The epidemiological picture of chronic kidney disease (CKD) has seen a dramatic change in the past two decades. Initially restricted to low-incidence diseases such as classical nephropathies (glomerulopathies, cystic diseases, interstitial nephropathies) and to a specialised field of medical care (nephrology), the currently predominant CKD affects a significant percentage of the population due to aging and three highly prevalent disorders including essential hypertension (HT), diabetes, and vascular disease. Many patients who are seen by multiple specialties, particulary Primary Care, have CKD. Patients with end-stage renal disease (ESRD) who are on renal-replacement therapies by means of dialysis and transplantation are considered the tip of the iceberg of the public-health problem, which is CKD in the population.
The terms nephrosclerosis or hypertensive nephropathy are usually applied to CKD associated to HT. In practice, nephrosclerosis is an entity with a non-specific clinical picture, which groups together hypertensive patients with CKD with those in whom no other recognisable causes of the pathology can be appreciated.1-3
In nephrosclerosis, the most characteristic microscopic lesion is hyalinosis of afferent arterioles. The vascular changes produce vasoconstriction, glomerular ischaemia (retraction of the glomerular tuft with focal or global sclerosis), and in some areas, interstitial fibrosis and tubular atrophy. Other authors point out that the hyalinisation of afferent arterioles initially causes vasodilatation, glomerular hypertrophy and, in the long term, glomerulosclerosis lesions that would favour the development of proteinuria and disease progression. These abnormalities are more frequent in black patients.4-7
Its causal relationship to HT is still under debate. It is not at all clear that treated HT can lead to ESRD.8-10 Therefore, some authors have postulated that renal structural abnormalities may precede hypertension and that nephrosclerosis is an intrinsic process of the preglomerular renal microvasculature with loss of self-regulatory capacity. This anomaly would result in excessive preglomerular vasoconstriction3,7 or persistent vasodilation of the afferent arteriole.5,6 Chronically impaired renal plasma flow in the long run would lead to hypertension and renal failure.
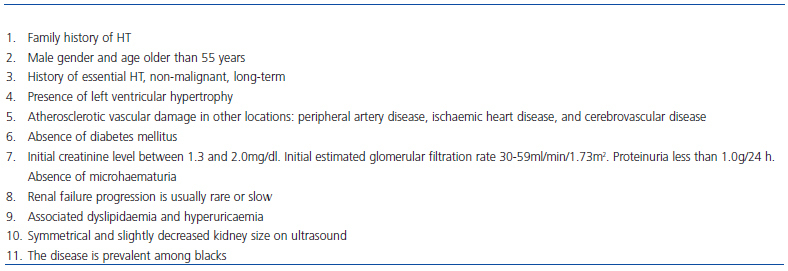
Vascular nephropathy in the United States, as well as in Europe and Spain, is the second most common cause of ESRD. However, this observation has been histologically confirmed in very few cases. The diagnosis of nephrosclerosis is usually made by exclusion in the absence of signs suggesting another type of nephropathy or another possible clinical situation (advanced age, long-standing hypertension, left ventricular hypertrophy, originally-mild renal insufficiency and proteinuria less than 0.5-1g/day). As with diabetic nephropathy, one almost never resorts to renal biopsy to confirm the diagnosis. This attitude may be reasonable in many cases but is undoubtedly a source of misdiagnosis.11,12 Compared with primary glomerular nephropathies or diabetic nephropathy, progression of renal failure is slow in most cases, especially in Caucasians. Kidney function can remain stable over long periods of time if HT is adequately controlled. However, in a poorly determined, but probably small proportion of cases, the disease progresses until it reaches ESRD.12,13 In patients with renal failure, vascular nephropathy is the most common indication for hospital consultations to nephrology services in our country. Up to 39% of cases of CKD have this aetiology, surpassing diabetic nephropathy (20%) and glomerular nephropathy (14%).14 Despite the small percentage of patients with disease progression, its high prevalence justifies its place as the second leading cause of ESRD.
