SARS-CoV-2 infection (COVID-19) has had a significant impact on transplant activity in our country. Mortality and the risk of complications associated with COVID-19 in kidney transplant recipients (KT) were expected to be higher due to their immunosuppressed condition and the frequent associated comorbidities. Since the beginning of the pandemic in March 2020 we have rapidly improved our knowledge about the epidemiology, clinical features and management of COVID-19 post-transplant, resulting in a better prognosis for our patients. KT units have been able to adapt their programs to this new reality, normalizing both donation and transplantation activity in our country.
This manuscript presents a proposal to update the general recommendations for the prevention and treatment of infection in this highly vulnerable population such as KT.
La infección por el SARS-CoV-2 (COVID-19) ha supuesto un importante impacto en la actividad trasplantadora en nuestro país. Era esperable que la mortalidad y el riesgo de complicaciones asociadas a la COVID-19 en el receptor de trasplante renal (TR) fueran mayores debido a su condición de inmunosupresión y a las frecuentes comorbilidades asociadas. Desde el inicio de la pandemia en marzo del 2020 hemos mejorado rápidamente nuestro conocimiento acerca de la epidemiología, características clínicas y manejo de la COVID-19 post-trasplante, redundando en un mejor pronóstico para nuestros pacientes. Las unidades de trasplante renal han sabido adaptar sus programas a esta nueva realidad, normalizándose la actividad tanto de donación como de trasplante en nuestro país.
Este manuscrito presenta una propuesta de actualización de las recomendaciones generales para la prevención y el tratamiento de la infección en esta población tan vulnerable como son los receptores de un trasplante renal.
The COVID-19 pandemic has created unprecedented challenges for solid organ transplant (SOT) programs. During the first months of the pandemic, most organ donation and transplantation programs reduced their activity, or suspended it altogether, due to the collapse of intensive care units and the almost exclusive dedication to the care of patients with COVID-19, especially severe in immunosuppressed individuals. Subsequently, activity has been recovering, and the transplant units have been adapting their programs to the new reality1,2 until the practical normalization of the activity.
It is well known that the incidence and mortality rate due to COVID-19 in the renal transplant population is higher than in the general population, and this worse prognosis has been associated with variables such as advanced age or severe pneumonia.3,4 For this reason, this was one of the groups prioritized to receive the first vaccines against SARS-CoV-2 in Spain.5 However, they have developed suboptimal immunization due to their inherent characteristics (immunosuppression, advanced age, chronic kidney disease [CKD]).6–8 This has meant that, despite high vaccination rates, renal transplant (RT) patients continue to have a higher rate of severe disease and even mortality due to SARS-CoV-2.9
This manuscript aims to update the recommendations already published in 202010 on SARS-CoV-2 infection in the RT-bearing population taking into account the current epidemiological context.
Specific considerations for donation and transplantationOne of the most important concerns of those responsible for RT is the need for safety of living donors, recipients and healthcare personnel involved in this activity.11 Transmission of COVID-19 infection through organ transplantation is exceptional, and there is increasing experience with the use of kidneys from COVID-19 positive donors, with no adverse reactions reported in recipients.12,13
The National Transplant Organization (ONT), in collaboration with the Autonomous (Regional) Transplant Coordination Offices and the Corona-SOT Group, have prepared a document14 containing recommendations regarding organ donation and transplantation in relation to COVID-19 infection.
Recommendations for evaluation and selection of deceased donor for SARS-CoV-2 infection- •
Evaluation of the potential organ donor requires a detailed clinical history and microbiological screening for SARS-CoV-2 to rule out, raise suspicion or, if necessary, confirm the diagnosis of COVID-19. Routine chest CT as a screening test in asymptomatic potential donors is not recommended.
- •
Universal microbiological screening of potential organ donors for SARS-CoV-2 will be performed. Screening should be based on real-time PCR (RT-PCR) on a respiratory tract specimen obtained, ideally, within 24h prior to organ extraction.
- •
In potential donors with no documented history of COVID-19 and negative RT-PCR for SARS-CoV-2 at screening, the organ donation will proceed.
- •
In donors with a previous diagnosis of COVID-19, organ donation will proceed provided that all of the following requirements are met:
- a
a minimum period of 14 days had elapsed since the onset of symptoms or microbiological diagnosis in the case of asymptomatic persons;
- b
that the potential donor has remained a minimum period of 72h free of symptoms;
- c
the potential donor has a negative result for SARS-CoV-2 by RT-PCR in a respiratory tract specimen obtained on the day of donation.
- a
- •
In donors with positive RT-PCR for SARS-CoV-2 (either in the context of a previous diagnosis of COVID-19 or if the diagnosis is made at the time of donation), renal donation will proceed, provided that death is NOT attributable to COVID-19. Organs from these donors will preferably be used in recipients who have positive serology against SARS-CoV-2 or have received a complete vaccination regimen (three or more doses) or have a previous history of COVID-19 infection. If transplantation is performed in this context, specific informed consent will be obtained and the recipient will be monitored with RT-PCR in a respiratory tract sample (before RT and on days 3 and 7 after transplantation).
- •
It is recommended that donor assessment for SARS-CoV-2 be performed under the same assumptions specified for the deceased donor.
