Anemia is a common complication of chronic kidney disease (CKD) and is associated with a decrease in quality of life and an increased risk of transfusions, morbidity and mortality, and progression of CKD. The Anemia Working Group of the Sociedad Española de Nefrología conducted a Delphi study among experts in anemia in CKD to agree on relevant unanswered questions by existing evidence. The RAND/UCLA consensus methodology was used. We defined 15 questions with a PICO structure, followed by a review in scientific literature databases. Statements to each question were developed based on that literature review. Nineteen experts evaluated them using an iterative Two-Round Delphi-like process. Sixteen statements were agreed in response to 8 questions related to iron deficiency and supplementation with Fe (impact and management of iron deficiency with or without anemia, iron deficiency markers, safety of i.v. iron) and 7 related to erythropoiesis stimulating agents (ESAs) and/or hypoxia-inducible factor stabilizers (HIF), reaching consensus on all of them (individualization of the Hb objective, impact and management of resistance to ESA, ESA in the immediate post-transplant period and HIF stabilizers: impact on ferrokinetics, interaction with inflammation and cardiovascular safety). There is a need for clinical studies addressing the effects of correction of iron deficiency independently of anemia and the impact of anemia treatment with various ESA on quality of life, progression of CKD and cardiovascular events.
La anemia es una complicación frecuente de la enfermedad renal crónica (ERC) y se asocia con una disminución en la calidad de vida y a un mayor riesgo de transfusiones, de morbimortalidad y de progresión de la ERC. El Grupo de Trabajo en Anemia de la Sociedad Española de Nefrología realizó un estudio Delphi entre expertos en anemia de la ERC para consensuar respuestas a preguntas relevantes que no se hubieran podido resolver con la evidencia existente. Se empleó la metodología de consensos RAND/UCLA. Se definieron 15 preguntas con una estructura PICO, seguida de una revisión en bases de datos de literatura científica. A partir de la evidencia se formularon enunciados. Diecinueve expertos los evaluaron mediante un proceso iterativo tipo Delphi a dos rondas. Se consensuaron 16 enunciados en respuesta a 8 preguntas referidas a la ferropenia y suplementación con Fe (impacto y gestión de ferropenia con o sin anemia, marcadores de ferropenia, seguridad de hierro i.v.) y a 7 relacionadas con agentes estimuladores de la eritropoyesis (AEE) y/o con estabilizadores del factor inducible por la hipoxia (HIF), alcanzándose consenso en todos ellos (individualización del objetivo de Hb, impacto y gestión de resistencia a AEE, AEE en el periodo inmediato post trasplante y estabilizadores de HIF: impacto sobre la ferrocinética, interacción con inflamación y seguridad cardiovascular). Existe una necesidad de estudios clínicos que aborden los efectos de la corrección del déficit de Fe con independencia de la anemia y el impacto del tratamiento de esta con diversos AEE sobre la calidad de vida, la progresión de ERC y los eventos cardiovasculares.
Anemia is a common complication of chronic kidney disease (CKD) and it is associated with decreased quality of life (QoL), increased risk of transfusions, as well as increased morbidity and mortality, and progression of CKD.1
The main cause of anemia in CKD is inadequate endogenous erythropoietin production; although in recent years it has been recognized the multifactorial origin of anemia. Other factors involved are, for example, a decreased erythropoietic response of the bone marrow, decreased availability of iron (Fe) for erythropoiesis and increased levels of hepcidin (absolute or functional Fe deficiency), a decreased half-life of red blood cells, or vitamin deficiencies (vitamin B12 or folic acid).1,2
The prevalence of anemia increases as renal function decreases and it is virtually universal in hemodialysis patients. Moreover, iron deficiency is already very common in earlier stages of CKD.3
The introduction of erythropoiesis-stimulating agents (ESA) in the late 1980s was a fundamental advance for renal patients; it increased hemoglobin (Hb) levels and quality of life and reduced morbidity and mortality and the need for transfusions.1 However, there are unresolved aspects in the management of renal anemia, such as individual treatment targets (Hb and ferrokinetics), hyporesponsiveness to ESA, variability in Hb levels, safety of ESA and ferrotherapy, among others. For this reason, the Spanish Society of Nephrology (S.E.N.), through its Anemia Working Group, proposed to carry out a DELPHI study among experts in anemia in CKD to reach a consensus on answers to relevant questions that could not be resolved with the available evidence from clinical trials.
MethodologyThis work was carried out following the consensus methodology developed by the RAND/UCLA.4 The Recommendations Elaborating Group (REG) comprised of 8 nephrologists (two coordinators and 6 advisors) with experience in the management of patients with CKD and anemia. At the first meeting in April 2021, 15 questions with PICO structure (patients, intervention, comparator, outcome) were defined.5
Based on the questions, a literature review was performed in the databases PubMed, Scopus, Web of Science (data collection was closed: June 2021) using controlled syntax (see Appendix Supplementary data 1), with a time limit of 10 years. In addition to the references found through the database search, the REG was able to put in studies identified by others means or that did not meet the presestablished criteria (e.g., temporal) but were considered relevant to answer questions related to the ongoing investigation. The process of selecting references is described in Appendix B Supplementary Fig. 1. The publications were then critically analyzed, relevant evidence was extracted, and statements were formulated that answered the questions.
Nineteen experts evaluated the proposed statements through a two-round iterative Delphi process according to a Likert scale from 1 to 9 (1: fully disagree; 9: fully agree) in an online questionnaire. Together with the link to the questionnaire, the panelists received a dossier with a summary of the evidence used by the experts for the formulation of the statements, with the related bibliographic references. The RAND/UCLA methodology was used for consensus analysis in Delphi panels.4 Each questionnaire item is classified according to the degree of agreement and the median score of the panel into Appropriate (median in range 7–9), Uncertain (median in range 4–6 or any median in disagreement) or Inappropriate (median in range 1–3). Agreement was achieved if at least one-third of the sample responded within the same score range as the median, disagreement if the median score fell in either extreme range and more than one-third of the sample responded in the opposite extreme range, or if the median fell in the middle range, and at least one-third of the sample responded in one of the other two ranges, and “neutral” if it did not meet any of the above criteria.4 Always being based on the comments received in the first round, it was decided to split one of the statements for clarity. A total of 16 statements (100%) proposed between the first and second Delphi rounds were agreed upon by the expert panel.
