Early biomarkers search for Diabetic Kidney Disease (DKD) in patients with Type 2 Diabetes Mellitus (T2DM), as genetic markers to identify vulnerable carriers of the disease even before Glomerular Filtration Rate (GFR) decline or microalbuminuria development, has been relevant during the last few years.
The rs5186 (A116C) polymorphism of the Angiotensin II Receptor Type I gene (AGTR1), has been associated to multiple effects of renal injury risk, commonly detected in patients with Diabetes Mellitus (DM). It has been described that rs5186 could have an effect in stability proteins that assemble Angiotensin II Receptor Type I (AT1), modifying its action, which is why it should be considered as a risk factor for Chronic Kidney Disease (CKD), characterized by a GFR progressive reduction. Even though, the association between rs5186 AGTR1 gene polymorphism and DKD in patients with T2DM has been controversial, inconclusive, and even absent. This disputable issue might be as a result of association studies in which many and varied clinical phenotypes included are contemplated as CKD inductors and enhancers. Although, the sample sizes studied in patients with T2DM are undersized and did not have a strict inclusion criteria, lacking of biochemical markers or KDOQI classification, which have hindered its examination.
ObjectiveThe aim of our study was to establish an association between rs5186 AGTR1 gene polymorphism and GFR depletion, assessed as a risk factor to DKD development in patients with T2DM.
MethodsWe analyzed 297 not related patients with T2DM, divided into 221 controls (KDOQI 1) and 76 cases (KDOQI 2). Arterial pressure, anthropometric and biochemical parameters were measured. rs5186 of AGTR1 genotyping was performed by TaqMan assay real-time PCR method. Allele and genotype frequencies, and Hardy-Weinberg equilibrium were measured. Normality test for data distribution was analyzed by Shapiro-Wilk test, variable comparison by Student’s t-test for continuous variables, and Chi-squared test for categorical variables; ANOVA test was used for mean comparison of more than two groups. Effect of rs5186 to DKD was estimated by multiple heritability adjustment models for risk variables of DKD. Statistical significance was indicated by p<0.05. Data was analyzed using Statistical Package STATA v11 software.
ResultsDominant and Over-dominant models showed a likelihood ratio to GFR depletion of 1.89 (1.05−3.39, p=0.031) and 2.01 (1.08−3.73, p=0.023) in patients with T2DM. Risk factor increased to 2.54 (1.10−5.89) in women in Over-dominant model.
ConclusionIn clinical practice, most of nephropathies progress at a slow pace into a total breakdown of renal function, even asymptomatic. This is the first study, reporting that rs5186 polymorphism of AGTR1 gene contribution to GFR depletion, and this could be evaluated as a predisposing factor for DKD in patients with T2DM.
La búsqueda de biomarcadores tempranos de Enfermedad Renal Diabética (ERD) en pacientes con Diabetes Mellitus tipo 2 (DMT2) como los marcadores genéticos para identificar pacientes vulnerables de la enfermedad incluso antes de la presencia de una disminución de la Estimación de Tasa de Filtrado Glomerular (TFGe) o presencia de microalbuminuria ha cobrado importancia en los últimos años.
El polimorfismo rs5186 (A1166C) presente en el gen Receptor Tipo 1 de la Angiotensina II (AGTR1) ha sido asociado a distintos efectos del riesgo de daño renal que suelen estar presentes en pacientes con Diabetes Mellitus (DM). Se ha descrito que el rs5186 podría influir en la estabilidad de las proteínas que conforman al Receptor de la Angiotensina II Tipo 1 (AT1) alterando su actividad, por lo que podría ser considerado como un factor de riesgo a Enfermedad Renal Crónica (ERC) caracterizada por una disminución progresiva de la TFG. Sin embargo, la asociación del polimorfismo rs5186 del gen AGTR1 con ERD en pacientes con DMT2 ha sido controversial, no concluyente, incluso nula. Las controversias podrían ser debidas a que los estudios de asociación y estimación del riesgo del rs5186 previamente reportados incluyen distintos fenotipos clínicos considerados como inductores y potenciadores de ERC, además, los tamaños de las muestras analizadas en pacientes con DMT2 eran pequeñas y no tenían un control estricto en su inclusión, careciendo incluso de marcadores bioquímicos o estadificación KDOQI que han dificultado su análisis.
ObjetivoDeterminar la asociación del rs5186 del gen AGTR1 con la disminución de TFGe considerada como riesgo al desarrollo de ERD en pacientes con DMT2.
Material y métodosSe analizaron 297 pacientes con DMT2 no emparentados, divididos en 221 controles (KDOQI 1) y 76 casos (KDOQI 2). Se determinaron parámetros de presión arterial, antropometría y bioquímicos. El genotipado del rs5186 del gen AGTR1 se realizó por PCR en tiempo real con uso de sondas TaqMan. Se determinaron frecuencias alélicas, genotípicas y equilibrio de Hardy-Weinberg. El análisis del comportamiento de los datos se determinó con Shapiro–Wilk, la comparación de las variables por t de Student para variables continuas, y chi-cuadrada (x2) para variables categóricas; se usó prueba ANOVA para comparación de medias de más de dos grupos. El efecto del rs5186 a disminución de TFGe se realizó por distintos modelos de herencia ajustados por variables de riesgo a ERD. Se consideró un valor de p<0.05 como estadísticamente significativo. Se utilizó el software Statistical Package STATA v11.
ResultadosLos modelos dominante y sobredominante mostraron un riesgo a la disminución de la TFGe de 1.89 (1.05−3.39, p=0.031) y 2.01 (1.08−3.73, p=0.023) en pacientes con DMT2. El riesgo aumentó a 2.54 (1.10−5.89) en el modelo Sobre dominante en mujeres.
ConclusiónEn la práctica clínica, la mayoría de las nefropatías progresan lentamente hacia la pérdida definitiva de la función renal, cursando incluso asintomáticas. El presente estudio es el primero en reportar una contribución del rs5186 del gen AGTR1 con una disminución de la TFGe y podría ser considerado como un riesgo a ERD en pacientes con DMT2.
It has been reported that the prevalence of chronic kidney disease (CKD) in adult population worldwide ranges from 9 to 13%. In 2017, Mexico reported a prevalence of CKD of 12.2%, with a mortality rate of 51.4% per 100 thousand inhabitants, being one of the countries with the highest prevalence and mortality rates associated with CKD in the world.1,2
It has been described that the most important etiological cause associated with CKD is diabetic kidney disease (DKD), mainly secondary to uncontrolled type 2 diabetes mellitus (T2DM). The metabolic and hemodynamic changes caused by hyperglycemia, hyperinsulinism and the accumulation of advanced glycation products3 characteristic of chronic uncontrolled diabetes mellitus (DM), lead initially to a gradual decrease in the estimated glomerular filtration rate (eGFR) and subsequently produces a severe structural and functional alterations of the renal microvasculature, conditioning a potential risk of advanced chronic kidney disease (ACKD).4
In recent years it has been observed that the use of angiotensin II receptor antagonist drugs (ARA-II) can delay and reduce the frequency of ACKD,5 however, not all patients with ESRD have responded well to this intervention; which may suggest that genetic factors could be involved as triggers or enhancers to the risk, development and progression of CKD.