There are no well-recognised factors for progression of the disease, which hampers the implementation of preventive measures. Some commonly cited risk factors are African race, the degree of renal failure at diagnosis, systolic blood pressure (SBP) and the degree of proteinuria.15-17 In the AASK study, patients with proteinuria below 0.3g/day and who had received an angiotensin converting enzyme inhibitor (ACE-I), ramipril, showed slower disease progression. In this study, age over 70 years was a factor that was inversely correlated with progression of renal failure.18,19
Among whites, there are only a few cases, perhaps in those who are genetically predisposed, of patients with an unfavourable clinical course. Disease progression can be aided by the concomitant presence of atherosclerotic lesions in the aorta and main renal arteries and processes such as type-2 diabetes, hyperuricaemia and dyslipidaemia. The age of onset for ESRD is between 45 and 64 years for African Americans, whereas it is over 65 years for Caucasian Americans.2,3
In the last decade, the disease is being diagnosed in patients older than 65-70 years of age with vascular disease in other locations. In these cases, nephrosclerosis could be the manifestation of diffuse atherosclerosis in renal arterioles.20 It has also been noted that the presence of concomitant cardiovascular disease is a risk factor for progression of renal failure. Elsayed et al., in a study of 13,826 subjects included in the Atherosclerosis Risk in Communities Study and the Cardiovascular Health Study, found that cardiovascular disease at baseline predicted the development of CKD (HR = 1.75, p < 0.001).21
The publication of an original study concerning nephrosclerosis in this issue of NEFROLOGIA should be welcomed, given the small number of publications on the subject—which is the Cinderella of renal diseases despite its high prevalence.22 Robles et al.23 conducted a retrospective analysis of 479 hypertensive patients with renal disease treated in an outpatient nephrology clinic for 17 consecutive years (1991-2007). This clinic cared for an area with approximately 650,000 inhabitants and, in that period, 5,071 patients were treated for unspecified conditions, so it is not possible to determine the overall prevalence of the disease. The diagnosis was made based on clinical criteria, except in 60 patients (12.5%) who, having proteinuria exceeding 1g/day, were subjected to biopsy. The average age of patients was 66 years, and 57% of them were men. According to the article, 34% had diabetes mellitus. The basic aim of the study was to examine the evolving implications of the disease over three consecutive 5- year periods. The authors verify that the average incidence of nephrosclerosis was 44 cases per million population (PMP) and that there was a progressive increase of it, from 31.8 PMP in 1991-1995 to 32.1 PMP in 1996-2000 and 54.4 PMP in 2001- 2006. The average age of the patients showed a “J” curve (69, 65, and 67 years, respectively). Atotal of 53 patients (11.1%) started renal replacement therapy. The mortality rate before the arrival of such treatment was 4, 16 and 19%, respectively. The authors concluded that the incidence of the disease has grown in recent years even though the preventive therapeutic measures in the most recent period were theoretically better.
In the last two decades, the continuous increase in life expectancy and the growing permissiveness for the entry of patients with stage 5 CKD into dialysis programs has enabled patients over the age of 65 to become the largest group in these programs. Vascular nephropathy, diabetes and CKD of unknown aetiology, which is predominant in patients over 65 years of age, are the main causes of ESRD.24 It is likely that a significant percentage of cases of unknown cause correspond to hypertensive nephropathy. Therefore, the true prevalence of this process is not known. As with the aforementioned study, the inclusion of patients is usually done exclusively by clinical criteria that, moreover, are not uniform across studies. The clinical-pathologic correlation is less obvious than that described in patients with diabetic nephropathy.
In nephrosclerosis, clinical markers are less consistent than those described in diabetics with established nephropathy (diabetic retinopathy, proteinuria exceeding 1g/day, and renal failure) (Table 1).25 However, it is possible that a large proportion of patients with stages 3-4 CKD and over 70-75 years of age who are treated in outpatient nephrology clinics correspond to cases of nephrosclerosis.14
The percentage of patients who progress to ESRD is also unknown. Since the disease rarely progresses, in many cases those patients who are in better clinical condition are discharged and therefore lost to follow-up. This may explain the difference in progression between the study by Robles et al. (11.6%) and a multicentre prospective study carried out in our country that excluded baseline cases of “historical” nephrosclerosis and included only incident cases (n = 430) over the course of one year. Preliminary results after two years of monitoring show that progression has been observed in only 3.9% of patients, with notable markers of progression being the presence of higher baseline SBP and a higher rate of associated cardiovascular events.26
