Statin intolerance affects 10–20% of patients who start treatment with these drugs.1 Discontinuation of the drug is mainly due to the muscular side effects associated with the medication.2
In recent years, studies have been conducted with proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) monoclonal antibodies3 that have proven to be effective in reducing levels of low-density lipoprotein cholesterol (LDL-C) and a safe alternative for those patients in whom the use of statins is contraindicated.
In addition, both the ODYSSEY4 (alirocumab) and FOURIER5 (evolocumab) trials showed a reduction in non-fatal infarction, ischaemic stroke and hospitalisation for stable angina, plus a 15% decrease in cardiovascular events with no reduction in mortality. Taking all this into consideration, in September 2015 the European Community authorised the use of alirocumab6 as a lipid-lowering treatment.
In these two trials, only patients with a glomerular filtration rate (GFR) of ≥30 mL/min/1.73 m2 and ≥20 mL/min/1.73 m2 were included, respectively. In addition, in the study with alirocumab,7 a subgroup analysis was performed according to their stage of chronic kidney disease (CKD),8 showing a similar reduction in LDL-C levels in both groups, without changes in kidney disease progression and with good drug tolerance and safety levels. None of these trials included patients on renal replacement therapy.8
We present the case of a 58-year-old male patient with a personal history of arterial hypertension being treated with three drugs, including an angiotensin II receptor antagonist and a diuretic. He has insulin-dependent type 2 diabetes mellitus with micro- and macrovascular involvement; a body mass index of 31.1 kg/m2; ischaemic-valvular heart disease with a revascularised anterior descending artery and surgical aortic valve replacement in 2012; preserved left ventricular ejection fraction; and chronic lower limb ischaemia due to femoropopliteal stenosis.
The patient has chronic kidney disease (CKD) stage 5D secondary to diabetic nephropathy, which has never been biopsied. He has been on peritoneal dialysis since October 2012, currently on automated peritoneal dialysis with a wet day. Total volume: 15 l. Tests reveal a Kt/V of 1.78 and a total weekly clearance of 67.52. The patient maintains a residual diuresis of 700−1,000 mL/day.
Regarding treatment, in 2004 he started treatment with statins (pravastatin) at 20 mg/day. Due to myalgia and elevated creatine kinase (CK), he was changed to atorvastatin 20 mg/day and he also presented levels of CK > 900 mg/dL, leading to discontinuation of the treatment. Subsequently, other drugs such as ezetimibe, fenofibrate and Omacor have been administered, without achieving LDL-C targets.
In November 2017, he had a total cholesterol (TC) level of 249 mg/dL, triglycerides (TGC) 473 mg/dL, high-density lipoprotein (HDL) cholesterol 30 mg/dL and LDL-C levels of 143 mg/dL. As a result, treatment with PCSK9i was proposed in order to achieve target levels in line with his cardiovascular profile (LDL-C 70 mg/dL).
There are no studies that analyse the use of PCSK9i in patients with stage 4–5 CKD or who are receiving renal replacement therapy, but the use of alirocumab is not contraindicated in its summary of product characteristics. As a result, after being approved by the pertinent committee, the decision was made to start treatment at a dose of 75 mg every 2 weeks, with continuation of treatment with ezetimibe.
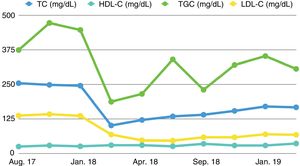
The patient's TC levels improved from the onset of treatment, reaching target levels of LDL-C from the third month of treatment that have been maintained to date, as can be seen in the lipid profile shown in Table 1 and Fig. 1. During this time, only a flu-like illness was detected as an adverse effect, with myalgia and cramps following the first administrations of the drug, with no subsequent symptoms or added adverse reactions. As such, the treatment has been maintained.
Lipid profile of a PD patient receiving treatment with alirocumab.
| Start of treatment with alirocumab | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aug. 17 | Nov. 17 | Jan. 18 | Feb. 18 | Apr. 18 | Jun. 18 | Sep. 18 | Dec. 18 | Jan. 19 | Mar. 19 | |
| TC (mg/dL) | 255 | 249 | 246 | 102 | 122 | 135 | 141 | 155 | 171 | 168 |
| HDL-C (mg/dL) | 26 | 30 | 27 | 31 | 31 | 27 | 36 | 30 | 30 | 37 |
| TGC (mg/dL) | 375 | 473 | 448 | 188 | 216 | 341 | 231 | 321 | 353 | 307 |
| LDL-C (mg/dL) | 138 | 143 | 137 | 69 | 48 | 47 | 59 | 59 | 70 | 68 |
In conclusion, in this patient on peritoneal dialysis, alirocumab treatment has proven to be useful and safe in lowering LDL-C levels, reaching the target levels for his cardiovascular risk profile and maintaining these figures during the follow-up period, with no associated complications.
This case may be of particular interest since there are no studies reported in the literature that analyse the use of this drug in patients on renal replacement therapy, mainly because these patients have not been included in clinical trials.9 In addition, it is noteworthy that the treatment continues to be effective in achieving LDL-C targets without the onset of side effects. It would be necessary to perform controlled and randomised clinical trials in patients with end-stage renal disease, GFR < 15 mL/min, in order to gather a more substantial case history with a longer follow-up period and assess whether the beneficial effects in terms of cardiovascular morbidity and mortality are reproduced without serious adverse effects.10
Please cite this article as: Rivas Oural A, Astudillo Cortés E, Bande Fernández JJ, Rodríguez Suárez MDC, Díaz Corte MDC. Tratamiento con alirocumab en paciente en diálisis peritoneal con intolerancia a estatinas. Nefrologia. 2021;41:76–79.