The rs5186 (A1166C) polymorphism present in the angiotensin II type 1 receptor gene (AGTR1) has been associated with different effects on the risk of renal damage that are usually present in patients with DKD and diabetic nephropathy (DN).6 Different studies have reported that rs5186 influences the stability and activity of the proteins that make up the angiotensin II type 1 receptor (AT1),7 although there is still controversy regarding its participation and whther it should be considered as a risk factor for kidney damage in patients with DKD.
Association studies of rs5186 of the AGTR1 gene with decreased eGFR associated with DKD, ND or CKD phenotypes in patients with T2DM are scarce, controversial and inconclusive, it has been suggested that the heterogeneity of results is due to age of onset, severity and progression of symptoms, as well as sexual dimorphism, ethnicity, environmental factors and epigenetic aspects.8
The aim of the present work was to estimate the risk of the presence of rs5186 of the AGTR1 gene in the decrease in eGFR considered as a risk for DKD in a sample of patients with T2DM in Mexico City. The type of approach of the present study would be of great importance, since it could guide us in the future in the predictive and personalized management of DRD, even in stages prior to the presence of its clinical manifestations. To date, there are no reported studies of this type in the Mexican or world population.
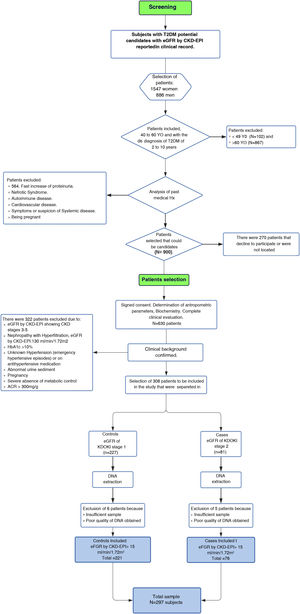
Material and methodsParticipantsThe study was conducted from 2017 to 2019 in patients of to the Family Medicine Unit number 31 (UMF 31) of the Mexican Institute of Social Security (IMSS) in Mexico City. For the identification of patients with KDOQI stages 1–2 we initially performed a screening and subsequently patients were selected (Fig. 1Flowchart).
Screening. Screening was performed in 2,443 adult patients with a diagnosis of T2DM who reported eGFR in the last three months according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 classification in the clinical record, which we considered as the baseline measurement. Patients were included in this phase of the study if they were between 40 and 60 years of age, as well as if the period of evolution of T2DM had been more than 5 and less that 15 years. Related patients were excluded, as well as those who reported other pathological conditions associated with alterations in eGFR or risk of ACKD, including KDOQI stages 3–5 considered as moderate, severe and advanced renal failure respectively according to the KDOQI guidelines,9 who reported electrolyte imbalance or other alterations of tubular origin, evidence of histological or structural damage by imaging tests, patients with use of renal replacement therapy in peritoneal dialysis, hemodialysis, renal transplantation, a rapid increase in proteinuria, nephrotic syndrome, presence of active urinary sediment, history of autoimmune diseases or signs and symptoms of another systemic disease, patients who reported arterial hypertension (HTN) with pharmacological treatment or without being controlled, history of acute myocardial infarction and cardiomyopathies. Patients who were candidates for screening were contacted, excluding those who did not wish to participate.
Selection of patients. Patients were selected for the study after signing a letter of informed consent. A total of 630 screened patients were selected. Anthropometric and biochemical parameters and eGFR were determined by the CKD-EPI 2009 formula to corroborate and select the stage of renal function evaluated three months earlier and reported in the clinical record. Finally, our study included a total of 297 unrelated patients, which were divided into two groups; 76 patients as cases for the presence of discrete or mild renal impairment presenting a decrease in GFRe ≤90 and ≥60mL/min/1.73m2 (KDOQI 2) and 221 were control patients considered to have a normal kidney filtration (≥90mL/min/1.73m2, KDOQI 1) using the CKD-EPI 2009 formula.
Patients with normal or slightly elevated (<30mg/g) or moderately increased (≥30 and <300mg/g) creatinine albumin coefficient (ACR) were included; patients with severely elevated ACR (≥300mg/g) were excluded.10 The study included 37 patients with HTN without severe uncontrolled hypertension and without the use of pharmacological treatment.
The present study is derived from the protocol under the title Study of Polymorphisms associated with chronic renal failure in Mexican patients with type 2 diabetes with registration number R-2016-785-022, authorized by the Research Ethics Committee of the Coordination of Health Research of the Centro Medico Nacional Siglo XXI (CONBIOETICA-09-CEI-009-20160601, respecting the guidelines and regulations of the Declaration of Helsinki.11
Anthropometric and biochemical measurementsWeight and height were determined using a digital scale and stadiometer (Seca, Hamburg, Germany). Body Mass Index (BMI) was calculated by the formula weight (kg)/height (m2) according to cut-off points described by the World Health Organization (WHO). Abdominal circumference was determined using a non-distensible tape measure (Seca, Umfangmessband 203cm, Hamburg, Germany), measured at the equidistant point between the lower edges of the ribs and the anterosuperior iliac crest.12 Blood pressure (BP) was determined by measuring systolic (SBP) and diastolic (DBP) blood pressure using a mercury sphygmomanometer (ALPK2, Tokyo, Japan) according to the guidelines described by the Eighth Joint National Committee for the Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 8).13
Each participant had blood drawn by venous puncture after an 8-h fast to determine serum concentrations of glucose, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, glycated hemoglobin (HbA1c%), urea, blood urea nitrogen (BUN), creatinine, and urinary albumin (UA), triglycerides, glycosylated hemoglobin (HbA1c%), urea, blood urea nitrogen (BUN), creatinine and urinary albumin (UA), the latter determined from a random urine sample, with the ILab 300 plus Clinical Chemistry System (Instrument Laboratory, Bedford, MA, USA).