- •
It is recommended that donation be delayed if there is a substantiated clinical suspicion or confirmed diagnosis of COVID-19 in the donorI, until a minimum period of 14 days has elapsed since the onset of symptoms and a minimum period of 72h free of symptoms. It is recommended that the potential donor present a negative result for SARS-CoV-2 by RT-PCR prior to donation. If this test is not negative, an individualized risk/benefit assessment will be made in each case.
- •
In the event that the potential donor was a close contact of a confirmed COVID-19 case, it is recommended that donation be delayed until seven days have elapsed and RT-PCR negativity for SARS-CoV-2 has been confirmed.
- •
Information on the relevant aspects related to COVID-19 infection should be included in informed consents for living donation.
- •
Before renal transplantation
- -
Exhaustive medical history.
- -
If the candidate presents a positive test for COVID-19, it is recommended to temporarily exclude the candidate until a minimum period of 14 days has elapsed since the onset of symptoms and a minimum period of 72h free of symptoms. It is recommended that the patient had a test negative for SARS-CoV-2 by RT-PCR prior to transplantation. In the absence of such a negative test, an individualized risk/benefit assessment will be made according to the clinical situation of each patient.
- -
In the event that the potential recipient had close contact of a confirmed COVID-19 case, it is recommended that transplantation be delayed until seven days have elapsed since the last contact and RT-PCR negativity for SARS-CoV-2 has been confirmed.
- -
Rule out active SARS-CoV-2 infection with a negative RT-PCR of nasopharyngeal exudate and absence of symptoms for at least 72h.
- -
Review information sheets and informed consent. In a pandemic situation it is advisable to incorporate specific information on COVID-19.
- -
Intensify the degree of information conveyed to patients, especially in relation with the risks of post-transplant COVID-19.
- -
In any elective RT situation (e.g., living donor), repeat RT-PCR and confirm negativity.
- -
Determine anti-IgG SARS-CoV-2 antibodies in serum; their presence suggests lower risk of severe infection.
- -
Limit the indication and the induction treatment dose with polyclonal antibodies.
- -
- •
After renal transplantation11
- -
Strict isolation measures during the immediate post-RT.
- -
If there is immediate kidney function, limit the hospital admission stay as much as possible (4–6 days).
- -
If there is delayed function, it is recommended outpatient follow-up, with hemodialysis on demand.
- -
After discharge, limit face-to-face visits as much as possible, encouraging telemedicine consultation.
- -
- •
The use of face masks (preferably FFP2) is recommended in closed spaces or in crowded areas.
- •
Maintain proper hygiene:
- -
Wash hands frequently with soap and water for at least 20s, or else with an alcohol-based hand sanitizer (at least 60% alcohol), especially: after using the toilet, before eating, after blowing the nose, coughing or sneezing, and after direct contact with sick people or their environment.
- -
Avoid touching eyes, nose and mouth before washing hands.
- -
- •
Avoid contact or maintain a distance of at least two meters with people suffering from symptoms of respiratory infection (fever, cough, generalized muscle pain, sore throat or respiratory distress), and do not share personal belongings with them.
- •
Follow a correct diet. Avoid sharing food and utensils (cutlery, glasses, napkins, handkerchiefs, etc.) and other objects without cleaning them properly.
- •
Avoid tobacco and alcohol consumption. In addition to being harmful to health, these substances weaken the immune system, making the body more vulnerable to infectious diseases.
- •
Avoid large crowds. It is recommended not to attend places where there may be crowds or excessive contact with other people.
- •
It is recommended to periodically ventilate the rooms with fresh air, open the windows.
- •
When SARS-CoV-2 infection is suspected in a renal transplant patient, a diagnostic test for active infection should be performed: a rapid antigen detection test or a viral RNA detection test by RT-PCR in nasopharyngeal exudate.
- •
In case of asymptomatic or mild infection, they should consult their transplant physician or primary care physician (preferably by telephone communication) for appropriate care.
- •
In case of severe infection, the patient should go to the emergency department of the hospital of reference.
Despite the new treatments against SARS-CoV-2, which we will discuss later, patients receiving RT continue to be a very vulnerable group, with rates of progression to severe COVID-19 and mortality much higher than the general population.15 Therefore, it is essential to develop preventive measures in this group of patients.
Until recently, vaccination was the only immunization option available. However, the effect of immunosuppression has a negative impact on the serologic response to vaccination in transplant recipients.16–19 Data from the SENCOVAC study reveal that up to 37.6% of RT recipients did not develop antibodies after 2 doses of an mRNA vaccine, leading to the administration of a third and fourth dose.20–23 The recent development of monoclonal antibodies capable of blocking viral entry into host cells has provided a promising alternative in the immunization of transplant recipients.16,24
VaccinesSince the approval of the first vaccines against SARS-CoV-2 in Europe, the Spanish Society of Nephrology has been in favor of priority immunization of patients with CKD, especially those on renal replacement therapy (RRT).25 Vaccination is currently indicated in all RRT patients and especially in those on the transplant waiting list, since it has been observed that the response rate achieved is higher than with post-transplant vaccination.23,26 Immunosuppressants, especially antimetabolites, rituximab and belatacept, are risk factors for non-response to the vaccine. A phase 4 clinical trial is currently underway to evaluate whether discontinuation of MMF improves vaccine response in this group of patients (https://clinicaltrials.gov/ct2/show/NCT05060991). The experience published so far is scarce, with small series in which does not appear to be differences, so we cannot make recommendations in this regard.27–29 To date the only strategy that has been shown to be effective in RT patients is the administration of repeated vaccine doses. Other risk factors identified for lack of seroconversion after vaccination are age, time post-transplant and renal insufficiency.16,18
Several types of vaccine have been available during these months (Table 1):
Immunization guidelines against SARS-CoV-2 in renal transplant recipients.
