Bisphosphonates are synthetic compounds similar to organic pyrophosphates. The bioavailability of intravenous preparations is 100%, whereas the availability of oral therapy ranges from 1 to 5%. About 50% to 80% of free bisphosphonates are incorporated into the bone. Because of their urinary elimination, bisphosphonates must be carefully administered in chronic kidney disease (CKD) patients. In spite of this, bisphosphonates can safely be used at all CKD stages, including dialysis and kidney transplant. Renal toxicity seems different among these compounds, and it is basically due to their protein binding and the average lifespan of renal tissues. In practice, renal toxicity has been associated with infusion speed and excessive dosage. In patients with CKD, it is very relevant to maintain infusion time and in haemodialysis patients we recommend administration during the haemodialysis session. When bisphosphonates are given to 4-5 CKD patients it seems reasonable to reduce the dose to 50%. No renal pathology has been associated to oral administration. The indications of bisphosphonates in CKD include: hypercalcaemia episodes, preventing bone loss after renal transplantation, treating low bone mineral density in all CKD stages including transplantation. They are also a promising therapy for calciphylaxis and to prevent vascular calcifications. When suppressed bone turnover is suspected, bone biopsy is mandatory before bisphosphonates therapy.
Los bifosfonatos son compuestos sintéticos análogos de los pirofosfatos. Mientras que la biodisponibilidad de una dosis endovenosa es del 100%, la biodisponibilidad oral es del 1 al 5%. Aproximadamente el 50-80% del bifosfonato disponible es captado por el hueso.
En pacientes con deterioro de función renal debemos ser cautelosos fundamentalmente porque se eliminan a nivel renal (se filtran por el glomérulo y secretan en el túbulo). Su diferente toxicidad renal puede deberse a factores como: diferente capacidad de unión a proteínas, distinta vida media en tejido renal, y diferente toxicidad renal acumulada. No obstante, la toxicidad se debe a la administración rápida y a dosis excesivas. En pacientes con filtrado glomerular inferior a 30 ml/min es aconsejable reducir la dosis a la mitad. Con la administración endovenosa es importante mantener el tiempo de infusión y en hemodiálisis administrar durante la sesion. Con el ibandronato, hasta el momento actual, no se ha descrito patología renal y con las formas orales de cualquiera de ellos tampoco.
Los bifosfonatos se han demostrado eficaces en la prevención de la pérdida ósea postrasplante, en tratamiento de calcifilaxis y prevención de las calcificaciones vasculares. En los pacientes con ERC avanzada ó en diálisis, los bifosfonatos estarían indicados, sobre todo, ante la presencia de franca disminución de la masa ósea y la existencia de factores de riesgo de osteoporosis junto a alto remodelado óseo. Se debe sopesar con cuidado su indicación en pacientes con sospecha de enfermedad ósea adinámica, siendo en este caso mandatoria la biopsia ósea.
INTRODUCTION
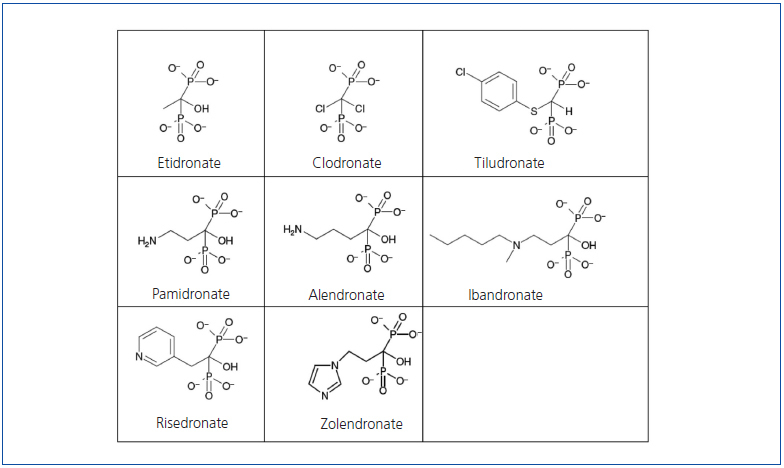
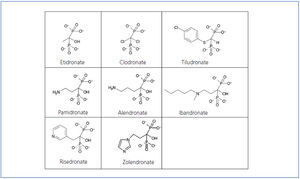
Bisphosphonates are synthetic pyrophosphate analogues with similar physicochemical effects. They were developed in the middle of this last century as growth inhibitors and subsequently they were found to decrease bone resorption too. Pyrophosphates, in turn, are organic compounds composed of two phosphoric acids linked by esterification to a molecule of oxygen (P-O-P structure) (Figure 1). They are detected in the blood and urine because they are byproducts of various physiological reactions.