The determination of serum insulin (μU/mL) was performed by chemiluminescence (IMMULITE® 1000 Immunoassay System, Siemens Healthcare Diagnostics). Insulin resistance (IR) was calculated by the Homeostatic Model Assessment (HOMA-IR) formula, being determined by the formula (fasting glucose [mg/dL]) (fasting insulin [μU/mL]/405).14 The ACR was performed by means of the albumin/creatinine formula recommended by the KDIGO guidelines.10 Cardiovascular risk (CVR) was estimated using the Framingham SCORE equation.15
DNA extraction, quantification, purity and genotypingDeoxyribonucleic acid (DNA) was extracted from peripheral whole blood using the AutoGen Flex Star kit (AutoGen, MA, USA) with QiagenFlexi Gene DNA AGF3000 reagents (QIAamp DNA Blood Midi/ Kit, Qiagen, Germany) according to the manufacturer's recommended specifications. DNA purity was determined by 260/280nm ratio spectrophotometry (BioTek Instruments, Winooski, VT, USA) and integrity by 0.8% agarose gel electrophoresis with SYBR Safe DNA Gel Stain (Thermo Scientific).
Genotyping was performed using 20ng/μL of DNA from each sample in a reaction mixture containing: Maxima Probe/ROX qPCR Master Mix (Thermo Scientific) and Taqman probe specific for the rs5186 polymorphism of the AGTR1 gene (C_3187716_10, TGCAGCACTTCACTACACCAAATGAGC[A/C]TTAGCTACTACTTTTTTCAGAATTGAAGGA Applied Biosystems CA, USA). The PCR reaction was performed on the 7900HT Fast Real-Time PCR system (Applied Biosystems, CA, USA) and allelic discrimination was performed with the Allelic Discrimination program of the SDS v2.4 software (Applied Biosystems).
Sample calculation and statistical powerThe total estimated sample was 324 patients, with a statistical power of at least 80% and a 95% confidence level, calculated according to the minor allele frequency (MAF) C of 0.29 of rs5186 reported for populations with Native American and European ancestry from the ALFA project (Latin American 2, https://www.ncbi.nlm.nih.gov/snp/rs5186?horizontal%20tab=true#seq_hash, Reference accessed and updated July 26, 2022),16 with an OR risk of 1.63 reported by Lin et al.,17 and a 3:1 ratio for controls and cases, respectively.
The total sample analyzed in our post-screening study was 297 patients, according to our results we obtained a statistical power of 83.4% and a safety level of 70%, taking into account the 3:1 ratio for controls and cases, respectively.
Statistical analysisAfter the analysis of the distribution of the data by the Shapiro-Wilk test, the comparison of the variables between groups was performed using Student's t-tests (parametric) for continuous variables and the X test2 for categorical variables. Comparison of anthropometric, biochemical and renal function parameters according to genotype was performed by ANOVA test for comparison of means of more than two groups. Allele frequencies, genotypic frequencies and Hardy-Weinberg equilibrium (EWH) of rs5186 were calculated and compared by X test2 for difference of proportions. Logistic regression analysis was used to calculate the risk to DKD defined as eGFR ≤90 and ≥60mL/min/1.73m2 (KDOQI stage 2), in relation to the risk effect of the presence of rs5186 in different genotypes determined in five inheritance models: codominant, dominant, recessive, overdominant and additive, which are expressed as Odds Ratio (OR) and regression coefficients adjusted for age, gender, BMI, DBP, SBP and ACR considered as variables to the risk of DKD.
The codominant model describes that each genotype provides a different and non-additive disease risk. The dominant model refers to the fact that a single copy of the C allele of rs5186 of the AGTR1 gene would be sufficient to modify the risk and being a carrier of two copies modifies it in equal magnitude. The recessive model estimates the risk modification to DKD if only CC are present, i.e. heterozygotes (AC) and homozygotes of the highest frequency allele (AA) would have the same risk, so that the CC genotype is compared with AC+AA. The overdominant model describes that individuals homozygous for the most frequent genotype (AA) and the variant genotype (CC) of the rs5186 of the AGTR1 gene will have the same risk. Finally, the additive model refers to each copy of C modifies the risk by an additive amount, therefore, CC homozygotes have twice the risk of AC heterozygotes. For all tests, a value of p<0.05 was considered statistically significant. Statistical Package STATA v11 software (Stata Corporation, Texas) was used for statistical analysis.
ResultsCharacteristics of the study sampleThe general characteristics of the population analyzed are presented in Table 1. The selection of the KDOQI 1 and 2 groups was made according to the confirmation of eGFR as described in the methodology. There were no statistically significant differences in age and gender between groups, as well as in different variables associated with the risk of decreased eGFR or renovascular complications, such as age at diagnosis of T2DM, time of evolution of HTN and T2DM, fasting glucose and insulin concentrations, HbA1c%, HOMA-IR, lipid parameters (TC, C-HDL and C-LDL), ACR and CVR.
Clinical, anthropometric and biochemical characteristics of the study population.
| ControlsKDOQI 1(n=221) | CasesKDOQI 2(n=76) | Value of p | |
|---|---|---|---|
| Age (years)d | 51.9±7.3 | 53.4±6.6 | 0.1153 |
| Gender (%)eMaleFemale | 70 (31.7)151 (68.3) | 30 (39.5)46 (60.5) | 0.21540.2154 |
| BMI (kg/m2) | 29.9±4.8 | 33.3±4.5 | <0.0001f |
| Abdominal circumference (cm)d | 95.4±10.1 | 103.8±9.7 | <0.0001f |
| Diagnosis of hypertension (%)b | 26 (11.7%) | 11 (14.4%) | |
| Age at diagnosis of hypertension (years)b,a | 48.59±7.4 | 45±15.6 | 0.0083f |
| Time of evolution of HTN (years)b,d | 6.30±7.6 | 7.20±9.1 | 0.4015 |
| SBP (mmHg)c,d | 118.7±14.1 | 125.2±18 | 0.0014f |
| DBP (mmHg)c,d | 81.3±9.1 | 84.9±9.9 | 0.0041f |
| Age at diagnosis of T2DM (years)d | 45.0±7.1 | 45.7±6.5 | 0.4505 |
| Time of T2DM evolution (years)d | 6.6±4.9 | 7.8±5.5 | 0.0803 |
| Fasting glucose (mg/dL)d | 158.8±65.8 | 159±73.7 | 0.9824 |
| HbA1c% HbA1cd | 7.6±2.7 | 7.2±2.2 | 0.2566 |
| Fasting insulin (mU/L)d | 19.1±14.6 | 19.8±11.6 | 0.7472 |
| HOMA-IRd | 6.67±4.9 | 6.91±4.1 | 0.7402 |
| Total cholesterol (mg/dL)d | 189.5±43.6 | 191.4±49.4 | 0.7520 |
| C-HDL (mg/dL)d | 42.1±12.2 | 41.1±11 | 0.5297 |
| C-LDL (mg/dL)d | 128.8±30.3 | 128.8±36 | 1.0000 |
| Triglycerides (mg/dL)d | 200.3±130.3 | 202.3±116.6 | 0.9058 |
| Creatinine (mg/dL)d | 0.7±0.1 | 0.9±0.1 | <0.0001f |
| eGFR (mL/min/1.73m2)a,d | 117.1±15.19 | 86.6±12.94 | <0.0001f |
| eGFR (mL/min/1.73m2)d | 102.6±7.2 | 82.3±6.56 | <0.0001f |
| ACR (mg/g)d | 33.5±156.7 | 78.1±295.8 | 0.1229 |
| BUN (mg/dL)d | 12.8±3.6 | 15.1±4.1 | <0.0001f |
| Urea (mg/dL)d | 27.5±7.8 | 32.3±8.9 | <0.0001f |
| RCV (%)e | 12.1±6.2 | 13.6±6.3 | 0.7333 |
Data are presented as mean±standard deviation, frequencies and percentages n (%).