| Vaccines | Primary vaccination | First reminder doseb | Second booster doseb | ||
|---|---|---|---|---|---|
| Vaccine | Vaccine type | Initial guideline | Additional dosea | ||
| Comirnaty® (Pfizer BioNTech) | mRNA vaccine | Two doses of 0.3ml, separated 21 days. | One 0.3ml dose of Comirnaty® and ≥28 days after the 2nd dose. (can be replaced by a 0.5ml dose of Spikevax)® | One dose of 0.3ml≥5 months from the last dose. (May be substituted by a 0.25ml dose of Spikevax® or 0.5ml dose of Novaxovid)® | One 0.3ml dose ≥5 months from the last dose (May be substituted by a 0.25ml dose of Spikevax)® |
| Spikevax® (Modern) | mRNA vaccine | Two doses of 0.5ml, separated by 28 days. | One 0.5ml dose of Spikevax® ≥28 days after the 2nd dose. (can be replaced by a 0.3ml dose of Comirnaty)® | One dose of 0.25ml≥5 months from the last dose. (May be substituted by a 0.25ml dose of Comirnaty® or 0.5ml dose of Novaxovid)® | One 0.3ml dose ≥ 5 months from the last dose (May be substituted by a 0.25ml dose of Spikevax)® |
| Vaxzevria® (AstraZeneca) | Viral vector vaccine | Two doses of 0.5ml, 4–12 weeks apart. | One dose of 0.3ml of Comirnaty® or 0.5ml of Spikevax® ≥ 28 days after the 2nd dose. | One dose of 0.3ml of Comirnaty®, 0.25ml of Spikevax® or 0.5ml of Nuvaxovid ≥3 months after the last dose. | One 0.3ml dose ≥5 months from the last dose (May be substituted by a 0.25ml dose of Spikevax)® |
| Jcovden® (Janssen [Johnson and Johnson]) | Viral vector vaccine | One dose of 0.5ml | One dose of 0.3ml of Comirnat and ® or 0.5ml of Spikevax® ≥28 days after the 2nd dose. | One dose of 0.3ml of Comirnaty®, 0.25ml of Spikevax® or 0.5ml of Nuvaxovid® ≥ 3 months after the last dose. | One 0.3ml dose ≥5 months from the last dose (May be substitute by a 0.25ml dose of Spikevax)® |
| Nuvaxovid® (Novavax) | Protein subunit vaccine | Two doses of 0.5ml, separated 21 days. | One 0.5ml dose of Nuvaxovid® ≥6 months after the last dose. | One 0.3ml dose ≥5 months from the last dose (May be substituted by a 0.25ml dose of Spikevax)® | |
| Monoclonal antibodies | Dose | ||||
| tixagevimab/cilgavimab (Evusheld)® | 150mg of tixagevimab and 150mg of cilgavimab administered as two separate sequential intramuscular injections, with possible repeat administration at 6 months. Ongoing evaluation on whether to increase the dose of Evusheld® to 300mg of cilgavimab and 300mg of tixagevimab to ensure efficacy against new variant SARS-CoV-2. | ||||
- •
BNTb162b2 (Comirnaty®; Pfizer-BioNTech): This was the first vaccine of this type to be approved. It demonstrated 95% effectiveness in reducing the number of symptomatic COVID-19 cases in groups at risk of progression to severe disease.30 Although the clinical trial did not include immunocompromised patients, we now have series reporting a reduction of approximately 54% in the rate of hospitalization and progression to severe disease in solid organ transplant (SOT) recipients,31 rising to 78% after the third dose.32 The 2-dose regimen does not appear to have demonstrated a survival benefit9 and the seroconversion rate after 3 doses in SOT recipients is around 70%.33
- •
mRNA-1273 (Spikevax®; Moderna): The serological response rate appears to be higher than that observed with the BNTb162b2 vaccine in SOT recipients, around 68%34,35; data from the Spanish COVID-19 registry reported higher survival in RT patients vaccinated with 2 doses of mRNA-1273 and SARS-CoV-2 infection versus those who had received 2 doses of BNTb162b2.9 Several explanations have been postulated for these results: the higher dose administered in each vial, the different lipid composition of the nanoparticles used to transport the mRNA content in each vaccine and, finally, the longer time period elapsed between doses for mRNA-1273.