Iron deficiency and iron supplementationAbsolute or functional iron deficiency (FeD) is a common condition in CKD. Nephrology clinical practice guidelines have considered iron therapy in the presence of iron deficiency and anemia.6 However, in recent years the relevance of FeD per se, independently of anemia, has been seen in other clinical situations, such as heart failure with reduced ejection fraction (HFrEF).7 The results of published clinical trials in patients with HFrEF or the PIVOTAL study in hemodialysis patients, as well as results from observational studies, suggest the convenience of treating iron deficiency beyond anemia in renal patients.8
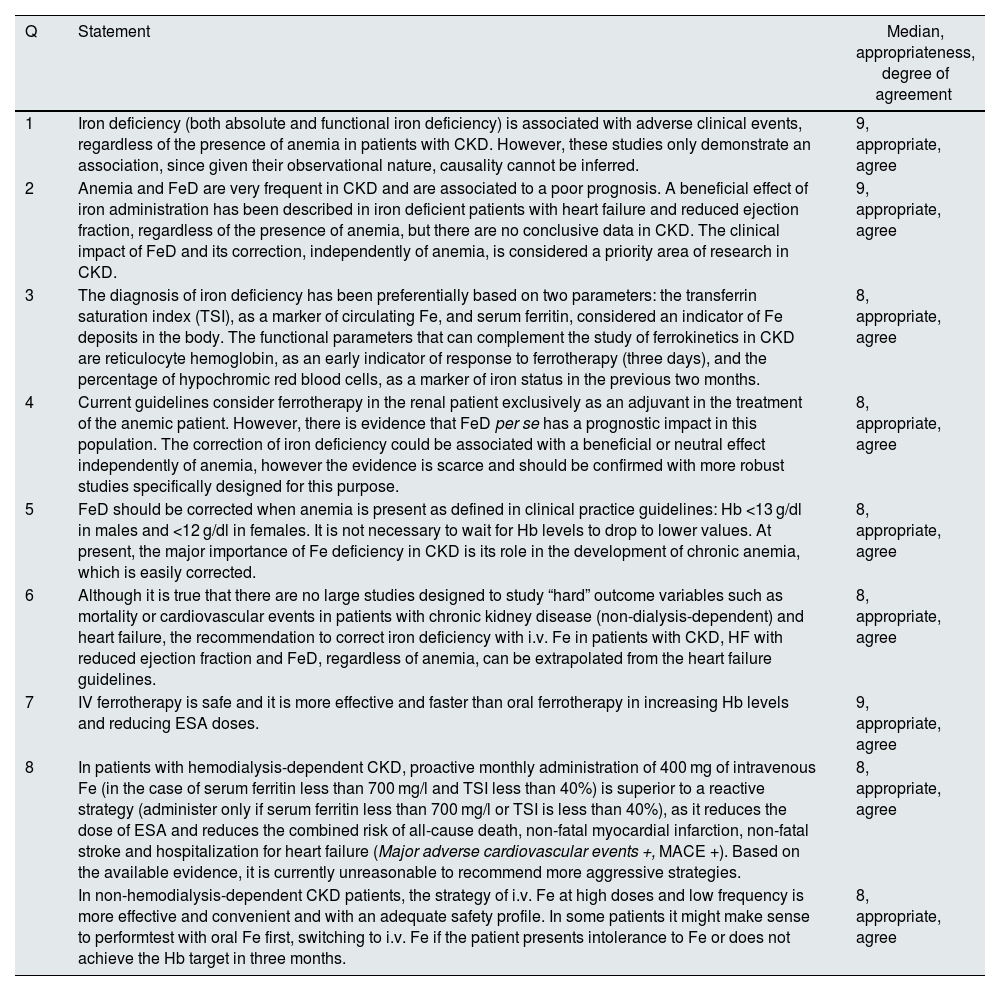
Recommendations related to this section can be found in Table 1
Statements related to iron deficiency and iron supplementation.
| Q | Statement | Median, appropriateness, degree of agreement |
|---|---|---|
| 1 | Iron deficiency (both absolute and functional iron deficiency) is associated with adverse clinical events, regardless of the presence of anemia in patients with CKD. However, these studies only demonstrate an association, since given their observational nature, causality cannot be inferred. | 9, appropriate, agree |
| 2 | Anemia and FeD are very frequent in CKD and are associated to a poor prognosis. A beneficial effect of iron administration has been described in iron deficient patients with heart failure and reduced ejection fraction, regardless of the presence of anemia, but there are no conclusive data in CKD. The clinical impact of FeD and its correction, independently of anemia, is considered a priority area of research in CKD. | 9, appropriate, agree |
| 3 | The diagnosis of iron deficiency has been preferentially based on two parameters: the transferrin saturation index (TSI), as a marker of circulating Fe, and serum ferritin, considered an indicator of Fe deposits in the body. The functional parameters that can complement the study of ferrokinetics in CKD are reticulocyte hemoglobin, as an early indicator of response to ferrotherapy (three days), and the percentage of hypochromic red blood cells, as a marker of iron status in the previous two months. | 8, appropriate, agree |
| 4 | Current guidelines consider ferrotherapy in the renal patient exclusively as an adjuvant in the treatment of the anemic patient. However, there is evidence that FeD per se has a prognostic impact in this population. The correction of iron deficiency could be associated with a beneficial or neutral effect independently of anemia, however the evidence is scarce and should be confirmed with more robust studies specifically designed for this purpose. | 8, appropriate, agree |
| 5 | FeD should be corrected when anemia is present as defined in clinical practice guidelines: Hb <13 g/dl in males and <12 g/dl in females. It is not necessary to wait for Hb levels to drop to lower values. At present, the major importance of Fe deficiency in CKD is its role in the development of chronic anemia, which is easily corrected. | 8, appropriate, agree |
| 6 | Although it is true that there are no large studies designed to study “hard” outcome variables such as mortality or cardiovascular events in patients with chronic kidney disease (non-dialysis-dependent) and heart failure, the recommendation to correct iron deficiency with i.v. Fe in patients with CKD, HF with reduced ejection fraction and FeD, regardless of anemia, can be extrapolated from the heart failure guidelines. | 8, appropriate, agree |
| 7 | IV ferrotherapy is safe and it is more effective and faster than oral ferrotherapy in increasing Hb levels and reducing ESA doses. | 9, appropriate, agree |
| 8 | In patients with hemodialysis-dependent CKD, proactive monthly administration of 400 mg of intravenous Fe (in the case of serum ferritin less than 700 mg/l and TSI less than 40%) is superior to a reactive strategy (administer only if serum ferritin less than 700 mg/l or TSI is less than 40%), as it reduces the dose of ESA and reduces the combined risk of all-cause death, non-fatal myocardial infarction, non-fatal stroke and hospitalization for heart failure (Major adverse cardiovascular events +, MACE +). Based on the available evidence, it is currently unreasonable to recommend more aggressive strategies. | 8, appropriate, agree |
| In non-hemodialysis-dependent CKD patients, the strategy of i.v. Fe at high doses and low frequency is more effective and convenient and with an adequate safety profile. In some patients it might make sense to performtest with oral Fe first, switching to i.v. Fe if the patient presents intolerance to Fe or does not achieve the Hb target in three months. | 8, appropriate, agree |
ESA, erythropoiesis-stimulating agents; FeD: iron deficiency; CKD, chronic kidney disease; Hb, hemoglobin; IV, intravenous; TSI, serum transferrin index; MACE, combined event, mortality or infarction or stroke; Q, question.