BMI: body mass index; HTN: hypertension; SBP: systolic blood pressure; DBP: diastolic blood pressure; T2DM: type 2 diabetes mellitus; HbA1c%: glycosylated hemoglobin %; HOMA-IR: homeostatic model for assessing insulin resistance; HDL-C: high-density lipoprotein cholesterol; C-LDL: low-density lipoprotein cholesterol; eGFR: estimated glomerular filtration rate by CKD-EPI formula; ACR: albumin/creatinine ratio; BUN: blood urea nitrogen; CVR: cardiovascular risk by Framingham SCORE.
There were no statistically significant differences (p≤0.05) in BMI and abdominal circumference parameters, with a excesive nutrition status and central obesity in patients with KDOQI 2, in addition, in this group of patients those diagnosed HTN despite having a younger age at diagnosis when compared to controls (p=0.0083) they were observed to have higher SBP and DBP than patients with KDOQI 1.
Analysis of renal damage markers, creatinine, urea and BUN were found to be increased in patients with KDOQI 2, while eGFR mL/min/1.73m2 by the CKD-EPI 2009 equation was higher in the control group, all variables showed statistically significant differences (p<0.0001). No significant differences were observed when comparing ACR in both groups (p<0.1229).
Although no statistically significant differences were observed in the estimation of CVR by Framingham SCORE, both groups are above the cut-off point of ≥10% which are considered to be at risk of coronary heart disease in patients with cardiometabolic disorders.
Modifications of the variables under study according to genotypeThe behavior of the variables under study according to genotype is shown in Table 2. Statistically significant differences were observed with an apparent increase in the CC genotype in BMI determinations (p=0.0483) and in Creatinine concentrations (p=0.0008) when compared to the AA and AC genotypes.
Analysis of differences in anthropometric and biochemical parameters with the presence of rs5186 genotypes of the AGTR1 gene.
| Genotypes | AA(n=151) | AC(n=112) | CC(n=34) | Value of p |
|---|---|---|---|---|
| BMI (kg/m2) | 30.4±4.7 | 30.7±5.2 | 32.2±5 | 0.0483c |
| Abdominal circumference (cm) | 96.8±10.3 | 97.6±10.8 | 100.5±11.3 | 0.0655 |
| Age at diagnosis of hypertension (years)a | 50.0±6.8 | 42.3±16 | 43.0±18.3 | 0.0003c |
| Time of evolution of HTN (years)‡ | 5.4±6.4 | 8.3±10.6 | 11.5±12 | 0.0001c |
| SBP (mmHg)b | 120±15.6 | 120.9±15.8 | 120±13.4 | 0.6474 |
| DBP (mmHg)b | 82.3±9.3 | 82.3±9.5 | 81.8±10.2 | 0.7823 |
| Age at diagnosis of T2DM (years) | 44.9±6.8 | 45.9±6.7 | 45.1±7 | 0.8787 |
| Time of evolution of T2DM (years) | 7±5.2 | 6.6±4.9 | 7.2±5.8 | 0.8438 |
| Fasting glucose (mg/dL) | 164±72.2 | 160±63.8 | 132.1±55.8 | 0.0167c |
| HbA1c % | 7.8±2.7 | 7.3±2.5 | 6.8±2.1 | 0.0491c |
| Fasting insulin (mU/L) | 18.2±10.6 | 19.06±11.6 | 26.23±18.7 | 0.0203c |
| HOMA-IR | 6.4±4.1 | 6.90±4.9 | 7.55±6.9 | 0.2783 |
| Triglycerides (mg/dL) | 195.2±122.4 | 219.4±145.2 | 164.4±53.7 | 0.0325c |
| Total cholesterol (mg/dL) | 187.5±47.6 | 193.5±45.5 | 189.7±31.5 | 0.3041 |
| C-HDL (mg/dL) | 42.4±12 | 40.2±12.2 | 44.5±10 | 0.0641 |
| C-LDL (mg/dL) | 126.5±36.1 | 131.0±36.7 | 132.1±25.7 | 0.3228 |
| Creatinine (mg/dL) | 0.7±0.1 | 0.7±0.1 | 0.8±0.1 | 0.0008c |
| Urea (mg/dL) | 28.8±8.9 | 28.5±7.3 | 29.2±9.1 | 0.6461 |
| BUN (mg/dL) | 13.4±4.1 | 13.3±3.4 | 13.6±4.2 | 0.6741 |
| eGFR (mL/min/1.73m2) | 97.67±11.83 | 97.81±10.73 | 95.52±11.60 | 0.0567c |
| ACR (mg/g) | 50.9±248.4 | 40.9±203.7 | 31.5±76.1 | 0.6535 |
| CVR (%) | 12.6±6.9 | 12.6±6.5 | 11.1±5 | 0.2377 |
Data are presented as mean±standard deviation.
BMI: body mass index; BMI: body mass index; HTN: hypertension; SBP: systolic blood pressure; DBP: diastolic blood pressure; DMT2: type 2 diabetes mellitus; HbA1c%: glycosylated hemoglobin %; HOMA-IR: homeostatic model for assessing insulin resistance; C-HDL: high-density lipoprotein cholesterol; C-LDL: low-density lipoprotein cholesterol; BUN: blood urea nitrogen; eGFR: estimated glomerular filtration rate by CKD-EPI formula; ACR: albumin/creatinine ratio; CVR: cardiovascular risk by Framingham SCORE.
No differences were observed in the SBP and DBP measurements; however, the three groups showed a tendency toward an increase in blood pressure. It was observed that the patients with a diagnosis of HTN who participated in the study and were carriers of the CC genotype had a lower age at diagnosis (p=0.0003) and a longer evolution (p=0.0001) of HTN when compared to carriers of the AA genotype.