- •
ChAdOx1 nCov-19 (Vaxzevria®; AstraZeneca): Since the initial clinical trials did not have data in groups at-risk and only included patients aged 18–55 years, mRNA vaccines were reserved for the most vulnerable groups and the indication for Vaxzevria® was maintained mainly for people in group 3 and group 4 (groups with essential function for society [law enforcement, education personnel]) aged 18–55 years.36 However, once the vaccination campaign began, there was a small percentage of RT patients who, sharing characteristics with these above mentioned groups and with the aim of achieving immunity in the shortest possible time, received Vaxzevria®.9,20,36–38 The series publish seroconversion rates significantly lower than those described with mRNA vaccines, around 15%.38
- •
Ad26.COV.2 (Jcovden®; Janssen [Johnson and Johnson]): The experience in immunosuppressed patients is very limited. However, given their composition, they are not contraindicated in RT patients. According to data from the SENCOVAC study, only 0.5% of patients with CKD in Spain have received this vaccine and there are no series published that provide real-life data.20 In a series that included 12 SOT, the rate of seroconversion rate was 17%.39,40
- •
NVX-CoV2373 (Nuvaxovid®; Novavax): Nuvaxovid contains a purified recombinant SARS-CoV-2 spicule protein together with saponin-based Matrix-M adjuvant. They trigger immune responses of B and T lymphocytes to the S protein. It was the last vaccine to be approved in Spain and to date we do not have specific data in transplanted patients.41 Its main indication is the use in patients with contraindication to available vaccines or incomplete vaccination due to severe adverse reactions to mRNA vaccines because of a history of allergy to some of its components (mainly polyethylene glycol [PEG] in the case of mRNA vaccines and polysorbate 80 in adenovirus vaccines). Nuvaxovid also contains polysorbate 80 and there is a possibility of cross-reactivity with PEG, although the evidence for this is still very limited.23
Initially, the proposed schedule was two doses for the Pfizer-Biontech, Moderna, AstraZeneca and Novavax vaccines and a single dose for the Jannsen vaccine. However, due to data showing a lack of response in a significant proportion of cases and a decrease in effectiveness over time, in September 2021, the administration of a booster dose was indicated, preferably with mRNA vaccines to be administered at least 28 days after the last dose received.21–23 Conversely, the publication of series with severe cases of SARS-CoV-2 infection and high mortality rates in RT patients showed a lower vaccine protection in this population.9 Thus, in February 2022, the administration of an additional dose in high-risk patients, including RT recipients, was approved. The mRNA vaccines or Nuvaxovid® can be used for this additional dose. It will be administered 5 months after the last dose in the case of patients who have received exclusively mRNA or Nuvaxovid® vaccines, while in patients who have received a dose of Janssen or 2 doses of Vaxzevria®, the additional dose can be administered after 3 months.21–23
The absence of pre-transplantation vaccination does not contraindicate RT, but, as mentioned, it is highly advisable that the patient receive vaccination before transplantation. In those patients who have not completed the vaccination treatment, it is advisable to continue with the vaccination after the third month post-transplantation. Likewise, it is advisable to wait this same period before administering the next dose in those who have received immunosuppressive corticosteroids or biological and non-biological immunomodulators. If rituximab has been administered, the next dose of the vaccine should be delayed for at least 6 months.23 In relation to a first vaccination, if the patient has passed the SARS-CoV-2 infection, the corresponding dose will be administered when he/she is completely recovered, without the need to wait.23
According to the latest recommendation of the Ministry of Health, a new booster dose is recommended for people at risk, regardless of the number of doses received and the number of previous infections.42 This booster dose is also recommended in cohabitants with persons with a high degree of immunosuppression and will be carried out with the new bivalent mRNA vaccines (against the original strain and against new omicron variants). In this September 2022 update, it is stated that “Moderna BA.1 and Pfizer BA.4/5 vaccines (currently available vaccines) are two suitable alternatives and there is no evidence to favor one or the other”; however, the currently predominant lineages in Spain are BA.4 and BA.5. In the case of a recent SARS-CoV-2 infection, this booster dose will be administered within 3 months after infection. It is recommended to be administered together with influenza vaccine and pneumococcal vaccines if possible. Those persons who have not completed the primary vaccination (including the additional dose in persons with immunosuppression) should complete it as soon as possible.42
Monoclonal antibodies- •
Tixagevimab/cilgavimab (Evusheld®): It is a combination of two long-acting IgG1k monoclonal antibodies, cilgavimab and tixagevimab, derived from B cells from convalescent patients with SARS-CoV-2 infection that are indicated as pre-exposure prophylaxis in groups at risk of developing severe disease. In Spain, it has been included in the Vaccination Strategy against COVID-19 as a complement to vaccination in persons aged ≥ 12 years and weighing at least 40kg with a high degree of immunosuppression and who do not respond adequately to vaccination, among whom are SOT recipients.24 They should be administered as separate sequential intramuscular injections at different injection sites in two different muscles, preferably in the buttocks. The efficacy and safety of Evusheld® has been evaluated in the Phase 3 PROVENT trial,43 demonstrating an 83% reduction in the risk of severe disease. In this study, participants in the Evusheld® group suffered more serious cardiovascular events than those in the placebo group, although a causal relationship between Evusheld® and these events has not been established.43 Prior to administration, the person must have received at least 3 doses of the vaccine, unless it was contraindicated, and preferably have a serological test performed at least 15 days after administration. Until now, it had been established that an antibody titer to protein S of less than 260BAU/ml was considered an inadequate response to vaccination. However, since the threshold against the new variants of interest has not been validated, this figure can be considered indicative and the degree of immunosuppression of the patient and individual risk of infection should be taken into account. Additional candidates would be those persons at risk in whom it is not possible to complete the vaccination regimen, either because of contraindication to vaccines against COVID-19 or because they have developed serious adverse reactions associated with vaccination. In the case of recent SARS-CoV-2 infection, they can receive Evusheld® at least 6 months after infection.43 In the latest recommendations of the European Union Vaccination Registry and Program Panel, following the review performed in September 2022, the dose in pre-exposure prophylaxis is maintained at 300mg of Evusheld® (150mg tixagevimab and 150mg cilgavimab) recommending administration of new doses also of 150mg tixagevimab/150mg cilgavimab after 6 months of the first administration.42
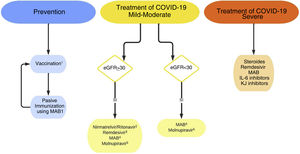
Vaccination and passive immunization with monoclonal antibodies are not mutually exclusive (Fig. 1). Administration of monoclonal antibodies is advised at least 15 days after vaccination, whereas COVID-19 vaccines can be administered at the same time or at any time after administration of Evusheld®.42,44
Recommendations on the prevention and treatment of SARS-CoV-2 infection.