Discussed in conjunction with question II.
Should iron parameters be measured in CKD patients regardless of the presence of anemia (preventive role of anemia)?FeD in patients with ESRD has been shown to have a negative prognostic impact and its correction with intravenous (i.v.) Fe improves the clinical situation and reduces morbidity, regardless of the presence of anemia.8 In addition there is growing evidence that FeD per se has negative prognostic implications in patients across the spectrum of CKD,9–21 as well as on health-related quality of life (HRQoL)22 or the general symptoms23 of patients with CKD. Since the definitions of FeD and study populations are not homogeneous, it is difficult to quantify the risk attributable to absolute or functional FeD, but they agree on an increased risk of total mortality,9–12,14,16,17,19,21 cardiovascular (CV) hospitalization,13 CV mortality,9,10,17,21 heart failure (HF)15,20 or CV events11,19 in the presence of FeD (absolute or functional) in most studies. However, these studies only demonstrate an association, because given their observational nature, causality cannot be inferred. There is also no clear evidence of the benefit of FeD correction on CV events, as the trials have been designed to assess its erythropoietic effects in the presence of anemia. The 2012 Kidney Disease Improving Global Outcomes (KDIGO) anemia guidelines6 recommend screening and monitoring of Iron parameters only for patients with anemia. In fact, the latest KDIGO controversies concluded that understanding the clinical impact of FeD and its correction, regardless of the existence of anemia, is a high priority area of research for future studies in patients with CKD.24
Should the new markers of iron deficiency (% hypochromic red blood cells/reticulocyte Hb) be incorporated in the study of ferrokinetics in CKD?The diagnosis of FeD has classically been based on IST, as a marker of circulating Fe (and available to the bone marrow) and serum ferritin, considered an indicator of Fe deposits in the body. However, these indicators are considered unreliable for estimating Fe stores (ferritin is an acute phase reactant that can increase in the presence of inflammation) or for predicting response to ferrotherapy in patients with CKD. Both reticulocyte Hb (CHr) and the percentage of hypochromic red blood cells are functional parameters that can help in the study of ferrokinetics in CKD, since they are more reliable parameters for the evaluation of Fe homeostasis except in the presence of thalassemia. Currently, most hematology laboratories are capable of measuring these indicators. Additionally, they have the advantage of low cost, low variability and are not influenced by inflammation or infections. However, apart from the diagnostic evidence, there is little experience about the implementation of these markers in clinical practice. It is recommended to use the percentage of hypochromic red blood cells, but only if it is possible to process the blood sample within 6 h of collection. Probably, the combination of all these ferrokinetic parameters will allow a more accurate algorithm for the diagnosis of Fe deficiency and the erythropoietic response to ferrotherapy in renal patients. However, according to the latest controversies on the management of anemia from the KDIGO Guide,24 the widespread clinical use of both parameters is limited by the absence of universal clinical decision limits, as well as by the requirement for their determination in fresh blood samples in the case of the percentage of hypochromic red blood cells. These areas have been identified as high priority for future research, so at the present it is difficult to recommend their widespread use and to replace ferritin and IST.
Is there evidence about correcting Fe deficiency independently of anemia and/or treatment with erythropoiesis-stimulating agents?In the PIVOTAL study,25 incident hemodialysis patients treated with ESA were randomized to a proactive group that received 400 mg/month of i.v. Fe sucrose until increasing ferritin levels >700 ng/ml or a TSI >40% vs the reactive group that received low-dose Fe sucrose if ferritin was <200 ng/ml or TSI 20%. After a follow-up of 2.1 years, 29.3% of the proactive group had a composite CV event (death, myocardial infarction, stroke or hospitalization for HF) versus a 32.3% of the reactive group (HR 0.85, 95% CI 0.73–1.0). An analysis of the events individually revealed a reduction in the risk of mortality, hospitalization due to HF or fatal and non-fatal myocardial infarction, with similar rates of stroke. The rates of hospitalization and/or infection were similar in the two groups. The authors concluded that, in hemodialysis patients, a high-dose i.v. Fe regimen administered proactively was superior to a low-dose regimen administered reactively and required lower doses of ESA (19% reduction), so the observed benefit could be, at least in part, due to the lower doses of ESA required.25
The FIND-CKD study26 includes 626 anemic patients with CKD no on dialysis (ND) (eGFR <60 ml/min/1.73 m2) and FeD (IST <20%, ferritin <100 ng/ml) patients were randomized into two different i.v. Fe strategies: high (400–600 ng/ml) or low (100–200 ng/ml) ferritin or FE oral. The superiority of the high ferritin strategy in preventing other anemia treatments was demonstrated,26 with no differences in safety after 1-year follow-up (serious adverse events [AEs], cardiac events, mortality, infections or progression of CKD) between the different groups.25,27
A pilot study28 analyzed the effect of a fixed dose of ferric citrate versus standard treatment in patients with advanced CKD. There were 203 patients randomized with eGFR ≤20 ml/min/1.73 m2, phosphorus ≥3.0 mg/dl, Hb >8.0 g/dl, IST < 55%. The group receiving ferric citrate significantly increased Hb, IST and serum ferritin, and significantly reduced serum phosphate and intact FGF23 (p < 0.001 for all). Compared to themusual treatment, ferric citrate treatment resulted in significantly fewer annual hospital admissions, less time spent in hospital, and a lower incidence of the combined endpoint of death, need for dialysis or transplantation (p = 0.002).28
A prospective, double-blind, randomized study in non-anemic patients with stage 3b-5 CKD and FeD investigated whether 1000 mg i.v. Fe (ferric derisomaltose –FDI–) could improve exercise capacity. After adjusting for baseline characteristics, there was no significant difference in the 6-min walk test (6MWT) between the two groups at 1 month (p = 0.736) or 3 months (p = 0.741). There were nonsignificant increases in 6MWT from baseline to 1 and 3 months in the FDI arm. Hb remained stable and there were statistically significant increases in ferritin and IST at 1 and 3 months (p < 0.001). There was a modest numerical improvement in QoL parameters. There were no adverse events attributable to FDI.29
Thus, the results point to a benefit of FeD correction in CKD. However, its effect is difficult to separate from Hb improvement or ESA dose reduction, so specifically designed randomized studies are needed to confirm these issues.