Two recent studies have provided a new approach to the pathogenesis of the disease, at least in the African-American race. The study by Kao et al., which included 1,372 patients, revealed a close relationship between the presence of ESRD secondary to hypertensive nephrosclerosis in patients without diabetes, as well as some polymorphisms of the MYH9 gene, located on chromosome 22, that encodes the heavy chain of the non-muscle protein myosin IIA.27 The study by Kopp et al. reported the exact same association between these polymorphisms of this gene and the presence of idiopathic focal segmental glomerulosclerosis (FSGS) or secondary to HIV infection.28 In another study, Freedman et al. confirmed the presence of MYH9 gene polymorphisms in 696 African-American subjects with hypertensive nephropathy and ESRD compared with 948 control individuals without CKD.29 The above mentioned MYH9 gene polymorphisms are less common in Caucasians, but they have been studied and it is not possible to determine whether they could also be markers of the disease. It appears that, in early stages, myosin IIA is present mainly in podocytes and causes structural abnormalities. Recently, the role of podocyte loss and dysfunction has been described in the pathogenesis of the disease.29-31
Based on these studies, some editorials have stated that nephrosclerosis should no longer be considered a disease secondary to HT. At least among patients of African descent, it appears to be a genetically based disease. Polymorphisms of this gene may be markers of various renal diseases that may be grouped in the same histologic group, the one that includes FSGS. This entity could include, in addition to the idiopathic form and the collapsing form as observed in HIV infection, hypertensive nephropathy, which would be a primitive renal disease. One may speculate that treatment could be approached with new perspectives and include more than blocking the renin-angiotensin system and strict control of blood pressure.32-35
However, there are still many unknowns about these findings. The studies referred to were performed in patients with nephrosclerosis that was not confirmed with renal biopsies. Clinical diagnosis of nephrosclerosis may hide cases of malignant hypertension, ischaemic nephropathy, atheroembolic nephropathy and some types of primary glomerular nephropathy. This marks the opportunity to reassess the cases in the AASK study, which is the only study with a high number of patients who underwent renal biopsy,15,17 and also the need to design prospective studies to further evaluate the relationship of this genetic polymorphism with disease progression.
In Caucasians almost everything still needs to be done. There are no studies to support that these or other MYH9 gene polymorphisms might be involved in disease. We do not know if the nephrosclerosis that is described in the African-American race with histological support of FSGS, high levels of proteinuria, and an abnormality in the MYH9 gene, is the same type of process which is seen more commonly in Caucasians: in elderly patients, those with significant vascular comorbidity, minimal proteinuria, and in whom progression of CKD is uncommon. It is possible that this process is merely a magnification of renal aging.
Finally, it should be noted that some studies carried out over a decade ago verified a direct relationship between nephrosclerosis and the DD genotype of the ACE gene in Caucasians. The D allele appeared to be predominant in hypertensive patients with nephrosclerosis and could be a marker of progression. Although the number of patients was small, the studies included histological support and control groups of hypertensive patients without renal impairment.36,37
In summary, it seems that prospective studies need to be designed in the future with prolonged follow-up intervals that allow us to know the true nature of the disease and limit the proportion of cases progressing to stage 5 CKD. Analysis of markers of progression must include both classical clinical markers and genetic markers described above, and it may be reasonable, at least in a subset of the population chosen at random, to obtain histological confirmation of the disease. This would be the basis for recognising whether renoprotective and cardioprotective treatments prescribed so far (in the form of renin-angiotensin system blockers, lipid lowering agents, antiplatelet agents, etc.) have had a real preventive role. In addition, it would be useful to clarify whether the objective of reducing blood pressure to below 130/80mmHg is effective in this disease and to investigate other potential therapeutic goals.
KEY CONCEPTS
1. Nephrosclerosis is seen in patients with chronic kidney disease and essential hype rtension with no other cause of kidney disease.
2. Nephrosclerosis is the second most frequent cause of terminal chronic kidney disease and the first cause of nephrology hospital consultations in our country. 3. The causal relationship with hypertension is still a subject for debate.
4. In Caucasians, progression of kidney failure is infrequent in most cases.
5. The factors that cause progression are not well recognised. Factors that are usually mentioned are: black race, degree of kidney failure on diagnosis, systolic blood pressure, degree of proteinuria and degree of associated cardiovascular comorbidity.
6. There is no evidence that a target blood pressure of <130/80 mmHg is more effective than a target blood pressure of <140/90 mmHg at preventing disease progression. Renin-angiotensin system blockers are used in the first step of treatment, although it has been shown to be effective only in cases with proteinuria (albumin/creatinine ratio >300 mg/g). The renoprotective effect of lipidlowering and antiaggregant agents requires greater research.
7. A relationship with disease has been found in Afro-Americans due to polymorphisms in the MYH9 gene. There have been no studies performed in Caucasians.
8. Prospective studies with histological support are necessary to recognise the clinical and genetic markers that condition progression in not Afro-American patients.
Table 1. Diagnosis of nephrosclerosis. Suspected clinical data