Bisphosphonates differ from the pyrophosphates in that the oxygen molecule has been replaced by a carbon (P-C-P structure) (Figure 1), making them difficult to break down and, moreover, giving them a high affinity for hydroxyapatite crystals. The central carbon is also joined by two side chains that vary for each type of bisphosphonate and determine their potency, duration of action, side effects, and other clinical or bone parameters (Figure 1). The most potent bisphosphonates have a hydroxyl group on one of their side chains that increase their ability to bind to calcium.
DISTRIBUTION
Bisphosphonates, since they are not biodegradable, are absorbed, stored and excreted by the body without being metabolised. While the bioavailability of an intravenous dose is 100%, the bioavailability of an oral dose is only 1 to 5%. Absorption is achieved through passive diffusion in the stomach and intestine, and decreases when the drug is administered with meals, especially in the presence of calcium, so it is recommended that its administration occurs at least 30 minutes before breakfast (although it can also be administered 2-3 hours after a meal), and only with water.
Approximately 50-80% of available bisphosphonate is taken up by bone. The remaining 30-50% is excreted in urine without being metabolised. The uptake of bisphosphonate by bone increases with high bone turnover or low renal excretion. The half-life in plasma is approximately 1-2 hours, while bisphosphonate in the bone usually persists for many years.1 In bone, bisphosphonates are bound with high affinity to the hydroxyapatite crystals on bone surfaces, inhibiting its breakdown. From there it is absorbed quickly and directed primarily to areas of active remodelling, acting as potent inhibitors of bone resorption. Bisphosphonates also prevent the formation of calcium phosphate crystals and inhibit apoptosis of osteocytes and osteoblasts.
In experimental models, they have been shown to inhibit soft-tissue calcification, prevent calcification induced by vitamin D in the aorta and the renal arteries, and other forms of ectopic calcification.1,2
MECHANISM OF ACTION
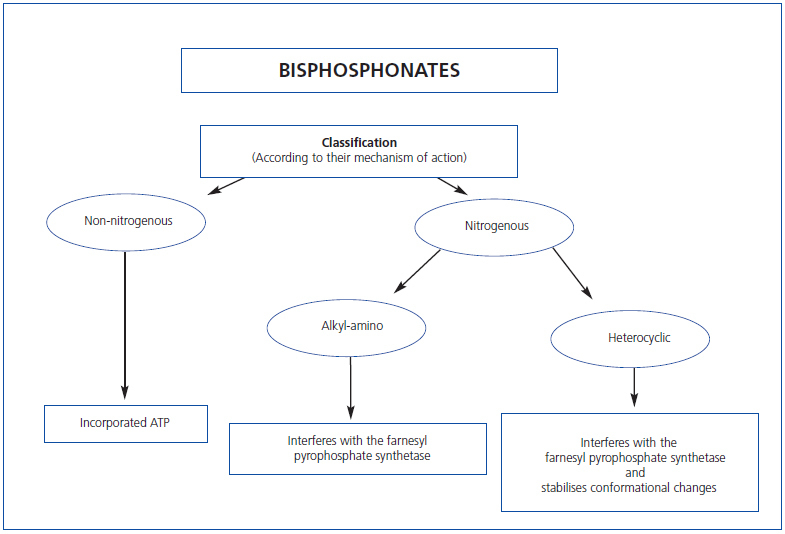
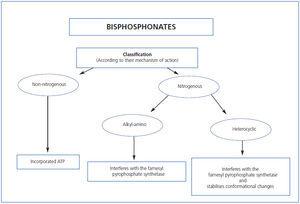
We have proposed two basic molecular mechanisms responsible for the effects of these drugs on osteoclast function (Table 1) that allow their classification: Bisphosphonates that do not contain nitrogen (etidronate, clodronate and tiludronate), considered “first-generation” bind ATP which, when incorporated in osteoclasts, are cytotoxic to these cells, disrupting cell function and causing apoptosis.