Should Fe deficiency be corrected at diagnosis of anemia according to general population parameters – male Hb <13 and female Hb <12 g/dl – in the patient with CKD?FeD may cause anemia and hyporesponsiveness to ESAs and should be corrected to ensure optimization of erythropoiesis in anemic patients with CKD.
In patients with anemia associated with CKD, ferrotherapy is aimed at ensuring adequate Fe reserves for erythropoiesis, avoiding or delaying the need for ESA and, in patients receiving ESA treatment, preventing the development of FeD and reducing ESA requirements. As mentioned above, anemia in CKD is associated with an increased risk of morbidity, mortality, and progression of CKD, so early correction is desirable.8
The recommendations of the clinical guidelines coincide on the administration of Fe to CKD patients with anemia. It is not appropriate to extrapolate the Hb thresholds applied for the initiation of ESA prescription (Hb <10 g/dl), which are lower, based on the results of clinical trials with these agents.30,31
Iron, unlike ESAs, is a factor necessary for efficient erythropoiesis, but it is not a growth factor, so there is no risk of exceeding the Hb level above the target limit29,32 (except in patients with polycythemia/polyglobulia).
Should Fe deficiency be corrected in CKD patients with heart failure without anemia?It is estimated that FeD is present in approximately 46% of non-anemic patients with stable HF. The presence of FeD, independently of the presence of anemia, is associated with increased symptomatology, decreased exercise capacity, QoL, and increased mortality and hospitalizations due to HF progression.33,34
In this regard, several randomized clinical trials such as FAIR-HF,35 CONFIRM-HF,36 EFFECT-HF37 and AFFIRM-AHF38 in patients with HFrEFr and FeD (but not specifically CKD) have demonstrated the safety of Fe carboxymaltose supplementation (FCM), as well as an improvement in symptomatology and in some CV outcome variables.
In a small trial analyzing patients with CKD, HF, anemia and FeD, i.v. Fe administration was associated with improvement in myocardial functional parameters and cardiac dimensions.39 In an observational pilot study in patients with HFrEFr, FeD and renal dysfunction (mean GFR 40 ml/min),) myocardial repletion of Fe was associated with improvement in left ventricular ejection fraction and its end-systolic volume, independently of the change in Hb levels,40 which has been confirmed in another recent larger study in patients with less renal function impairment.41 In CKD patients, a strategy of high-dose FDI versus repeated low-dose Fe sucrose was associated with a more rapid increase in Hb and ferric parameters, as well as fewer CV events, including HF.42 A meta-analysis on the effect of i.v. ferrotherapy in patients with HFrEFr and FeD43 demonstrated an improvement in functional class, symptomatology, exercise capacity and QoL. It also demonstrated a reduction in the combined outcome of all-cause death and CV hospitalization, in the risk of the combined outcome of CV death and hospitalization for worsening HF, and a reduction in the risk of HF hospitalization. Another more recent meta-analysis of FCM studies in patients with HFrEF and FeD showed similar clinical results, although the beneficial effect was smaller in patients with TSAT > 20%.44
The IRONOUT HF study45 studied 225 patients with symptomatic HF and FeD who were randomized to receive 150 mg of oral Fe polysaccharide or placebo. Patients who received oral Fe failed to improve Fe stores or their functional capacity.
For all these reasons, the HF guidelines of the European Society of Cardiology (ESC) recommend considering treatment with i.v. FCM in patients with HFrEF who are symptomatic and present FeD. However, the same guidelines point out that the studies on which the recommendation is based did not have sufficient statistical power to evaluate mortality or CV events, or to analyze the independent effects of Hb levels.46
In hemodialysis patients, the PIVOTAL study has demonstrated a reduction in HF hospitalizations (this study excluded patients with New York Heart Association functional class IV HF) with proactive i.v. Fe administration versus reactive administration only in case of iron deficiency.25
All of which supports the administration of i.v. FCM in patients with HFrEFr and CKD, extrapolating the results obtained in patients with HF.
Is i.v. Fe more effective than oral Fe in the correction of anemia in CKD (3–5) or dialysis?Qunibi et al.47 compared the efficacy and safety of high-dose i.v. FCM vs. oral Fe in 255 patients with ND-CKD. After 8 weeks, Hb increased more with FCM than oral Fe (1.31 g/dl vs. 0.83 g/dl, p < 0.01) and Hb increased ≥1 g/dl in a higher percentage of patients receiving FCM than oral ferrotherapy (60.4% vs. 34.7% of patients), regardless of whether they were receiving ESA. Similar results were observed in the PROGRESS study in 351 CKD-ND patients with i.v. Fe isomaltoside and similar period of follow-up.48
In the FIND-CKD study,49 the FCM i.v.-high ferritin group had higher Hb and ferritin levels than the other two groups, with no differences in terms of AEs or serious AEs between the different groups, although there was a higher rate of dropouts in the oral Fe group, especially due to gastrointestinal AEs.49 The meta-analysis by Shepshelovich et al.50 included 24 trials, 13 with 2369 patients with ND-CKD and 11 including 818 patients with CKD-5D. Patients treated with i.v. Fe were more likely to achieve an increase in Hb >1 g/dl and ferritin levels were significantly higher in all i.v. Fe groups vs. the oral Fe group. Regarding safety, the analysis demonstrated comparable rates of AEs and serious AEs between i.v. and oral Fe. However, as in many studies the limitation was that the follow-up periods were generally limited to three months, so there are insufficient data on the safety of pre-dialysis i.v. Fe administration in the medium to long term.
Only two randomized clinical trials in CKD patients have addressed the impact of i.v. Fe on outcomes other than anemia correction. The REVOKE study51 was a single-center, randomized, clinical trial that compared the effect of i.v. Fe sucrose versus oral ferrotherapy in 137 patients with CKD-ND on renal function as a primary event at 104 weeks. The study was stopped prematurely because of an increased risk of AEs in the group treated with i.v. Fe. However, some methodological limitations should be considered; most AEs occurred long after the intervention was completed, all AEs had been included (even if they were repeated in the same patient) instead of period of time to the first AE. Furthermore, the number of patients with severe AEs was similar in both groups and the difference was significant only after adjusting. The other clinical trial is the aforementioned PIVOTAL25 which demonstrated that the high-dose proactive i.v. Fe regimen was superior to the low-dose reactive regimen in reducing the rate of combined CV event and all-cause death in hemodialysis patients with anemia treated with ESAs. In this study, the proactive group achieved Hb targets earlier, ESA dose was reduced and was not associated to an increased risk of vascular access thrombosis, infections or hospitalization.