Neither were significant differences in genotypes in relation to the diagnostic age and time of T2DM evolution, as well as in HOMA-IR. However, a greater increase in fasting glucose and HbA1c% concentrations was observed in subjects carrying the AA genotype (p≤0.05), although the presence of fasting hyperinsulinism (mU/L) was observed in all three genotypes, and it was greater in CC carriers as compared to AC and AA carriers with statistically significant differences (p=0.0203).
Analysis of the lipid profile showed that triglyceride concentrations were higher in the AC genotype (p=0.0325), whereas comparison of genotypes showed no differences in total cholesterol, C-LDL and C-HDL concentrations.
The analysis of renal damage markers only showed that creatinine concentrations were higher in CC carriers (p=0.0008) as compared to AA and AC genotypes, so eGFR was also lower in that group. The rest of the renal damage markers BUN, urea, ACR and CVR by Framingham SCORE (%) did not present statistical differences.
Association of the rs5186 variant of the AGTR1 gene with DKD riskThe analysis of allele frequencies, genotypic frequencies and the EHW of the total study sample is shown in Table 3. The allele frequencies (%) were similar to those reported for populations with Native American and European ancestry from the ALFA project (Latin American 2, https://www.ncbi.nlm.nih.gov/snp/rs5186?horizontal%20tab=true#seq_hash, Reference consulted and updated on July 26, 2022)16 without showing statistically significant differences when comparing the groups, in addition, it complied with the EHW (p=0.074).
Allele, genotypic and EHW frequencies of rs5186 (A1166C) of the AGTR1 gene of the total sample under study.
| Allele frequencies (%) | p | EHWp | Genotypic frequencies (%) | ||
|---|---|---|---|---|---|
| CasesKDOQI 2n=76 | KDOQI 1 Controln=221 | CasesKDOQI 2n=76 | ControlKDOQI 1n=221 | ||
| A110 (72.4) | A304 (68.8) | 0.406 | 0.074 | AA44 (57.9) | AA107 (48.4) |
| Ca42 (27.6)b | Ca138 (31.2)b | AC22 (28.9) | AC90 (40.7) | ||
| CC10 (13.2) | CC24 (10.4) | ||||
Data expressed in frequencies and percentages.
n: (%); EHW: Hardy-Weinberg equilibrium for X2.
The association and estimated effect (OR) of rs5186 of the AGTR1 gene on the decrease in eGFR is shown in Table 4. Logistic regression analysis of the dominant and overdominant models corrected for age, gender, BMI, DBP, SBP and ACR showed a risk of the presence of rs5186 of the AGTR1 gene with decreased eGFR of 1.89 (95% CI 1.05−3.39, p=0.031) and 2.01 (95% CI 1.08−3.73, p=0.023), respectively.
Association and risk estimation of rs5186 of the AGTR1 gene with decreased eGFR.
| Model | Genotypes | OR | Value of p |
|---|---|---|---|
| (A1166C) | (95% CI) | ||
| Codominant | A/A | 1 | 0.08 |
| A/C | 1.88 (1.10−3.93) | 0.06 | |
| C/C | 2.13 (1.12−4.05) | ||
| Dominant | A/A | 1 | 0.031a |
| A/C-C/C | 1.89 (1.05−3.39) | ||
| Recessive | 0.95 | ||
| A/A-A-A/C | 1 | ||
| C/C | 0.97 (0.41−2.33) | ||
| Overdominant | A/A | 1 | 0.023a |
| C/C | 2.01 (1.08−3.73) | ||
| Additive | A/C | 1 | 0.12 |
| C/C | 1.40 (0.91−2.16) |
eGFR: estimated glomerular filtration rate; OR (Odds Ratio). Logistic regression corrected for age, gender; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACC, albumin/creatinine ratio.
The analysis of allelic and genotypic frequencies, the EHW and the risk estimation of the decrease in eGFR due to the presence of rs5186 of the AGTR1 gene in women (46 cases vs. 151 controls) is shown in Table 5. The risk of the rs5186 variant of the AGTR1 gene of a decreased eGFR was observed in the overdominant model corrected for age, gender, BMI, DBP, SBP and ACR with an OR of 2.54 (95% CI 1.10−5.89, p=0.023).
Allele, genotypic and EHW frequencies of rs5186 (A1166C) of the AGTR1 gene in females.
| Cases | Control | Value of p | EHW | |
|---|---|---|---|---|
| KDOQI 2 | KDOQI 1 | p | ||
| n=46 | n=151 | |||
| Allele frequencies (%) | A | A | 0.684 | 0.06 |
| 67 (0.73) | 210 (0.70) | |||
| Ca | Ca | |||
| 25 (0.27)b | 92 (0.30)b | |||
| Genotype frequencies (%) | AA | AA | ||
| 28 (60.9) | 75 (49.7) | |||
| AC | AC | |||
| 11 (23.9) | 60 (39.7) | |||
| CC | CC | |||
| 7 (15.2) | 16 (10.6) |
| Association and risk estimation of rs5186 of the AGTR1 gene with decreased eGFR in women. | |||
|---|---|---|---|
| Models | Genotypes | OR | Value of p |
| (A1166C) | (95% CI) | ||
| Codominant | A/A | 1 | 0.09 |
| A/C | 1.80 (1.08−3.86) | 0.075 | |
| C/C | 2.51 (1.06−5.96) | ||
| Dominant | A/A | 1 | 0.095 |
| A/C-C/C | 0.93 (0.31−2.81) | ||
| Recessive | A/A-A-A/C | 1 | 0.49 |
| C/C | 0.68 (0.23−1.98) | ||
| Overdominant | A/A | 1 | 0.023c |
| C/C | 2.54 (1.10−5.89) | ||
| Additive | A/C | 1 | 0.38 |
| C/C | 1.27 (0.74−2.16) | ||
eGFR, estimated glomerular filtration rate; OR, Odds Ratio; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACR, albumin/creatinine ratio. Logistic regression corrected for age; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; ACC: albumin/creatinine ratio; EHW: Hardy-Weinberg equilibrium by X2.
Despite showing allele frequencies (%) without statistically significant and similar differences reported in the NCBI databases, in addition to EHW (p=0.064), no association of the rs5186 variant of the AGTR1 gene with decreased eGFR in men (30 cases vs. 70 controls) was observed in any of the risk estimation models corrected for age, gender, BMI, DBP, SBP and ACR (Table 6).