Nitrogenous bisphosphonates, called “second and third generation” (pamidronate, alendronate, ibandronate, risedronate and zolendronate) are more potent than their predecessors. They inhibit farnesyl pyrophosphatase synthase and other final steps of the intracellular mevalonate pathway whose end product is cholesterol. Depending on the different side groups, bisphosphonates vary in their affinity for mineral, farnesyl pyrophosphate synthase inhibition, and hydroxyapatite binding capacity, all of which determine its potency and effects. If we regard etidronate as having a potency of 1, pamidronate has a potency of 100; alendronate 1,000; risedronate 5,000; ibandronate 10,000; and zolendronate 20,000.
Theoretically, the greater the drug potency, the lower the dose and frequency of administration should be, although the risk of accumulation and the possible toxicity and side effects are also increased.
BISPHOSPHONATES IN CHRONIC KIDNEY DISEASE
In patients with impaired renal function, caution should be used when administering these drugs, mainly because bisphosphonates, in their renal elimination, are freely filtered by the glomerulus and actively secreted in the tubule. This does not mean they are contraindicated in these patients.3
Experimental studies in animals have shown that high doses of bisphosphonates can cause decreased glomerular filtration rate and abnormalities in renal histology.4
However, not all bisphosphonates behave the same. In experimental animals, administration of high doses of ibandronate (1mg/kg) produced no deterioration of renal function, whereas a slight deterioration was observed with the administration of zolendronic acid (1mg/kg).5 In these same studies, higher and repeated administration of zolendronic acid yielded renal histological abnormalities with the presence of degeneration and tubular atrophy, findings that were evident only with repeated doses of ibandronate.5
Clinical experience
On review of the studies published so far on intravenous bisphosphonate administration, a slight deterioration of renal function is seen in 6-10% of all patients, except in the case of ibandronate in which the percentage is only 2-3%.6-10 Oral forms have not been reported as a cause of impairment of renal function.
In cancer patients, intravenous administration of high doses of bisphosphonates has been associated with some degree of renal toxicity. Pamidronate, when administered at well above recommended doses, has resulted in nephrotic range proteinuria and collapsing glomerulonephritis in some cases.11-14 Cases of acute tubular necrosis with high-dose intravenous zolendronic acid have also been published.15 Side effects with intravenous ibandronate have not been reported.16 In terms of oral bisphosphonate administration, although some cases of renal involvement have been reported, these occurred in patients with previously established nephrotic syndrome and confirmed renal disease and in whom the association between deterioration of renal function and bisphosphonates cannot be wellestablished.17
Pathophysiologically, differences in renal toxicity of these drugs may be due to various factors such as:
1. Their different protein-binding capacity, with ibandronate having the highest percentage (87%).
2. Their distinct half-life in renal tissue, which is low with ibandronate (24 days) when compared with the 150-200 days of zolendronic acid.
3. The difference in cumulative renal toxicity, which is also lower in the case of ibandronate.9 Taking all this into account, and in general, it must be noted that toxicity is usually secondary to excessive doses and rapid administration of the drug.
Dosing
There have been no prospective trials in patients with impaired renal function, although it has been observed that, in general, at recommended doses, these drugs do not cause impairment of renal function. No dose adjustment is necessary in patients with mild or moderate renal failure (Clcr greater than or equal to 30ml/min), although there may be increased risk of renal toxicity in elderly patients or in patients simultaneously receiving other nephrotoxic drugs.18-20
In patients with clearance rates below 30ml/min renal elimination is decreased, the concentration being approximately two times higher than in patients with normal renal function, so it is advisable to reduce the dose by half.15-21
Curiously, in some patients with multiple myeloma, it has been observed that not only is there improvement of serum calcium but also of renal function with the administration of ibandronate.22
Infusion Time
Although a recent study with ibandronate did not show any differences when comparing an infusion period of 60 minutes with a period of 15 minutes,23 in general, the infusion rate may also determine renal toxicity, with less observed toxicity when the infusion is slower.24,25
Elimination in chronic kidney disease
Renal clearance of bisphosphonates has a linear relationship with creatinine clearance.