MAB: monoclonal antibodies; IL-6: Interleukin-6; JAK: Janus kinase.
1Vaccination and passive immunization with MAB are not mutually exclusive. At least 15 days after vaccination is advised to administer MAB; COVID-19 vaccines can be administered simultaneously or at any time after MAB.
2Avoid it if there are pharmacological interactions, so it is not usually indicated as a first option in transplant recipients. There are proposals for dose adjustment in GFR<30ml/min/1.73m2, that are not supported in clinical practice.52
3Although limited, some published experiences do not show greater toxicity in advanced CKD.54,55 Some guidelines propose its use with GFR<30ml/min/1.73m2, evaluating the risks-benefits.45
4Indicated, in general, if the anti-S IgG antibody response is considered inadequate for the patient's degree of immunosuppression (Table 2).
5Lower efficacy than other anti-COVID-19 therapies.
Information on the treatment of COVID-19 is continuously evolving. There are many ongoing randomized controlled studies of different therapies for SARS-CoV-2 infection, so the evidence in this field is rapidly changing. Currently, treatments widely recommended a few months ago, such as hydroxychloroquine, lopinavir/ritonavir, azithromycin or interferon, have been discarded due to lack of efficacy.15,16 The use of hyperimmune plasma is also increasingly discussed and was considered not to be indicated before the arrival of Omicron, however in the current epidemiological situation there is not enough data and therefore its use could be considered, together with other strategies, in situations of severe immunosuppression.15,16,45,46
Treatment of patients with non-severe COVID-19SOT recipients are considered high-risk patients for progression to severe COVID-19 and therefore treatment with antivirals or neutralizing monoclonal antibodies (MAB) may be indicated and has been approved by the Spanish Agency of Medicines and Health Products (AEMPS).47
The therapeutic options currently available are detailed below (Fig. 1). The doses and interactions with immunosuppressive treatment are summarized in Table 2.
Treatment guidelines and recommendations on antivirals and monoclonal antibodies used against SARS-CoV-2.
| Drug | Administration period from the onset of symptoms | Dosage | Interactions with anticalcineurinic drugs and mTOR inhibitors | Recommendations | ||
|---|---|---|---|---|---|---|
| GFR (ml/min/1.73m)2 | ||||||
| >60 | 59−30 | <30 | ||||
| Nirmatrelvir/ritonavir | First 5 days | Nirmatrelvir 300mg/2 cp+ritonavir 100mg/1 cp/each 12h | Nirmatrelvir 150mg/1 cp+ritonavir 100mg/1 cp/each 12h | Not recommended | High interaction with increased immunosuppressant exposure. | Use in mild-moderate COVID-19. |
| Route: oral | Duration: 5 days | Requires adjustment according to levels. | ||||
| Duration: 5 days | Use with great caution in transplanted patients. | |||||
| Preferable MAB or remdesivir in the transplanted patient. | ||||||
| Remdesivir | First 7 days for mild-moderate COVID-19. | Day 1: single dose of 200mg. | Equal dose | Not recommended | No interaction | Indicated in mild-moderate COVID-19 if serology is not available or IgG anti-S>260 BAU/ml. |
| First 10 days for severe COVID-19 | From day 2: 100mg/once a day. | Can also be used in severe COVID-19. | ||||
| Route: IV infusion | ||||||
| Duration: In mild-moderate COVID-19, 3 days. In severe COVID-19, 5 days (may be extended to 10). | ||||||
| Molnupiravir | First 5 days | 800mg/4 cp/each 12h. | Equal dose | Equal dose | No interaction | Use in mild-moderate COVID-19. |
| Route: oral | Lower efficiency. | |||||
| Duration: 5 days | Limited experience in transplant recipients | |||||
| Sotrovimab | First 5 days | 500mg. | Equal dose | Equal dose | No interaction | Indicated in mild-moderate or severe COVID-19. |
| Route: IV infusion | Perform anti-S IgG serology. | |||||
| Duration: single dose | Indicated if serology negative or anti-S IgG antibody titer <260 BAU/ml.a | |||||
| Bebtelovimab | First 7 days | 175mg. | Equal dose | Equal dose | No interaction | Approved in the U.S. in mild-moderate COVID-19 only if nirmatrelvir/ritonavir or remdesivir is not considered suitable. |
| Route: IV infusion | No current EMA approval | |||||
| Duration: single dose | ||||||
| Tixagevimab/cilgavimab | First 7 days | 300mg/300mg | Equal dose | Equal dose | No interaction | Approved by EMA for COVID-19 that does not require oxygen supplementation, with risk of progression to severe disease. |
| Via: IM | ||||||
| Duration: single dose | ||||||
Cp: tablets/capsules; EMA: European Medicines Agency; eGFR: estimated glomerular filtration rate; IM: intramuscular; IV: intravenous; MAB: monoclonal antibody.
