Vascular access (VA) is a sine qua non state for patients with chronic kidney disease (CKD) in treatment with haemodialysis (HD) and it is the most important factor that determines the failure or success of chronic HD programmes.1 Of the three types of VA currently used, specifically, arteriovenous fistula (AVF), polytetrafluoroethylene (PTFE) synthetic graft, and central catheter, there is broad consensus that AVF is the VA of choice.2-4 The Guidelines of the Spanish Society of Nephrology (SEN) on VA, currently under review by the VA Working Group, considered the following factors as quality indicators: 80% or greater of patients receiving permanent VA (AVF or graft) or with existing AVF, and 10% or less of patients with a tunnelled catheter (TC).2
The studies presented in this issue of NEFROLOGÍA have been conducted by the Madrid Society of Nephrology (SOMANE) VA Study Group and were supported by the Regional Ministry of Health of the Autonomous Community of Madrid (CAM).5 This is a multicentre retrospective study of 2,332 patients from 35 centres and it was carried out with a survey distributed to HD units of the CAM. Its purpose is to analyse various VA management models for HD in the CAM and their impact on various VA quality indicators.5
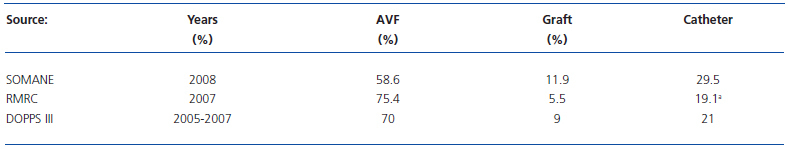
In this study in the CAM, centres were classified into three evaluation levels (good, sufficient, or insufficient) from a scoring obtained from the following three variables5: 1) Nephrologic Organisation: Advanced CKD structured consultation, complete multidisciplinary protocol and database with systemic collection of quality indicators; 2) Level of satisfaction achieved with the associated surgical service; 3) Level of satisfaction achieved with the associated radiological service. In relation to sites rated as “insufficient”, centres rated as “good” had lower levels of catheter use, lower thrombosis rates, and higher prevalence of treatment for VA, both electively for dysfunction and as rescue for thrombosis.5 The current status of VA for HD in the CAM that is presented in this issue of NEFROLOGÍA is not optimal.5 In this study, 45% of patients started HD through a catheter in 2008 and, from the previous year (31 December 2007 to 31 December 2008), an increased percentage of catheters was found from 24.7% to 29.5%, as well as a reduction in the percentage of AVF from 62.3% to 58.6%.5 Unfortunately, similar results have been shown in other Autonomous Communities in Spain. According to data from the Kidney Disease Registry of Catalonia (RMRC),6 which is one of the most prestigious kidney disease registries in Europe, between 2002 and 2005, the percentage of patients who started HD in Catalonia with AVF was always less than 50% (varying between 44% and 48%) while an increase in TC was seen at the expense of non-tunnelled catheters.6The percentage of AVF in patients in Catalonia has been progressively decreasing over the years from 86% (31 December 1997) to 75.4% (31 December 2007), in a manner that is inversely proportional to the gradual increase in TC.6 The results of the multicentre study prompted by the SEN Working Group on Quality in Nephrology that looked at VA for 2,516 patients in 28 HD units in Spain for the year 2007, showed that no centre met the goal of having more than 80% of patients with mature VA at the start of HD.7 Table 1 shows the distribution of VA in the CAM, Catalonia and in Spain as a whole according to DOPPS III (2005-2007).4-6
Regarding AVF, the presence of a venous catheter for HD has been associated with numerous complications that translate into high morbidity and mortality.4,6,8-10 From the data obtained by the RMRC and through the CHOICE study (Choices for Healthy Outcomes In Caring for ESRD), it has been shown that starting HD programmes through a central catheter means, compared with starting HD through an AVF, increased mortality risk of 30% and 50%, respectively, after properly adjusting for various variables.6,11 One could argue that this increased mortality risk associated with catheters does not depend solely on the catheter itself but rather on the fact that patients who have a catheter also have a precarious cardiovascular status, related to older age and greater comorbidity that first prevents the construction of the AVF and, secondly, confers increased mortality. However, Allon et al. have shown that the increased mortality risk in these patients, adjusted for the considered variables, decreased or increased by changing the catheter to permanent VA and vice versa, respectively.12
Therefore, given its potential complications, it is a moral obligation to maximally restrict the rate of existing catheters.8 In line with other authors, we believe that the cause of catheter excess is multifactorial, specifically, that there are several contributing factors that are responsible for this situation and, as a result, we must simultaneously act on several fronts to try to improve the situation.13,14 Below, we examine several factors of greater or lesser specific weight that are involved in the increase in catheters, both in incident and prevalent patients in HD (Table 2).