What is the safety profile of high-dose i.v. Fe compared to oral Fe or low-dose i.v. Fe in patients with CKD and anemia?In the PIVOTAL study,25 those patients randomized to receive high and proactive doses of i.v. Fe sacharose had a lower incidence of MACE than patients assigned to low reactive doses of i.v. Fe, with a similar safety profile, demonstrating the superiority of the proactive strategy in reducing CV events.
In the FIND-CKD study in patients with ND-CKD, already mentioned, there were no differences in safety between groups. Furthermore, a post hoc analysis showed that only 21.6% of patients treated with oral Fe achieved an increase in Hb ≥1 g/dl in 4 weeks and among those who did not respond in that time, less than 30% achieved it at the end of the study, at week 52.49 This finding would suggest that an early ineffective response to treatment with oral Fe could be a reason to consider an early switch to i.v. ferrotherapy.
In the FERWON-NEPHRO study, patients were randomized to receive either a single dose of 1 g Fe isomaltoside i.v. or up to 5 doses of 200 mg Fe suscrose i.v. over a two-week period. The high single dose of Fe isomaltóside i.v. induced non inferior hematologic response in 8 weeks with low and similar rates of hypersensitivity reactions with a significantly lower incidence of the composite CV adverse event (MACE, unstable angina, HF, atrial fibrillation, hypertension or hypotension).52
The REPAIR IDA study53 randomized to 2 doses of FCM 750 mg i.v. over 1 week vs Fe sucrose 200 mg i.v. administered in up to 5 doses during 14 day period. No differences were found in the composite safety event (CV death, infarction and stroke), but there were more episodes of transient hypertension in the FCM i.v. group. In terms of efficacy, more patients in the FCM group achieved an increase in Hb ≥1 g/dl.
With these data, it can be recommended that when choosing i.v. Fe therapy in non dialysis patients, the choice should be high dose and low frequency. As recommended by the National Institute of Health and Care Excellence (NICE) guidelines,31 high dose and low frequency is a scheme in which at least 500 mg of Fe is administered in each infusion, with a maximum of two infusions, a pattern that has been shown to be safe and effective.
Erythropoiesis-stimulating agents (ESA) and hypoxia-induceble factor stabilizers (HPI-HIF)The recommendations related to this section can be found in Table 2
Statements related to erythropoiesis-stimulating agents (ESAs) and hypoxia-induced factor stabilizers (HPI-HIFs).
| Q | Statement | Median, appropriateness, degree of agreement |
|---|---|---|
| 9 | There are no specific studies that individualize the Hb target for different patient profiles. Certain comorbidities in which the possible negative effects of high hemoglobin levels are known, such as diabetes, cardiovascular disease and cancer, among others, should be taken into account. The patient’s perspective should be incorporated into therapeutic decisions, according to his or her functional level and quality of life. | 9, appropriate, agree |
| 10 | It is estimated that around 12% of patients do not respond to ESAs or require a higher than usual dose of ESAs to achieve a given Hb level. This condition, known as ESA hyporesponsiveness or resistance, is associated with increased morbidity, mortality, and health care costs. | 9, appropriate, agree |
| 11 | Since there are no specific studies available that compare the impact of the intervention (suspend ESA, maintain or increase the dose) on clinical events, there is no consensus recommendation in the guidelines. KDIGO proposes not to continue increasing doses and ERBP recommends downward adjustment of the Hb target. In patients with ESA resistance (defined by a dose of 300 IU/kg/week of EPO or equivalent without reaching the target) and after correcting other associated causes (iron deficiency, inflammation, vitamin deficiencies, etc.) it is recommended to carefully evaluate the risk-benefit of side effects in each patient and not to continue increasing the ESA dose. | 8, appropriate, agree |
| 12 | Treatment with erythropoiesis-stimulating agents may be useful immediately after renal transplantation and should be used. This field of application constitutes an area of research for interventional studies. | 8, appropriate, agree |
| 13 | HIF stabilizers seem to show better management of ferrokinetics than ESAs, with a later use and lower doses of i.v. Fe or similar results in patients with partial Fe deficiency. These results need to be confirmed in their wide use in clinical practice. | 8, appropriate, agree |
| 14 | HIF stabilizers, by blocking hepcidin production, show a better response in patients with mild or moderate inflammation. These results need to be confirmed in i populations with the comorbidity and variability typical of routine clinical practice. | 8, appropriate, agree |
| 15 | There is insufficient evidence to define a stabilizing class effect of HIF on MACE as compared to ESAs. | 8, appropriate, agree |
ESA, erythropoiesis-stimulating agents; ERBP, European Renal Best Practice; Hb, hemoglobin; HIF, hypoxia-inducible factor; i.v., intravenous; MACE, Combined Event Mortality or infarction or stroke; Q, question.
According to the KDIGO 2012 guidelines, ESAs should be indicated in ND-CKD patients with Hb levels <10 g/dl and in CKD-5D patients with Hb of 9.0–10.0 g/dl. The maintenance target of Hb is 10–11.5 g/dl in the population of CKD patients.54 It is also stated that Hb levels >13 g/dl should not be intentionally achieved with ESA in adult patients.
Additionally, the European Renal Best Practice (ERBP) guidelines indicate that ESAs should be indicated when the Hb concentration is <10 g/dl in all patients with CKD, proposing individualization of both initiation (earlier in low-risk active individuals) and Hb targets, although without exceeding Hb of 12 g/dl in any case.
Finally, the recent NICE guidelines31 recommend individualizing the targets also taking into account the patients’ opinion, symptoms and comorbidities. They recommend an overall Hb target of 10–12 g/dl and do not escalate ESA doses indefinitely if pre-specified therapeutic targets are not achieved.
None of the three guidelines reviewed provide results of studies with specific objectives by patient profile.
What is the percentage of resistance to ESA in treated patients?Discussed in conjunction with question XI.
In patients with ESA resistance, should treatment with high doses of ESA be maintained, even if the Hb target is not achieved?