Allele, genotypic and EHW frequencies of rs5186 (A1166C) of the AGTR1 gene in males.
| Cases | Control | Value of p | EHW | |
|---|---|---|---|---|
| KDOQI 2 | KDOQI 1 | p | ||
| n=30 | n=70 | |||
| Allele frequencies (%) | A | A | ||
| 43 (0.72) | 94 (0.67) | 0.6791 | 0.64 | |
| Ca | Ca | |||
| 17 (0.28)b | 46 (0.33)b | |||
| Genotype frequencies (%) | AA | AA | ||
| 16 (0.53) | 32 (0.46) | |||
| AC | AC | |||
| 11 (0.37) | 30 (0.43) | |||
| CC | CC | |||
| 3 (0.10) | 8 (0.11) |
| Association and risk estimation of the of rs5186 of the AGTR1 gene with decreased eGFR in men. | ||||
|---|---|---|---|---|
| Models | Genotypes | OR | Value of p | |
| (A1166C) | (95% CI) | |||
| Codominant | A/A | 1 | 0.88 | |
| A/C | 1.12 (1.44−3.12) | 0.78 | ||
| C/C | 1.39 (0.55−3.52) | |||
| Dominant | A/A | 1 | 0.5 | |
| A/C-C/C | 1.35 (0.56−3.22) | |||
| Recessive | A/A-A-A/C | 1 | 0.96 | |
| C/C | 1.04 (0.25−4.34) | |||
| Overdominant | A/A | 1 | 0.51 | |
| C/C | 1.35 (0.55−3.30) | |||
| Additive | A/C | 1 | 0.60 | |
| C/C | 1.20 (0.61−2.32) | |||
eGFR, estimated glomerular filtration rate; OR, Odds Ratio; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACR, albumin/creatinine ratio. Logistic regression corrected for age; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; ACR: albumin/creatinine ratio; EHW: Hardy-Weinberg equilibrium by X2.
The present study is the first to demonstrate an association of the rs5186 of the AGTR1 gene with different risk estimation models of a decrease in eGFR in T2DM patients, the main predisposing sign of progression to ESRD and ACKD.
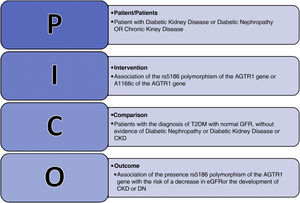
The originality of the research was demonstrated, after the background search using the PICO strategy with the Boolean operators: Diabetic Kidney Disease OR Chronic Kidney Disease OR Diabetic Nephropathy, AND AGTR1 OR rs5186 OR A1166C, searched in the PubMed database between the years 2010–2022 (Fig. 2).
According to this search, we identified a total of six studies that reported association of rs5186 of the AGTR1 gene with DKD and CKD; none reported any analysis of the association of the polymorphism with DKD in patients with T2DM. Prior to 2010, more than six meta-analyses and systematized studies had been reported which included different phenotypes and syndromes associated with renal damage, generating discrepancies and no association of rs5186 of the AGTR1 gene with CKD secondary to the progression of DKD in patients with T2DM.
After a fine characterization of the analyzed sample based on a screening and selection of patients KDOQI stages 1 and 2, our study is the first to describe, an association of rs5186 of the AGTR1 gene with the decrease or decline of GFRe considered as risk for CKD. The exclusion of KDOQI stages 3–5 and other factors that may have triggered or enhance CKD should provide more evidence to our findings because we assumed that advanced stages of CKD would be more exposed to overt ACKD.
We should consider that the search for early biomarkers has become more important in recent years, because traditional markers associated with CKD such as urinary albumin in DKD have been controversial for their clinical application as a diagnostic or prognostic method in this disease.
Although it has been described that ACKD in patients with a diagnosis of T2DM could be present 10–20 years after the identification of glycemic alterations. In addition there are patients with ACKD with a recent diagnosis of T2DM who usually present different degrees of renal damage associated with DKD, even of the early stages in which in addition to the decrease in eGFR there is proteinuria that correlates positively with metabolic dyscontrol and is considered in turn as the first clinical evidence of DKD and CVD. However, the controversy between the presence of microalbuminuria and its association with DKD in patients with T2DM lies in the fact that a considerable percentage of patients with diabetes have CKD without albuminuria.18 Therefore, the presence of an initial subclinical phase of hyperfiltration usually followed by a mild or moderate albuminuria (ACR 30–300mg/g) and subsequent progressive loss of glomerular filtration rate may not be related to DKD secondary to T2DM because sometimes the diagnosis of this type of diabetes is made in an individual with a decrease in eGFR already established.19 In addition, the presence of albuminuria in patients with T2DM is usually reversible, since it can be modified by controlling the triggering or potentiating factors associated with the diabetic phenotype, such as hyperglycemia, high BP, dyslipidemia, and even with the use of certain drugs such as angiotensin-converting enzyme inhibitors (ACE inhibitors) or ARBs II.
In our study we did not observe statistically significant differences in the variables considered as enhancers and triggers of DKD such as fasting glucose concentrations, HbA1c%, HOMA-IR, C-LDL and triglycerides, however, according to different clinical practice guidelines, these are above the cut-off points for optimal metabolic control in patients with T2DM.
Although the lack of metabolic control observed in the groups analyzed could be conditioning a decrease in eGFR even with characteristics of incipient CKD, the logistic regression adjusted for variables considered to be causal or potentiators of renal damage showed that the presence of rs5186 of the AGTR1 gene conditions a risk of a decrease in eGFR showing an OR between 1.89 and 2.01 (Table 4). Despite the controversies on associations reported in previous studies, this expected outcome could be explained by the mechanism of action of AT1 when analyzing the pathophysiology of CKD. Furthermore, we should remember that the T2DM phenotype is commonly accompanied by obesity, dyslipidemias, hyperinsulinism, and IR, that are described as factors favoring susceptibility, initiation, enhancement, or progression to CKD, as they promote increased AT1 expression and activity.20,21
We must recognize that the alteration of the renin-angiotensin-aldosterone system (RAAS) is considered the most important pathophysiological mechanism associated with the decrease in eGFR and implicated in the development of CKD in patients with T2DM, in which angiotensin II (AngII) is the main regulatory peptide, also identified as the most potent vasoconstrictor in the body and the main effector of vascular and renal damage associated with CKD. The action of AngII is exerted by activation of AT1, which is present mainly in heart, lung, muscle, adipose tissue, kidney and blood vessels, organs vulnerable to be damaged by advanced glycation products in patients with uncontrolled T2DM.