This cut-off point is indicative given the great variability of commercial tests. In addition to assessing the quantification of protein S antibody titers, the degree of immunosuppression of the patient must be taken into account. That is, the interpretation of the serology result will have to be assessed together with the patient's characteristics in terms of their degree of immunosuppression, the individual risk of infection and in case the result is greater than 260BAU/ml it should be assessed on an individualized basis.4,76
- •
Nirmatrelvir/ritonavir (Paxlovid®): Nirmatrelvir co-administered with ritonavir has demonstrated to produce a 89% reduction in hospitalization or death in patients with mild to moderate COVID-19 at risk of progression to severe disease when administered within 5 days of the onset of symptoms.48 However, ritonavir is a potent inhibitor of cytochrome P450 (CYP A3) and P-glycoprotein, leading to a strong interaction with calcineurin inhibitors and mTOR inhibitors, requiring temporary discontinuation and/or dose reduction of these immunosuppressants. Once treatment with ritonavir has been completed, CYP A3 activity needs a few days to recover. For these reasons, the administration of nirmatrelvir/ritonavir requires adjustment of the doses of immunosuppressants and monitoring the levels of these drugs, which may complicate their use in transplant patients with COVID-19, although it does not contraindicate it.49 The New York Presbyterian Hospital group has proposed a protocol for the management of tacrolimus and cyclosporine in patients treated with nirmatrelvir/ritonavir, and the published results are acceptable.49,50 However, the experience published so far in transplant recipients is very limited. The American Society of Transplantation recommends other treatments (MAB or remdesivir) in preference to nirmatrelvir/ritonavir, due to the mentioned risks associated with this drug, nevertheless it also suggests strategies for its use in case it is chosen.51 Moreover, in patients with moderate renal insufficiency the dose of this medication should be reduced (with a dose adjustment that has not been clinically tested) and is not recommended with estimated glomerular filtration rate (eGFR) below 30ml/min/1.73m2. Based on the pharmacology of nirmatrelvir, Hiremath et al make a proposal for dose adjustment in patients with eGFR less than 30ml/min/1.73m2 or on dialysis.52 However, so far there are no studies that support this proposal, so its use in these cases should be carried out with extreme caution, closely assessing risks and benefits and giving priority to other therapeutic options that are not contraindicated in advanced CKD.
- •
Remdesivir (Veklury®): It is a viral RNA-dependent RNA polymerase inhibitor that inhibits virus replication, with in vitro activity against SARS-CoV-2. It has been shown to accelerate healing in hospitalized patients with COVID-19 pneumonia, especially in those who do not require oxygen or who require low-flow oxygen.45,46 More recently, the PINETREE study showed that a 3-day course of treatment in outpatients reduced the risk of hospitalization or death by 87% as compared to placebo.53 Based on this, a 3-day course of treatment with this antiviral is considered indicated in immunosuppressed patients at risk of progression to severe disease, such as transplant recipients, in the first 7 days from the onset of symptoms, if effective MAB treatments are not available.45–47 Clinical trials have excluded patients with eGFR less than 30ml/min/1.73m2. It has been proposed that remdesivir could cause dose-dependent tubular damage or by accumulation of the excipient sulfobutylether-beta-cyclodextrin in patients with decreased renal function, so it is considered contraindicated in these cases.15,16,45,46 However, the results are contradictory and recent experiences do not show greater toxicity when used in patients with advanced CKD.54,55 Therefore, the U.S. National Institute of Health (NIH) guidelines, raise the possibility of its use in patients with GFR less than 30ml/min/1.73m2 if it is considered that the benefits may outweigh the risk, and it should be discontinued if there is a greater than 10-fold increase in ALT values or other evidence of hepatic inflammation is detected.45
- •
Molnupiravir (Lagevrio®): A ribonucleoside antiviral that has also been approved for patients with non-severe COVID-19, within 5 days of symptom onset; however it appears to be less effective than other antivirals and sotrovimab.56,57 It is not excreted through kidneys so it is considered safe for use regardless of renal function. It is contraindicated in pregnancy because it is teratogenic, and patients should be warned about this.45,46 Experience in transplant recipients is limited.58 It is recommended in those cases in which nirmatrelvir/ritonavir, sotrovimab or remdesivir cannot be used.15,16,45–47
In transplant patients the response to the vaccine is very poor, especially after 1 or 2 doses. In fact, the data from the COVID-19 registry of the Spanish Society of Nephrology showed no improvement of survival in renal transplant patients who had received two doses of vaccine versus those that did not receive the vaccine.9 This has generated great interest in passive immunization. MABs directed against the SARS-CoV-2 spike protein have emerged as a new therapy to reduce viral load and decrease the risk of progression to severe disease. Several formulations have been emerging in recent months (bamlanivimab, casirivimab/imdevimab, bamlanivimab-etesevimab, sotrovimab, bebtelovimab, tixagevimab/cilgavimab). They are anti-spike MABs designed to block the receptor-binding domain of the virus, thus interfering with its entry into the cell. They have demonstrated efficacy in hospitalized seronegative patients requiring low-flow oxygen.15,16,45,46 Some studies suggest an adverse effect if administered to patients who have developed endogenous neutralizing antibodies, although the possible mechanisms of this damage and whether or not it is specific to some MABs are unknown.45 In non-hospitalized patients with recent infection (3–7 days from symptom onset) and with risk factors for progression to severe forms of COVID-19, have been observed to significantly reduce this progression and reduce hospitalizations. However, the rapid emergence of mutations has meant that most of these MABs lack efficacy against new strains of the virus. Only sotrovimab, bebtelovimab and tixagevimab/cilgavimab have maintained potential activity against omicron, although with limitations that will be discussed below.15,16,45–47
- •
Sotrovimab (Xevudy®): It is approved in Spain for the treatment COVID-19 in patients with negative or low titers antibodies against SARS-CoV-2 who present mild-moderate or severe disease, recent onset (5 days from the onset of symptoms) and risk factors for poor outcome (such as transplant recipients).47 We have recently reported experience with this MAB in 82 renal transplant recipients with COVID-19.59 We observed excellent tolerance even in patients with very high comorbidity or advanced stages of CKD, and administration in the early stages of the disease was associated with a decrease in the need for ventilatory support and less ICU admissions and mortality.