There is no universally accepted definition of ESA hyporesponsiveness or resistance. In practice, it means not achieving a target Hb concentration despite receiving a higher than usual dose of a given ESA, or requiring very high doses to maintain a target Hb level. The main guidelines define ESA hyporesponsiveness differently: NKF-KDOQI55 and NICE56 as erythropoietin doses higher than 300 or 450 U/kg/week if given s.c. or i.v. respectively, NICE also includes a darbepoietin dose >1.5 μg/kg/week (once a known cause of resistance such as chronic bleeding or maintained comorbidity has been ruled out). The KDIGO guidelines6 define it as no increase in Hb after treatment with ESA for 1 month at appropriate doses according to weight.
Despite the absence of a precise definition of hyporesponsiveness to ESA, it has been used the ratio between the epoetin dose in IU/kg/week (or the equivalent for other ESA) and the Hb concentration in g/dl, known as the erythropoietic resistance index (ERI). Resistance is defined as an ERI greater than 12,720 IU per week of epoetin/kg body weight/g/dl of Hb.57
The prevalence of resistance to ESA treatment in patients with CKD and anemia will vary depending on the definition and the population studied. For example, recent prevalence of ESA resistance estimates a range from 12.5% (when both Hb level and ESA dose were included in the definition57), to 30.3% (when it is only considered the change from baseline in Hb with baseline Hb <11 g/dl58). Regardless of the definition, hyporesponsiveness to ESA is associated with increased all-cause mortality59–63 probably as a consequence of underlying comorbidity. Recently, a similar association has been demonstrated in HF patients treated with ESAs.64 However, a recent analysis of the DOPPS study demonstrates that ESA resistance in most cases is transient and improves with correction of its causes.65
NICE guidelines recommend considering discontinuation of ESA therapy in cases of resistance in patients requiring frequent transfusions and high doses of ESA, provided that all reversible causes of ESA resistance have been ruled out and treated.31
Increasing the dose of ESA does not improve prognosis in patients with high ERI. In fact, several studies show that the use of high-dose ESA can produce adverse clinical outcomes. In the CHOIR study,66 patients with ND-CKD were randomized to achieve a target Hb of 11.3 or 13.5 g/dl with epoetinα given once weekly. The incidence of the primary outcome (combined death, HF, stroke, and acute myocardial infarction) was higher in patients assigned to the higher Hb target. Further analysis showed that patients receiving high doses of epoetinα (≥20,000 U/week) had more adverse events.67 Failure to achieve target Hb and protocolized use of high-dose epoetin were associated with an increased risk of the combined event in unadjusted analyses. However, in adjusted models, only high-dose epoetinα was associated with a significantly increased risk of reaching the combined event, suggesting that the use of high-dose ESAs in the context of hyporesponsiveness may be more harmful than beneficial. In the TREAT study in patients with diabetes mellitus, treatment with darbepoetin-α in patients assigned to the high Hb correction group (Hb of 13.0 g/dl) doubled the risk of stroke as compared to placebo,68 and in those with baseline hyporesponsiveness was associated with an increased cardiovascular risk.60 Similar results were observed in the RED-HF study in HF patients using the TREAT inclusion criteria.69 These data suggest that the risks of administering very high doses of ESAs outweigh the benefits in patients with ESA hyporesponsiveness,70 and support the recommendations of the KDIGO guidelines of not to increase ESA doses indiscriminately in case of hyporesponsiveness.6
Is it convenient to maintain ESAs in the immediate post-transplant period to improve evolution?Post-transplant anemia (PTA) affects 30%–45% — of renal transplant recipients and it is associated with increased morbidity71–73 but only a minority of them receive ESA treatment.73 The reasons could be the lack of clear evidence on the risk-benefit of the therapy, as well as the cost of the treatments. At present there are no specific recommendations for the treatment of PTA.6
The neo-PDGF study randomized 104 patients The use of high-dose epoetinβ (4 doses of 30,000 IU, approximately 430 U/kg and dosed over 2 weeks) during the first 2 weeks post-transplant significantly increased Hb levels 1 month post-transplant compared to the no administration (mean Hb 11.1 vs. 10.5 g/dl; p = 0.038) but with no significant difference in the incidence of initial graft dysfunction or glomerular filtration rate.74 Similarly, intraoperative administration of high-dose epoetinα (40,000 IU single dose, approximately 570 U/kg) to 72 renal transplant recipients did not improve the incidence of baseline graft dysfunction, renal function at 1 month, or Hb at 1 month post-transplant.75 The limited sample size of these studies precludes a proper assessment of the potential risks of such high doses of ESA.
In a retrospective cross-sectional cohort study of 1794 renal transplant recipients, anemia (Hb <12.5 g/dl) was significantly associated with increased mortality. In patients without ESAs, spontaneous increase in Hb was associated with decreased all-cause mortality. In ESA-treated patients, improvement in anemia (Hb up to 12.5 g/dl) was also associated with decreased mortality, although achieving Hb >14 g/dl significantly increased the risk of death.76 In the CAPRIT trial,77 125 renal transplant recipients were randomized to epoetinβ with Hb targets of 13–15 g/dl (full correction) or 10.5–11.5 g/dl (partial correction). Compared — with the partial correction group, the complete correction group had a smaller decrease in estimated creatinine clearance (5.9 vs. 2.4 ml/min/1.73 m2), a lower rate of terminal CKD (21% vs. 4.8%), and longer graft survival (80% vs. 95%). There was also a significant improvement in QoL. With respect to CV safety there were no cardiac events (HF, arrhythmia or myocardial infarction) in the complete correction group as compared to the partial correction group (4 patients, 8%). These data are consistent with a study in Japan of 127 kidney transplant recipients treated with ESA for three years.78 More recently, another trial has been published in 55 kidney transplant patients with PTA at three months.79 Patients were randomized to receive epoetinβ with a target of 11.5–13.5 g/dl or no treatment for anemia. After two years of follow-up there were no differences in the progression of renal function between the two groups, with a similar rate of decline in glomerular filtration rate; there were also no differences in proteinuria or blood pressure control. A more marked improvement in QoL was indeed observed in the ESA-treated group. The small number of participants in these trials does not allow to obtain adequate information on the CV safety in trasplanted patients with higher Hb target levels.
The results of these studies, as well as those from the large observational study by Heinze et al.76 suggest that the optimal target Hb level in APT is higher than the suggested target in CKD and should probably be up to 12–13 g/dl. A larger clinical trial, designed like the CAPRIT study, with longer follow-up could help to define the target Hb level. The ALERT study demonstrates that anemia is a risk factor of renal graft loss but not for CV morbidity and mortality; Hb levels are inversely associated to graft loss (HR 0.86 [0.80–0.92] per 1 g/dl Hb p < 0.001).80
Thus, although the data on ESA treatment of anemia in post-transplant kidney patients suggest that it is benefitial, the advantage of maintaining ESAs in the immediate post-transplant period requires specific studies to substantiate its benefit.