We know that the population analyzed in our study has a high genetic susceptibility to the T2DM phenotype,22 and therefore to its complications. Although some patients with T2DM will have a good response to the use of ACEI or ARB II drugs to prevent or delay DKD; however, a percentage of these patients will develop the disease and even progress to ACKD. So far it is not possible to predict which patients will develop DKD, but it is agreed that the search for and validation of genetic biomarkers directly involved in the RAAS could be clinically useful to identify patients vulnerable to DKD even before a decrease in eGFR or presence of microalbuminuria with the intention of making a timely referral to the nephrology service from the first level of care. We consider that the AGTR1 gene could be a candidate as a genetic biomarker, because it is responsible for the coding of structural proteins of AT1,6 in addition, different polymorphisms located in this gene have been associated with direct alterations of the RAAS that promote renal damage and CKD, such as vasoconstriction, inflammation, and hydroelectrolyte imbalance.23
It has been reported that the rs5186 polymorphism located in the 3'-UTR region of the AGTR1 gene can influence the stability and translation of the mRNA of the proteins that form AT1,7 therefore, its altered activity is associated with phenotypes that affect the renovascular system mediated by the RAAS, including cardiovascular and autoimmune diseases.24–26 However in meta-analysis studies have shown that its association with diabetic vasculopathies such as retinopathy, neuropathy, heart disease, DKD, DN, and ACKD is controversial, even some studies describe this association as null or non-significant. The discrepancies shown in the meta-analyses of the association of different genetic variants of the AGTR1 gene with DKD, DN or CKD could be due to ethnicity, stratification and sample size of the populations analyzed,27 sample heterogeneity due to the inclusion in most of the studies of multiple diseases linked to CKD, age distribution, variables that limit the analysis of the association of rs5186 with DKD or DN secondary to T2DM2.8,24,28
In comparison with other studies our aim was to analyze the association of rs5186 of the AGTR1 gene with decreased eGFR predisposing to DKD and ACKD in a sample of patients with T2DM from Mexico City. We inferred that the selection of patients after screening for KDIGO stages 1 and 2 in our study may have influenced the genotype-phenotype association result.
Recently, the meta-analysis by reported no presumed association of rs5186 with ACKD regardless of etiology using different methods of randomized and multivariate effect.29 In contrast with the study by Braliou et al., we did not include patients with renal transplantation, on renal replacement therapy (dialysis or hemodialysis) or pediatric patients, moreover, our control group were T2DM patients with discrete reduction in eGFR (≥90mL/min/1.73m2, KDOQI 1). In addition, the meta-analysis included a sample of Caucasian, Asian and African populations, therefore, it was unknown what could be the contribution to ERD risk of rs5186 in populations with T2DM and Native American ancestry such as Mexican. This is important since it has been described that ethnicity can influence the staging and behavior of eGFR independently of its etiology.4 The eGFR by the Modification of Diet in Renal Disease (MDRD) equation, which has been the most widely used in different studies of genetic association with CKD or ACKD even in advanced stages of renal replacement therapy,30 has shown significant differences with other eGFR formulas. Furthermore, MDRD formula has not yet been validated for the Latin American, Mexican or Mexican-American population.31 Our study used the CKD-EPI equation for eGFR, which according to expert panels is the most recommended formula for the Latino population with Native American lineage.32 According to previous studies of the analysis with lineage panels in the population of Mexico City, the percentage of miscegenation in our analysis could oscillate between 70% Native American and 30% European,33,34 so the use of CKD-EPI in the present study is justified.
The study by Moradi et al. in 2015 did not report association of a direct risk of rs5186 with T2DM and DN, however, they concluded that patients with T2DM carrying the AC+CC genotype presented macroalbuminuria when HbA1c% levels were higher than 7.5%, and that carriers of the C allele had higher values of SBP and serum creatinine concentrations as compared to carriers of the A allele.35 However, it can not be concluded that rs5186 influence the presence of micro- or macroalbuminuria in patients with T2DM, because the authors stated that they had limitations in the analysis due to insufficient sample size in the study groups, mainly in the controls. Our study, as compared with that of Moradi et al.35 did not include subjects without the diagnosis of DM as control, nor the diagnosis of CKD by the presence of albuminuria, because this may depend on different variables such as lack of metabolic control among others that have been described already. In addition, the decrease in eGFR and the presence of micro or macroalbuminuria is usually dependent on the time of evolution and the glycemic control in patients with T2DM; our groups did not show statistically significant differences in age at diagnosis (p=0,4505) and the time of evolution (p=0.0803) of T2DM, however, they did present hyperinsulinism with IR estimated by HOMA-IR, which represented a poor fasting glycemic control and in HbA1c% in both groups.14,36 T2DM is usually characterized by the presence of hyperinsulinism, hyperglycemia and IR, phenomena that contribute to essential hypertension by increasing renal damage, due to the altered activity of the sympathetic nervous system, which is usually also dependent on insulin concentrations and activity. In our study, the presence of IR estimated by HOMA-IR could be influencing the elevation of renal and peripheral vascular resistances, accelerating the presence of endothelial dysfunction and CVR in both groups37; in addition, we observed that in both groups the CVR by Framingham Score was above 10%, which is considered a high risk, regardless of the fact that this estimate is usually very subjective in patients with T2DM2.38
Our study showed that patients carrying the C allele had a younger age at diagnosis and a longer time of evolution of essential HTN (Table 2). Different studies have assumed an
Association of polymorphisms of the AGTR1 gene with essential HTN, suggesting that individuals with risk genotypes have a greater predisposition to the development of this clinical entity.39,40 The association of rs5186 with HTN could have a biological explanation. It has been described that some regulatory molecules such as microRNA-155 can suppress the translation of proteins that conform the structure of AT1 by coupling with AGTR1 mRNA as long as the A allele is present, however, this action is not performed when the C risk allele of rs5186 is present, causing and increase in blood pressure (BP)41 and contributing to renal damage in vulnerable patients due to the presence of the risk allele. In addition, it has been reported that in the Mexican population with T2DM there is a decrease in the expression of microRNA-155, which could contribute to an increase in blood pressure, playing an important role in the pathogenesis of DKD in patients with T2DM accompanied by inappropriate metabolic control.42 Perhaps this could be the explanation why not all patients with T2DM and essential hypertension achieve their clinical control, that is, we infer that the presence of rs5186 promotes alterations in the activity and expression of AT1, the homeostasis of the RAAS and in turn promotes renal damage independently of pharmacological intervention.
However, in our study we could not conclude that the presence of essential HTN could be an indirect risk for development of DKD as reported in other studies,43 because not all patients included in our study with the C allele have the diagnosis of HTN; in addition, the number of patients with diagnosis of essential HTN was 37 and their BP was not out of control and did not have prescribed antihypertensive or nephroprotective pharmacological treatment of the ACEI or ARA II type; therefore, this small sample could not interfere with the results obtained.