Change in the profiles of patients with chronic kidney disease
Some authors have justified the excess of catheters by stating that the current CKD patient is “clinically different” from that of 10-20 years ago due to older age and higher prevalence of diabetes mellitus and cardiovascular comorbidities.4,11,14,15 It has been argued that the poorer clinical situation of the current CKD patient could result in an unfavourable vascular tree that hinders construction and/or maturation of a normally functioning permanent VA.14,15 In this respect, of 616 patients included in the CHOICE study (66.6% with catheter and only 13.8% with AVF), those patients who started HD with a catheter had a worse comorbidity score compared with those who started HD with an AVF.11 According to data obtained in the three phases of the DOPPS study, the probability of a patient being dialysed with an AVF is less if the patient is female, older, and has obesity, diabetes mellitus, peripheral artery disease, and recurrent cellulitis.4
If higher prevalence of diabetes mellitus and cardiovascular comorbidities had a decisive impact on the excessive catheter use, it would be logical to assume that non-diabetic patients without cardiovascular comorbidities would have very low rates of catheter insertion. However, data from Catalonia from the period of 2000-2007 have shown that, in the best possible scenario, specifically, in the normal course of renal disease, with nephrologic follow-up of greater than 2 years and the absence of diabetes mellitus and cardiovascular comorbidities, the percentage of AVF and central catheters in patients in Catalonia was 66.9% and 31.4%, respectively.6 In this regard, an AVF construction programme carried out in 121 patients already being dialysed with a TC, Asif et al. showed that 95% of patients with a TC who were evaluated with vascular mapping (physical examination and venography) had suitable veins for the construction of an AVF.16 Therefore, there appear to be other causes besides the “patient factor” that are responsible for the current rate of catheter use.
The centre factor
As in the CAM5, there are also notable differences in the rest of Spain when comparing different HD units in terms of the distribution of VA type in the new patient as well as in the existing patient on HD.6,7 For example, according to data presented in the Fifth Congress of the Vascular Access Society in 2007, 94% of existing patients in an HD unit in Murcia were dialysed with an AVF.17 In 2007, HD via catheter was started in Catalonia in between 20% and 100% of patients, depending on the HD unit being studied.6
As suggested from the CAM,5 there may be several causes acting simultaneously at the same centre that result in excessive catheter use and, at the same time, these causes may be different at the various HD units being studied. Although some studies highlight VA surgery to explain the inequalities between HD units,18,19 there are other relevant elements associated with the “centre factor” such as, for example, the lack of an advanced CKD consultation service or the lack of a VA monitoring programme. In any case, it is not acceptable from any point of view that patients of the same age and comorbidity have differing mortality risks based on the “centre factor” according to whether HD is initiated through an AVF or a central catheter.8
Vascular access surgery
In the study from the CAM,5 the nephrologic organisation was considered to be good or sufficient in most centres (80%, 28 of 35 centres). Satisfaction of nephrologists with the support from vascular radiology was good or sufficient in 74% of centres (26 of 35 centres). In contrast, more than half the centres in the CAM (57%, 20 of 35) considered the support from the surgical services to be insufficient.
The role of the surgeon, typically the vascular surgeon, is key to changing the excessive catheter dynamic.13,19 It is very important that the vascular surgeon be part of the multidisciplinary team. His or her activity is crucial for obtaining functioning permanent VA and must be involved, along with the vascular radiologist, both in elective surgery on significant VA stenosis as well as in the urgent VA rescue after thrombosis.