Is there a differential effect on ferrokinetics of HIF stabilizers versus ESAs?There is pathophysiological support for the effect of hypoxia inducible factor (HIF) prolyl hydroxylase inhibitors (HPI), also called HIF stabilizers, on ferrokinetics. HIF stabilizers increase HIF expression, allowing its dimerization and translocation to the cell nucleus. This triggers gene activation that promotes an increase in the production of EPO and expression of its receptor, and other actions on ferric metabolism such as increased production of iron transporters in the cell membrane (DMT1, DcytB), increased levels of transferrin and its receptor, and blockade of hepcidin,30,81,82 favouring the absorption and mobilization of Fe and its transport to the marrow for erythropoiesis.
A recent meta-analysis included a total of 30 studies with 13,146 patients treated with roxadustat, daprodustat, vadadadustat, molidustat, desidustat or enarodustat. HIF stabilizers reduced hepcidin, ferritin and serum iron levels, while total iron binding capacity and transferrin levels increased versus those in the placebo or ESA group.83 However, the results with the different molecules (such as daprodustat or vadadustat) are not uniform which could be due to differences between the molecules or differences in protocol design.
Is there an independent effect of inflammation on the correction of anemia by HIF-stabilizers (IPH-HIF)?Preclinical studies demonstrate that increased HIF expression reduces hepcidin levels, an acute phase reactant that is elevated in CKD and has been implicated in functional Fe deficits.84 A recent meta-analysis demonstrates that treatment with HIF stabilizers can reduce hepcidin levels, both versus placebo and ESA.83 In the studies compared with placebo the reduction of hepcidin is maintained in all subgroups. For both reasons we can speak of a class effect.
In the studies of roxadustat in ND-CKD, patients were stratified by C-reactive protein (CRP) quintiles and it was shown that they maintained the results of efficacy and dosing in all subgroups. Similarly, while patients with high baseline CRP treated with roxadustat maintained the same dose, those receiving ESAs required dose increases during follow-up.85
In the vadadustat studies, there was no correlation between baseline CRP levels and Hb increase.86
It should be clear that the phase 3 studies were not designed to measure the erythropoietic response in patients resistant to ESAs and that their post-hoc analysis,83 only highlights a lower impairment of the erythropoietic response by intercurrent inflammatory events in patients treated with HIF stabilizers.
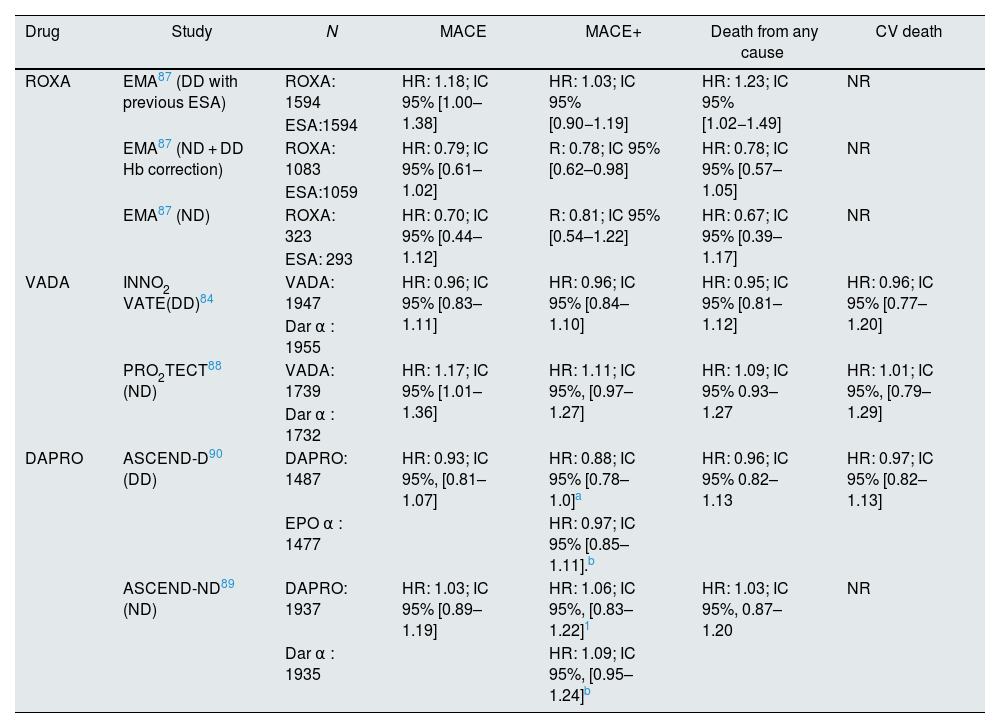
Can we consider that there is a class effect (HIF stabilizers) in terms of response in Major Adverse Cardiovascular Events (MACE)?There are no comparative CV safety studies between the different HIF stabilizers. Table 3 summarizes results on CV events of the different clinical trials of HIF stabilizers. After the results of the different clinical trials and analysis by the regulatory agencies84,87–90 it can be concluded that the different molecules present a non-inferiority profile with respect to MACE in DD patients, but their results in ND are more controversial. This fact would justify further studies in order to guarantee the safety of these molecules in all patients with CKD.
Summary of results obtained in clinical trials with HIF stabilizers compared to ESAs.
