticeable in cases of hypercalcemia.33
In addition, in the thick ascending limb of the loop of Henle, CaSR reduces the active reabsorption of Na+ and Cl− by inhibition of the ROMK potassium channel (and indirectly of the NKCC2 cotransporter), which induces a decrease in the luminal load positive required for passive reabsorption of Ca2+, Mg2+ and Na+.34 This defect of reabsorption of Na+ would be accompanied by an obligatory loss of water.35
In our study, renal water management was altered in 31% of children and 24.1% of adults, so activation of cell surface CaSR should not be ruled out, at least in pediatric patients with IH. In addition, in this study, no differences were observed in the values of the maximum UOs in childhood as compared with adults. Even the V/FGR was lower in adulthood (Table 2).
Our group, in 2 previous cross-sectional studies in IH patients, found a reduction of the maximum UOs in 14% of the children (n=42; 8.3±3.1 years) and in 48% of the adults (n=52; 39.7±11.8 years).11.12 Although the results are not exactly comparable because in the present study we have also included the value of V/TFG; the frequency of alteration in the RWH in adults included in the first work was much higher than in the current one.
In that previous study, it was observed that in adults with IH with impaired ability to concentrate had a higher urinary clearance of prostaglandin E2 and a higher frequency of ultrasound stones in the renal parenchyma than IH adults with normal RWH.11,12 In the current study, the maximum UOs in adulthood was significantly reduced in those who had renal colic as compared to those who did not (p<0.04).
In the current study we observed a less frequency of defects in the ability to concentrate in adulthood compared to the previous cross-sectional study. This finding can be explained by 3 reasons:
- (a)
A younger age in (mean: 28.2 years) than in the previous study (mean: 39.7 years), which, at least in theory, reduces the possibility of calcium deposits formation in the renal parenchyma which may alter the mechanism to concentrate urine. In fact, in the current study a significant negative correlation was observed between age and maximum urinary osmolality in adulthood.
- (b)
Lower calciuria in this study (19 patients had normalized the calcium/creatinine ratio) than the observed in patients in the initial cross-sectional study in which all were hypercalciuric.
- (c)
The possible preventive effect of the recommended dietary and pharmacological treatment for our pediatric patients could have reduced crystalline deposits in the renal parenchyma.
Finally, although it was not the purpose of the study, the results show a point that had previously observed in our clinical experience regarding urinary excretion of calcium and citrate.36 We refer to the fact that, for unknown reasons, some patients with IH diagnosed during childhood can normalize the urinary elimination of calcium in adolescence and adulthood without receiving pharmacological treatment at that time. At the same time, the urinary elimination of citrate decreases and even increases the lithogenic risk (higher calcium/citrate ratio) (Table 1). The observation of hypocitraturia in the absence of distal renal tubular acidosis in IH patients, has been previously described in the literature.37 In our study, an alteration of the RWH was observed in approximately one third of the patients with IH, and this did not changed 20 years after the diagnosis. In adults, it seems to be related to urolithic disease. It is difficult to know if the favorable results respond to a certain compliance with the recommended protective diet and the pharmacological treatment administered at the diagnosis of IH in the pediatric age.
Conflict of interestNot declared.
Please cite this article as: Pérez Suárez G, Serrano A, Magallanes MV, Arango Sancho P, Luis Yanes MI, García Nieto VM. Estudio longitudinal del manejo renal del agua en pacientes diagnosticados de hipercalciuria idiopática en la infancia. Nefrologia. 2020;40:190–196.