In multivariate regression analysis performed in the study by Prischi et al., conducted with 108 patients dialysed with radiocephalic AVF, the only relevant prognostic parameter for AVF permeability was the surgeon.20 According to Basile and Lomonte, the surgeon is the main determining factor in AVF maturation.21 In the study by Feldman et al, by optimising surgical technique, it is possible to increase the likelihood of successful AVF maturation from 55.5% up to 84%.15 According to Allon and Robbin, one of the factors necessary for obtaining a mature AVF and prolonged survival is to restrict VA surgical procedures to surgeons with demonstrable interest and experience.22 In Linda Francisco’s opinion, the vascular surgeon should meet the following three conditions23:
1. Commitment to VA for haemodialysis.
2. Familiar with the basic principles of HD and the problems of patients on HD. 3. Expert in performing all of the required surgical procedures.
Consultation for advanced chronic kidney disease
In the CAM,5 a significant percentage of centres (45.7%, 16 of 35) did not have a structured consultation process for advanced chronic kidney disease (advanced CKD). This consultation is very important, both for early indication for AVF construction as well as for periodic monitoring during the maturation stage.10,24 In a nationwide series, 73% of patients who had been previously evaluated with an advanced CKD consultation were started on HD through an AVF.9
The fewer nephrologic visits to an advanced CKD consultation, the less likely it is to start HD with permanent VA. In the study by Stehman-Breen et al., patients with only one visit to the nephrologist were 79% less likely to start HD with permanent VA than those patients with more than five visits.24
Vascular mapping
To reduce the catheter rate, it is essential to have both an arterial and venous vascular map for all patients with CKD who are consulting for advanced CKD.25,26 In addition to the physical examination, vascular evaluation with Doppler ultrasound should be performed in most patients.27
The vascular map is essential for the conversion from TC to AVF in the existing renal patient.16,28 In the above mentioned series by Asif et al., of the 86 patients dialysed with TC and subjected to vascular evaluation through a physical examination and venography, a normal functioning AVF was obtained in 77% of cases.16
Late referral of patients for consultation for advanced chronic kidney disease
The dedication of the nephrologist to the advanced CKD patient depends on early or late referral for consultation. There is an inverse relationship between follow-up time of the patient with CKD by the nephrologist and the probability and starting HD through a catheter.4,6,11 According to data from DOPPS, the percentage of patient who start HD through a central catheter in Spain is very different based on the whether the first visit to the nephrologist occurred 4 or more months beforehand (25.6%) or less than one month before HD initiation (81%).4 According to data from the RMRC, the percentage of patients who started HD through a catheter from 1997-2007 in Catalonia was progressively higher based on whether the nephrologic follow-up time was greater than two years, between one and two years, and less than one year.6
The multidisciplinary team
One of the main factors for reducing catheter rates and increasing AVF rates both in the new and prevalent HD patient is the creation of multidisciplinary teams for VA management.10,17,22,27 All the professionals responsible for the patient’s VA must be represented on this team, specifically, nephrologists, vascular surgeons, vascular radiologists, and HD nursing staff.17,22 Usually, this team is coordinated by a nephrologist or by an HD nursing professional.27 The most important roles of the interdisciplinary team are the following:
1. To establish consensus protocols for action.
2. To manage the waiting list for VA intervention.
3. To decide on the type, location, and timing of permanent VA construction based on the result of vascular mapping.
4. Permanent VA follow-up in the advanced CKD consultation from construction up to the start of puncture (before starting the HD programme) and in the HD room (for existing patients).
5. To ensure early diagnosis of cases of significant permanent VA stenosis through evaluation of the screening methods used in the advanced CKD consultation (before starting the HD programme) and in the HD room (for existing patients).
6. To guarantee elective treatment of significant permanent VA stenosis with radiology and/or vascular surgery before its thrombosis.
7. To ensure urgent rescue treatment with radiology and/or vascular surgery for cases of VA thrombosis without needing to place a central catheter.
8. To maintain an up-to-date database of VA for every patient.
9. To periodically evaluate the proposed goals.
In the CAM, less than half of the centres (48.6%, 17 of 35) had consensus protocols for action between the services of nephrology, vascular surgery, and vascular radiology.5 In Spain, some structured multidisciplinary teams have already been in operation for 10 years, such as in the Alcorcón Foundation Hospital (Madrid), the Reina Sofía Hospital (Murcia), or the Terrassa Hospital (Barcelona). In the Barcelona Clinical Hospital, a multidisciplinary Vascular Access Functional Unit has been created, with the goal of improving the VA situation both within the hospital and in other centres in Catalonia.