It has been described that in the Mexican-American population the presence of rs5186 is associated with a higher BMI, which is recognized as a subclinical factor that favor renal damage.44 In the groups analyzed in the present study It is evident the presence of overweight and obesity as determined by BMI, that is accompanied by the presence of high concentrations of C-LDL and triglycerides (Table 1), which together could promote the future development of atherosclerosis, vascular alterations, CVR and kidney damage45 by persistent abnormalities in the activation of the RAAS secondary to increased synthesis, absorption and storage of fatty acids and triglycerides.46
We do not know whether the lack of lipid control in the sample of subjects analyzed could also be influenced by the presence of the rs5186 variant, since it has been reported that in the Mexican population carriers of the AC genotype have a slow metabolism of atorvastatin, whereas the metabolism is fast in the C/C or A/A+C/C genotypes.47 Thus, the presence of hypercholesterolemia, which has been associated with glomerulosclerosis, could also be considered as a pathogenic factor of progressive renal failure in patients carrying rs5186, being an additive risk factor to DKD in patients with T2DM independent of the use of pharmacological treatment of hypercholesterolemia. In the present study, we do not know the contribution of rs5186 as an additive effect to the development of DKD in T2DM and dyslipidemia patients, with or without treatment; however, we did observe that genotypes with the presence of the C risk allele presented more lipid abnormalities (Table 2).
According to some studies in Mexico, CKD is more likely to develop in women than in men, with an average prevalence of 14% and 12%, respectively. However, the number of women on dialysis is lower than the number of men.
An important finding in our study was that the presence of rs5186 in women with T2DM (Table 5) could be an important risk factor for the development of DKD (OR 2.54, 1.10−5.89; p=0.023, adjusted for age, BMI, BP and ACR). There is little published evidence about the association of rs5186 and kidney damage in women. The study by Cooper et al. reported in 2009 that the presence of the CC risk genotype of rs5186 is associated with impaired renal function in women with preeclampsia,48 it has even been suggested that this polymorphism may be a risk marker in women for the presence of essential HTN and preeclampsia when there is a family history of HTN and an elevated BMI pre-pregnancy.49 Although systematic studies and meta-analyses cast doubt about this association.50
Our study showed no association of the C risk allele of rs5186 with the risk of DKD in men, which has been controversial in different studies. Lin et al. in 2009 reported in a population of 733 adult males with T2DM from the Health Professionals' Follow-Up Study (HPFS) an association of the C allele of rs5186 with decreased eGFR < 60mL/min/1.73m2 (1.63 [1.01, 2.65]) and coronary heart disease (OR 1.57 [1.10, 2.24]).17 However, the genotype-gender interaction on CKD risk in male rs5186 carriers seems to vary according to their etiology and ethnicity.
The study by Möllsten et al. in 2011 reported in a sample of 3,561 European patients with type 1 diabetes mellitus (T1DM1) that the risk to DN in males increases if they carry the AA genotype of rs5186 (OR=1,27, 95% CI, 1.02–1.58, p=0.03), adjusted for age at diagnosis, time of evolution of DM, HbA1c%, smoking and ethnicity, but the results were not the same in women (OR=0.89, 0.71–1.11, p=0.30).6
Finally, although it has been described that the risk allele of rs5186 to ERD and CKD could be C, the presence of the ancestral A allele also plays an important role in patients with T2DM. The systematic review and meta-analysis by Smyth et al. reported in 2019, based on four studies that analyze the contribution of rs5186 to the development of DN, concluded that the presence of the A allele in South Asian population represented a protective or low-risk factor to the development of DN (p=0.001; OR 0.71; 95% CI 0.58−0.87; I2=37%).51 Although our analysis did not report any protective association with the A allele, it could have a better population validation not observed in other studies, since it shows similar EHW to that reported in different databases.
It could be reasonable to assume that the presence of rs5186 in the population analyzed influences the activation of the RAAS and the presence of renal damage by different pathophysiological mechanisms, including preexisting metabolic alterations. Thus, our analysis corrected for those variables considered as risk factors for DKD such as age, gender, BMI, DBP, SBP and ACR becomes more important when observing the association of the genetic variant rs5186 with the decrease in eGFR in the dominant and overdominant model of the total population (Table 4). Whether environmental factors could contribute to the predisposition of risk to develop DKD in patients with T2DM carrying rs5186, as a gene-environment interaction, is still under discussion, therefore, we acknowledge that our study might have the following limitations:
- 1
Our study could be considered as a pilot study with suitability sample, because the calculated sample was not reached. However, we consider that fine selection by screening and screening represented a better estimation of risk to eGFR decrease, even with risk ORs of 1.89 and 2.01 in the dominant and overdominant models, respectively, in the total population and is higher than that reported in other studies. Increasing the N could have also provide greater statistical power and safety of the results obtained.
- 2
Being a cross-sectional study, it only demonstrates an association, but not causality or effect.
- 3
We do not know the effect and response to DKD risk in patients with T2DM considered vulnerable because they carry rs5186 but use ARA-II or ACEI as monotherapy or first-line combination therapy for the treatment of CKD as a nephroprotective use.
- 4
Since we did not analyze the effect and concentrations of medications for glucose control or metabolic alterations, we do not know if these could have generated changes in the results obtained.
- 5
Food intake, physical activity or additive salt consumption were not quantified, which could have influenced the results.
- 6
Finally, there are no ultrasonography or histological studies to associate them with any structural damage secondary to DKD such as DN.
Despite these weaknesses, we suggest that the presence of the rs5186 variant could be a possible early risk marker for the development of DKD and ACKD due to its association with decreased eGFR in patients with T2DM. Our results could encourage further studies to justify rs5186 in the future as a possible genetic biomarker for screening, prognosis or timely referral to the nephrology service, because patients with T2DM carrying the risk allele could be considered vulnerable to the development of ESRD, ACKD and even renal failure. This information could also be useful for future systematic or meta-analysis studies.
ConclusionIn clinical practice, most nephropathies progress slowly to a definitive loss of renal function, even when asymptomatic. The present inter-institutional study is the first to report a contribution of rs5186 of the AGTR1 gene with a decrease in eGFR considered as a risk for ESRD in patients with T2DM. In the future, it would be of utmost importance to develop prevention studies using genetic screening in patients vulnerable to the development of DKD, which could lead to better clinical management and reduce the economic impact of future complications for public health institutions.
FinancingThe present study is derived from the protocol under the title Study of Polymorphisms associated with chronic renal failure in Mexican patients with type 2 diabetes with registration number R-2016-785-022, authorized by the Research Ethics Committee of the Coordination of Health Research of the Centro Medico Nacional Siglo XXI (CONBIOETICA-09-CEI-009-20160601. Declaring that the present research did not receive grants or funding from public sector agencies, commercial sector or non-profit entities.
Conflict of interestNo author declares any conflict of interest.
To all the personnel who supported the Medical Research Unit in Biochemistry of the Centro Médico Nacional Siglo XXI and the Family Medicine Unit number 31 of the IMSS.