Sotrovimab has been designed to bind to a more conserved region of the receptor-binding domain present in other sarbecoviruses, conferring potential protection to maintain its activity against new mutations.47,59 However, its efficacy against the BA.2 subvariant is under discussion. Although in vitro studies show less neutralizing activity against these recent omicron sublineages, the in vivo effects are under investigation. Recent data reveal that, despite decreased neutralizing activity in cell culture, sotrovimab reduced virus and proinflammatory cytokine levels in the lung of BA.2-infected mice demonstrating these differences between loss of potency in vitro and protection maintained in vivo.60 However, the Food and Drug Administration (FDA) withdrew the authorization for use in the USA in April 2022.61 In contrast, in Europe, the European Medicines Agency (EMA) has maintained the authorization of sotrovimab based on the lack of knowledge about the clinical relevance of the decrease in neutralizing activity against omicron BA.2.62 A recent French multicenter study compared the efficacy of sotrovimab in preventing severe COVID-19 in high-risk patients infected with omicron BA.1 or BA.2, showing a low rate of hospitalizations and a similar reduction in nasopharyngeal viral load in both groups.63 The impact of the new omicron sublineages BA.2.11, BA.2.12.1 and BA.4/5 on the neutralizing capacity of Sotrovimab does not appear to be superior to that observed with BA.2. Early data on the recent BA.2.75 (centaurus) subvariant are contradictory: some results point to a loss of neutralizing activity that is lower than that detected with the previous omicron sublineages while other studies find very little activity.64,65 Updated information on the susceptibility of the different variants and subvariants of the virus to MAB can be found at https://covdb.stanford.edu/page/susceptibility-data/.
- •
Bebtelovimab: in vitro data on this MAB suggest that it maintains its neutralizing activity against the new omicron subvariants, but there are no controlled studies available that evaluate its use in patients at high risk of progression to severe COVID-19.45,64 So far it has been evaluated only in low-risk patients showing good tolerance and a more rapid decrease in viral load as compared to placebo.45 It has been approved in the U.S. the use in the first 7 days of symptoms and only if nirmatrelvir/ritonavir or remdesivir are not considered appropriate. Initial experience in transplant patients shows results that appear similar to those obtained with other MABs.66,67 At the time of this review in September 2022, it has not been approved in Europe.
- •
Tixagevimab/cilgavimab (Evusheld®): Tixagevimab shows significant loss in its neutralizing capacity against the new Omicron variants while for cilgavimab and the tixagevimab/cilgavimab combination the reduction of neutralizing capacity appears to be mild-moderate (https://covdb.stanford.edu/page/susceptibility-data/). Very recently the results of the TACKLE study have been published, this is a randomized versus placebo study in which patients, unvaccinated, with mild-moderate COVID-19 without oxygen requirement were treated with 600mg of tixagevimab/cilgavimab (2 consecutive intramuscular injections of 300mg cilgavimab and 300mg tixagevimab) in the first 7 days from onset of symptoms. The variants of virus preceded the omicron. Severe COVID-19 or death occurred in 4% of those treated with tixagevimab/cilgavimab vs. 9% in the placebo group, with a 50.5% decrease in relative risk (P=.0096).68 The number of patients in the study included in the category of “immunosuppressed” was very small (5%). The reported experience in transplantation is still very limited.69 The EMA has approved the use of tixagevimab/cilgavimab for treatment while the FDA maintains the use only for pre-exposure.70
In situations of more severe COVID-19 (dyspnea, respiratory rate greater than 22rpm, basal SatO2<94%) supportive treatments such as oxygen therapy, thromboembolic prophylaxis and/or antibiotic treatment should be considered if there is suspicion of bacterial superinfection. As for specific therapies there is evidence regarding the following:
- •
Steroids: These have been shown to reduce mortality in patients requiring supplemental oxygen, with maximum benefit in those requiring mechanical ventilation. However, in less severe forms without the need for oxygen therapy, no benefit has been demonstrated, and an even detrimental effect cannot be ruled out.16,46,65 The use of dexamethasone is preferably recommended as it is the steroid most tested in clinical trials showing the greatest advantages in survival. The risk of hyperglycemia and secondary infections must be monitored.