| Drug | Study | N | MACE | MACE+ | Death from any cause | CV death |
|---|---|---|---|---|---|---|
| ROXA | EMA87 (DD with previous ESA) | ROXA: 1594 | HR: 1.18; IC 95% [1.00–1.38] | HR: 1.03; IC 95% [0.90−1.19] | HR: 1.23; IC 95% [1.02−1.49] | NR |
| ESA:1594 | ||||||
| EMA87 (ND + DD Hb correction) | ROXA: 1083 | HR: 0.79; IC 95% [0.61–1.02] | R: 0.78; IC 95% [0.62–0.98] | HR: 0.78; IC 95% [0.57–1.05] | NR | |
| ESA:1059 | ||||||
| EMA87 (ND) | ROXA: 323 | HR: 0.70; IC 95% [0.44–1.12] | R: 0.81; IC 95% [0.54–1.22] | HR: 0.67; IC 95% [0.39–1.17] | NR | |
| ESA: 293 | ||||||
| VADA | INNO2 VATE(DD)84 | VADA: 1947 | HR: 0.96; IC 95% [0.83–1.11] | HR: 0.96; IC 95% [0.84–1.10] | HR: 0.95; IC 95% [0.81–1.12] | HR: 0.96; IC 95% [0.77–1.20] |
| Dar α : 1955 | ||||||
| PRO2TECT88 (ND) | VADA: 1739 | HR: 1.17; IC 95% [1.01–1.36] | HR: 1.11; IC 95%, [0.97–1.27] | HR: 1.09; IC 95% 0.93–1.27 | HR: 1.01; IC 95%, [0.79–1.29] | |
| Dar α : 1732 | ||||||
| DAPRO | ASCEND-D90 (DD) | DAPRO: 1487 | HR: 0.93; IC 95%, [0.81–1.07] | HR: 0.88; IC 95% [0.78–1.0]a | HR: 0.96; IC 95% 0.82–1.13 | HR: 0.97; IC 95% [0.82–1.13] |
| EPO α : 1477 | HR: 0.97; IC 95% [0.85–1.11].b | |||||
| ASCEND-ND89 (ND) | DAPRO: 1937 | HR: 1.03; IC 95% [0.89–1.19] | HR: 1.06; IC 95%, [0.83–1.22]1 | HR: 1.03; IC 95%, 0.87–1.20 | NR | |
| Dar α : 1935 | HR: 1.09; IC 95%, [0.95–1.24]b |
ESA, erythropoiesis-stimulating agent; DAPRO, daprodustat; Dar: darbepoietin; DD, dialysis-dependent CKD (hemodialysis or peritoneal dialysis); EPO, erythropoietin; ND, non-dialysis-dependent CKD; MACE, combined event mortality or stroke; ROXA, roxadustat; VADA, vadadustat.
The DELPHI work performed has made it possible to review the available evidence on current treatments in the management of renal anemia and to formulate recommendations agreed upon by experts based on a systematic review of the literature. Additionally, we recommend further studies with the main objective of evaluating the impact of anemia treatment on health-related quality of life and progression of CKD, and also analyze the effects of FeD correction independently of anemia on the prognosis of these patients. The novel mechanism of action of HIF stabilizers opens a new stage for research in this field and offers a therapeutic alternative that requires further studies to evaluate its potential benefits (e.g., reduced need for ferrotherapy, ESA-resistant patients).
FinancingVifor Pharma and Astellas have provided the S.E.N. with an unconditional grant for the development of this project, and in no way have been involved in the choice of PICO questions and bibliographic selection. They have not been present in the discussions during the development of the project.
Author/collaboratorsAll authors have made substantial contributions in each of the following aspects: 1) conception and design of the study, or analysis and interpretation of the data, 2) drafting of the article or critical revision of the intellectual content, 3) final approval of the version presented.
Conflict of interestJose Portolés reports having participated in pivotal studies of anemia-related drugs for Janssen-Cilag, Roche, AMGEN, Vifor Pharma, Astellas, Otsuka and GSK; for having participated in trainng and consulting activities for Astellas, GSK, Otsuka and Sanofi, Novartis and Vifor Pharma. Alejandro Martín Malo declares having received fees for conferences and consultancies from AstraZeneca, Astellas, Baxter, Medtronic and Vifor Pharma. Leyre Martín reports receiving payment or consulting fees from Astellas and BD-Bard; research grant funding from Vifor Pharma. Support for meeting attendance from Baxter and Vifor Pharma. Gema Fernández-Fresnedo declares having received payment or honoraria for conferences and presentations from Vifor Pharma, AstraZeneca, Novo Nordisk, Novartis, Boehringer-Ingelheim. Patricia de Sequera declares that she has received payment or honoraria for lectures, presentations from Vifor Pharma, Amgen, Fresenius, AstraZeneca, GSK, Braun and Baxter. Support for attendance to meetings of Nipro, Vifor Pharma, Amgen, Fresenius, AstraZeneca and Baxter. For participation in Advisory Board of Astellas, Vifor Pharma, Baxter, AstraZeneca. J. Emilio Sánchez declares having received payment or fees for conferences, presentations from Vifor Pharma, Amgen, AstraZeneca, Novo Nordisk, Astellas and Baxter and for participation in the Advisory Board of Astellas, Vifor Pharma and GSK.
Alberto Ortiz-Arduan declares having received funding in the form of a research grant from Sanofi and for consultancy, conference or meeting attendance from Advicciene, Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex and Vifor Fresenius Medical Care, Menarini, Mundipharma, Kyowa Kirin, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex and Vifor Fresenius Medical Care Renal Pharma and is Director of the Mundipharma-UAM Chair of Diabetic Kidney Disease and the AstraZeneca-UAM Chair of CKD and Alterations of CKD.
Aleix Cases declares having received research grant funding from Vifor Pharma, by consultancy from Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, GSK, Novo Nordisk, Otsuka and Vifor Pharma, by lectures from: Astellas, AstraZeneca, Amgen, Bayer, BMS, Medscape, Novo Nordisk, Sanofi Mexico, Vifor Pharma, as well as other concepts from Arbor Research, Astellas, Boehringer-Ingelheim, Diaverum, GSK, Novo Nordisk, Otsuka, Sociedad Española de Nefrología and Vifor Pharma.
The panelists members of the DELPHI-GAS study group include the following co-authors: José Luis Gorriz MD PhD Prof H. Clínico de Valencia; José Herrero MD PhD H. Clínico de Madrid; Manuel Macia MD PhD Hospital Universitario Nuestra Señora de Candelaria; Marco Montomoli Hospital Clínico Universitario de Valencia; Miguel Pérez Fontán MD PhD Prof C.H.U A Coruña-CHUAC; María Auxiliadora Bajo MD PhD Prof H.U La Paz, Madrid; Borja Quiroga MD PhD H. U. La Princesa, Madrid; Sagrario Soriano MD PhD H. U Reina Sofía Córdoba; Nuria Areste MD PhD Hospital Universitario Virgen Macarena; Marta Crespo MD PhD H. del Mar Barcelona; Marta Cesarpo MD PhD H. del Mar Barcelona; Marco Montomoli Hospital Universitario del Mar. La Princesa, Madrid; Cesar Remón MD PhD Hospital Puerta del Mar, Cádiz The authors would like to acknowledge the support and collaboration of Medical Statistics Consulting (MSC) in the development of the Delphi methodology, as well as the collaboration of María Giovanna Ferrario, PhD and Antoni Torres-Collado, PhD (MSC) in the coordination and editing of the manuscript.