In patients with normal renal function, bisphosphonates are rapidly eliminated from plasma during the first 2 hours after administration, renal excretion being the main route of elimination and bone being the tissue that retains it. Uptake in the skeleton is 47-82% and depends on bone remodelling. Therefore, more remodelling means increased uptake. In haemodialysis patients the elimination of various types of bisphosphonates is similar. No differences have been found between the clearance of these substances in haemodialysis patients as compared with a population with normal renal function.26-30 If this fact is combined with the fact that the duration of the sessions is equivalent to the elimination period of bisphosphonate in patients with normal renal function, we believe these drugs should be administered in the early hours of dialysis.
In connection with peritoneal dialysis, there is little information, except a study with clodronate, in which no differences were observed in the action of the drug compared to patients on haemodialysis.31
INDICATIONS IN PATIENTS WITH CHRONIC KIDNEY DISEASE
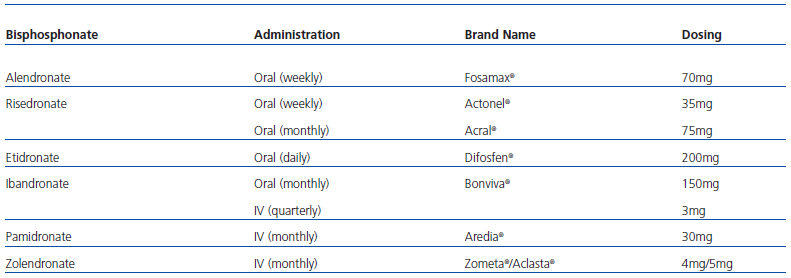
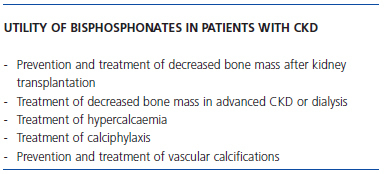
The method of administration and dosing varies between different types of bisphosphonates (Table 2). Possible indications for bisphosphonates, both for oral and intravenous administration, in patients with CKD are as follows (Table 3):
Prevention and treatment of bone loss after renal transplantation
Bisphosphonates have proven effective in preventing bone loss after transplantation. Intravenous pamidronate (60mg on day 0 and 30mg/month from months 1 through 6), IV ibandronate (1mg on day 0 and 2mg on months 3, 6 and 9), or oral risedronate (35mg/week) all prevent loss of bone mineral density (BMD) at different skeletal sites in the first 2 years after transplantation.32,33
However, this does not justify the indiscriminate use of bisphosphonates after transplantation. It seems that its use is recommended in patients with osteoporosis and/or other risk factors, such as: patients with type 1 diabetes, men over age 65 and women over age 45, those with preexisting stress fractures, and those with immunosuppression with high-dose steroids.
Treatment of decreased bone mass in chronic kidney disease stages 3-5 and dialysis
There are few data on these patients, which is paradoxical, considering that patients with CKD are at increased risk of fractures relative to the general population. Studies conducted in this population on patients with impaired renal function have demonstrated improvement in BMD and reduced fracture risk, independent of renal function.34 Furthermore, in the few published studies of dialysis patients, an improvement in BMD has also been observed, especially when the patients had high parathyroid hormone (PTH) levels.35-38
It is unknown whether the accumulation in bone increases with deterioration of renal function. It should be considered in these patients whether the accumulation results in microfractures and bone quality deterioration. On the other hand, it is also true that in situations of low remodelling, bisphosphonate accumulation is lower.
In summary, in patients with advanced CKD or on dialysis, bisphosphonates are indicated, especially in the presence of frank decrease in bone mass (z-score < 2.5) and the presence of risk factors for osteoporosis (bone fractures, type 1 diabetes, men over 65, and women over 45 years of age), along with high bone remodelling (PTH > 450pg/ml). In situations with PTH below 100, indicative of probable low remodelling, although to date the use of bisphosphonates has been highly discouraged, it is appropriate to treat on a case-by-case basis. One should consider and, in this case, assess the possibility of bone biopsy and treatment with parathyroid hormone. In situations with PTH between 100 and 450, one should also treat on a case-bycase basis, but one may be more lax.