- •
Tocilizumab: Interleukin-6 inhibitor that seems to reduce the need for mechanical ventilation and the length of hospital stay, but with doubtful effect on mortality.15 Currently it can be indicated together with dexamethasone in cases of severe hypoxemia and CRP greater than 75 or if mechanical ventilation is required. It is also recommended in patients that get worse despite treatment with dexamethasone.46 In transplant patients its efficacy has not been clearly established, but it has been used without specific side effects in this population.16,71
- •
Baricitinib: It is a drug also with anti-inflammatory activity, a Janus Kinase inhibitor, used so far in the treatment of rheumatoid arthritis. It has similar benefits as tocilizumab and it can be used instead of tocilizumab.72 The combined use of both is not recommended because, as they are potent immunosuppressants, the risk of infections can be increased.17
- •
Anakinra: Acts by inhibiting interleukin-6. It can be used if PLAUR is available and the result is greater than 6ng/ml.46 The evidence with this drug is more limited than with tocilizumab and baricitinib.73
- •
Remdesivir: It has not been shown to reduce mortality, but it reduces recovery time. It is recommended in patients requiring low-flow oxygen, in a 5-day treatment regimen and during the first 10 days of symptom onset.15,46,47 Although there is no clear evidence, prolonging administration for 10 days could be indicated in severe cases with mechanical ventilation or ECMO or patients with severe immunosuppression.47
- •
Sotrovimab: Could be used, with the same considerations as above, in critically ill hospitalized patients with negative serology or low level of antibodies to SARS-CoV-2.47,48 However, the efficacy of sotrovimab has not been demonstrated in patients hospitalized with severe or critically ill COVID-19.
Due to immunosuppression, RT patients have an altered immune response, particularly the T-cell mediated immune response, thus patients are exposed to a higher risk of infections.53 The first step in the treatment of any infectious disease of viral origin is prevention, through strategies such as vaccination, antiviral prophylaxis and control of the disease with general measures.74 However, if this fails and the patient develops infection, treatment of the infection includes not only targeted antiviral treatment but also reduction or modulation of immunosuppression.
In this regard, the accumulated evidence on the management of immunosuppression in kidney transplant patients with SARS-CoV-2 infection in these first 2 years of the pandemic has focused on the publication of observational, retrospective, non-randomized studies that have helped to clarify initial doubts about the immunosuppressive strategy to be followed and its medium-term repercussions.75–84 All published information recognizes that the reduction of immunosuppression has been one of the pillars of therapeutic management, in the absence of specific antiviral treatment, and this reduction has been carried out according to the clinical situation of the patient, the period of time after transplantation, the immunological risk of the patient and always under the supervision of a nephrologist.
In a Spanish multicenter study,82 which collected the experience of immunosuppression management of the majority of COVID-19 infected RT patients in Spain during the first and second waves of the pandemic. It was observed that immunosuppression management was mainly based on discontinuation of mycophenolic acid and very discrete reductions or discontinuations of calcineurin inhibitors. These supervised and intentional changes in immunosuppressive therapy did not influence renal function or HLA sensitization at 6 months after COVID-19 diagnosis. In another French multicenter study84 it was also observed that, after modulation of immunosuppression during coronavirus infection, the incidence of de novo donor-specific antibodies (de novo DSA) after SARS-CoV-2 infection was 4%, which was very similar to the incidence of de novo DSA in non-COVID-19 RT patients (5%) and renal function remained stable in all of them, This indicates that this practice of reducing immunosuppression in situations of severe infection does not seem to imply a greater immunological risk for the graft and allows the patient to acquire, in a short period of time, the cellular immunity necessary to control the infection and thus avoid its progression and its vital complications.
In the above mentioned Spanish study82 there was no common protocol for the management of immunosuppression; however, in all centers immunosuppression was progressively reduced as the patient's clinical situation worsened. Based on this study, which brings together the clinical practice of most of the RT units in Spain, we should recommend the following immunosuppression management guideline during SARS-CoV-2 infection:
- -
In cases of SARS-CoV-2 infection with mild or asymptomatic symptoms, it is not necessary to modify the patient's baseline immunosuppression.
- -
In cases of moderate infection with pneumonia and hypoxemia requiring oxygen therapy with nasal goggles. If the patient is at low immunological risk it is recommended to suspend immunosuppressive treatment with mycophenolic acid or m-TOR inhibitors, reduce the dose of calcineurin inhibitor (by 30%) and associate or increase the dose of steroids (prednisone or methylprednisolone to 20mg/day).
- -
In cases of severe infection with pneumonia requiring mechanical ventilation or ICU admission, it is recommended a complete and temporary suspension of immunosuppression and increased steroids (prednisone or methylprednisolone at 20−40mg/per day). In highly sensitized patients with severe infection, they could receive intravenous nonspecific immunoglobulin every 15 days if possible, until recovery of immunosuppression.
Regarding the re-instauration of immunosuppressive therapy, it should be done once the patient has shown clinical, analytical and radiological improvement, which will depend on the baseline situation of each patient and the associated comorbidity. The full dose of calcineurin inhibitors should be reestablished first, and if the patient remains clinically stable, immunosuppressive treatment with antimetabolites or m-TOR inhibitors should then be restarted, and reduce progressively the dose of steroid to its usual dose.
Conflict of interestThe authors declare that they have no conflicts of interest related to the contents of the article.