Positive results have been shown for implementing prioritisation strategies for waiting list management for VA intervention.27,29 According to data from DOPPS, there is an inversely proportional relationship between the probability of starting HD with permanent VA and elapsed time between patient referral and surgeon evaluation and also between surgical evaluation and VA construction.3 As a result of VA management through five prioritisation criteria by the multidisciplinary team at the Hospital Parc Taulí de Sabadell, 80% of patients started HD with an AVF.29
Prevention of non-anatomical causes of thrombosis
In about 15-20% of cases, thrombosis of the permanent VA in patients already on HD is due to non-anatomical causes, specifically, not provoked by progression of a significant VA stenosis. The most commonly involved non-anatomical causes are hypotension, extracellular dehydration, heart failure, extrinsic compression of the VA, local infection, blood coagulation abnormalities, and polycythaemia in some patients dialysed with synthetic PTFE grafts and being treated with erythropoiesis stimulating agents.2,3
The nephrologist must address these causes in order to avoid VA thrombosis and eventual implantation of a central catheter.
VA monitoring programmes
All the nephrology services in Spain should develop permanent VA follow-up programmes both during the maturation stage in the advanced CKD consultation and during the chronic HD programme.2,3 The goal of these programmes is the early diagnosis of significant VA stenosis and its elective repair prior to thrombosis. These programmes are based on the application of various screening methods for detected VA stenosis and on preventive intervention through techniques of radiology and/or vascular surgery.2,3
VA follow-up should be performed in the advanced CKD consultation for early diagnosis of permanent VA maturation failure related to the presence of stenosis. Usually, the monitoring methods of choice are physical examination and Doppler ultrasound.26,30 Elective intervention on these cases of maturation failure could avoid the need to initiate HD through a central catheter.31
The most common cause of VA thrombosis (80-85% of cases) in the prevalent HD patient is significant VA stenosis, specifically, reduction of 50% or more of the vascular calibre. VA monitoring programmes should allow for diagnosis of subclinical stenosis through the use of various screening methods and its elective repair via techniques of vascular radiology and/or vascular surgery.32 Without the establishment of a structured VA follow-up programme in HD units, it is not possible to reduce either the thrombosis rate or the percentage of catheters in HD patients.
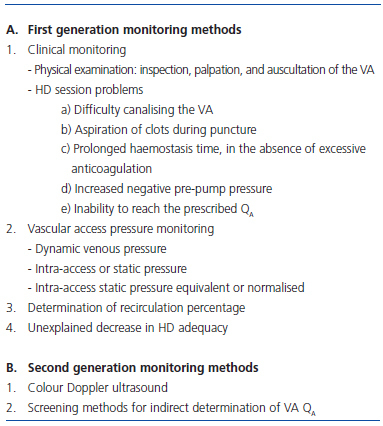
VA monitoring methods can be classified as first and second generation (Table 3).33 In the CAM, first generation monitoring methods were used preferentially, such as screening methods to diagnose VA dysfunction.5 The most widely used follow-up method was decreasing pump blood flow (QB) of the HD monitor (95.7%, 30 of 35). Determination of VA blood flow was only used (QA) as a second-generation method in a third of centres (11 of 35), despite it being the method of choice for permanent VA follow-up.2,3 No centre used Doppler ultrasound for VA monitoring. Preventive or elective treatment of VA dysfunction was not considered at several centres in the CAM.5
To avoid VA thrombosis and therefore reduce the catheter rate, it is necessary to introduce secondgeneration methods into HD units. Screening methods for indirect determination of VA QA are dilutional techniques that have become the techniques of choice for monitoring VA.2,3,34,35 In the presence of a significant stenosis, and in contrast to first generation methods, the QA always decreases independently of the type of VA (AVF or PTFE graft), location, and topography of the stenosis (feeding artery, anastomosis, arterialised vein, or central vein).3,33,36,37 Meanwhile, we must maximise the performance of Doppler ultrasound and the portable ultrasound must enter the HD room, once and for all.38
According to data from the CAM,5 a negative correlation was found between the rate of preventive treatment for dysfunction and the rate of VA thrombosis. The use of VA follow-up programmes has resulted in, at minimum, a 40% reduction in VA thrombosis rates.1 In a prospective case control study carried out in Mollet del Vallès (Barcelona), lower thrombosis rates were shown in VA that was monitored through QA determinations using the Delta-H method.34