TREATMENT OF CALCIPHYLAXIS
Some studies have shown that bisphosphonates may have a beneficial effect in the treatment of calciphylaxis. Inhibition of bone resorption caused by bisphosphonates could reduce the concentration of calcium in blood, and thereby reduce the tendency of mineral nuclei to form and grow on arterial walls. On the other hand, bisphosphonates could inhibit the secretion of proinflammatory cytokines in the vascular wall and thus improve the overall situation.39 Both intravenous (pamidronate) and oral (alendronate or risedronate) bisphosphonates have been used with similar results.40-42
In our experience, seven cases of calciphylaxis have been resolved, five of them in patients on dialysis and two with functioning kidney transplantations, with the administration of alendronate over 6 months in one case, risedronate in three others, and ibandronate in the last two cases.
Treatment of hypercalcaemia
In CKD patients, one may encounter hypercalcaemia when using high doses of calcium salts as phosphorus binders, with high doses of vitamin D, hypercalcaemia of malignancy, multiple myeloma, and some cases of primary hyperparathyroidism. The administration of intravenous bisphosphonates may be useful as a complement to other strategies.
Pamidronate is a bisphosphonate with which there is more experience in the treatment of hypercalcaemia, although zoledronate and ibandronate have also been used.43 It should be administered for very short periods to prevent hypocalcaemia in the medium term. In our experience, and due to its margin of safety, we recommend IV ibandronate 6mg as a first option.
Prevention and treatment of vascular calcification
The mechanism by which bisphosphonates inhibit vascular calcification has not been elucidated. The various mechanisms proposed are: a) inhibition of bone resorption leading to a decrease in the calcium and phosphorus leaving bone which limits their availability and deposition in the vascular tree, b) modulation of the activity of the NaP cotransporter of smooth muscle cells, c) direct effect on the vascular wall preventing the formation of hydroxyapatite crystals, and d) positive effect on osteoprotegerin/RANK-L.44
Dialysis patients have lower serum pyrophosphate levels, which is one of the possible mechanisms contributing to higher rates of vascular calcification. The administration of bisphosphonates could reset the pyrophosphate levels.45 There are very few studies in dialysis patients, each of which has a small number of patients. In most, low-dose bisphosphonates appear to have beneficial effects on vascular calcification, and improvement in coronary and aortic calcifications has been demonstrated.46-50 It remains a promising alternative that requires larger prospective studies for validation.
OSTEONECROSIS OF THE MANDIBLE
Mandibular osteonecrosis deserves special mention, although its incidence is very low. It is unusual for it to occur with oral bisphosphonates. The predisposing risk factors include the administration of intravenous bisphosphonates over long periods of time, high doses of steroids, alcohol and/or tobacco (snuff) abuse and especially local factors such as periodontal disease, tooth extraction and maxillofacial surgery.51-53
Prevention is important in patients with CKD, although no case has been reported to date. Basic guidelines have been developed for the treatment of this complication in patients treated with bisphosphonates54:
Before initiating treatment with bisphosphonates, teeth with poor prognosis should be extracted and surgical dental procedures performed.
As a preventive measure, during treatment with bisphosphonates and in the case of dental surgery, administer antibiotics before and after the procedure for 10 days. It is essential in all cases to maintain good oral hygiene.
KEY CONCEPTS
1. Bisphosphonates can be used at different stages of chronic kidney disease, including dialysis and renal transplantation.
2. In patients with glomerular filtration rates below 30ml/min, it is advisable to reduce the dose to half of that recommended for patients with normal renal function.
3. While very few negative effects on the kidney have been reported, it is important to maintain and even extend the infusion time to avoid side effects with intravenous administration. With ibandronate no renal effects have been reported to date.
4. Oral administration does not appear to alter renal function.
5. Patients on haemodialysis should be administered the drug during the session.
6. The indication for bisphosphonates must be weighed carefully in patients with suspected adynamic bone disease, in which case bone biopsy must be performed.
Figure 1. Molecular structure of bisphosphonates.
Table 1. Classification of bisphosphonates by their mechanism of action
Figure 2. Classification of bisphosphonates by their mechanism of action.
Table 2. Bisphosphonates marketed in our country, route of administration, and brand name
Table 3. Possible indications for bisphosphonates in nephrology