In the CAM, the most common cause of performing HD through a central catheter at the time of study was the exhaustion of vascular capital without the possibility of surgery to create an AVF or PTFE graft (in 44% of patients).5 According to data from DOPPS, the probability that an HD patient will undergo dialysis through a catheter is directly proportional to the number of prior permanent VA.4 It is possible that if a strict VA follow-up programme had been developed, most cases of thrombosis could have been avoided and these patients currently would be receiving dialysis through an AVF or PTFE graft. Rescue of thrombosed VA
It is very important for thrombosed VA that are diagnosed in the advanced CKD consultation to be rescued with radiological and/or surgical techniques in order to avoid the patient starting HD with a central catheter.31
Thrombosis of a permanent VA in an existing patient should not be synonymous with central catheter placement. In these cases, rescue of the thrombosed VA should be attempted urgently in order to avoid implantation of a central catheter and to get the patient to the next HD session with the recanalised VA.39
Renal patient education
As in other studies,16,28 the refusal of the patient to change VA was one of the causes (4%) of the persistence of the central catheter in the prevalent HD patient in the CAM.5 The nephrologist should identify why the patient prefers the central catheter and, using various strategies, has a moral obligation to try to persuade the patient to change it to a permanent VA.8 Renal patient education was one of the cornerstones in the programme implemented by Asif et al., to convert TC to permanent VA in the prevalent HD patient.16
Continuing medical education of professionals who care for renal patients Some VA improvement programmes to change the distribution of AVF and TC are based on promoting continuing medical education of professionals who care for renal patients.40 In this respect, one of the goals of the current VAWorking Group of the SEN, which has been reorganised in a multidisciplinary format, is to promote courses and conferences on VA throughout Spain, as well as symposia on VA at each SEN National Congress.
The study performed by the nephrologists in Madrid is the first of its kind in Spain, since it provides us with a precise picture of the current VA situation in an Autonomous Community.5 Now the main deficiencies of the CAM are known and three key points have been identified to improve VA. Based on the results obtained in this study, an improvement plan has been put into place supervised by the Regional Ministry of Health of Madrid and sponsored by SOMANE, in collaboration with ALCER-Madrid. If the administration and the specialists involved with VA management give it their all, the current situation can be reversed and the current results improved.
Key concepts for improving the current VA situation in Spain
1. Reduce the percentage of catheters both in new and prevalent HD patients, since their use increases morbidity and mortality.
2. Create an advanced CKD consultation service and a multidisciplinary team in charge of VA in all nephrology services.
3. Obtain integral involvement of the vascular surgeon in VA management.
4. Introduce vascular mapping of patients with Doppler ultrasound in the advanced CKD consultation.
5. Early construction of permanent VA 4-6 months before first HD.
6. VA maturation follow-up from construction up to first puncture.
7. Elective or rescue treatment with radiology and/or vascular surgery of a non-developed or non-functioning VA, respectively, before the start of chronic HD.
8. Maximally reduce cases of non-anatomical VA thrombosis.
9. Put VA monitoring programmes into place for prevalent HD patients.
10. Modernise HD units through the introduction of secondgeneration monitoring methods.
11. Increase the diffusion of screening techniques based on indirect determination of VA blood flow (QA).
12. Introduce portable ultrasound in HD rooms.
13. Guarantee elective or rescue treatment with radiology and/or vascular surgery of a significantly stenosed or thrombosed VA, respectively, in the prevalent HD patient.
14. Specific renal patient education related to their VA.
Table 1. Distribution of current VA in the CAM according to SOMANE, in Catalonia according to the RMRC, and throughout Spain according to DOPPS III. Data obtained from references 4, 5, and 6.
Table 2. Factors associated with the current rate of AVF and catheters both in the new and prevalent HD patient
Table 3. Classification of VA monitoring methods. Adapted from reference 33