El 4-10% de los pacientes incidentes en diálisis portan un injerto renal no funcionante y hasta en el 32% de los casos, según las series, se requiere la realización de trasplantectomía por diversas causas. La mortalidad de estos pacientes es significativamente mayor que la de aquéllos con injerto funcionante o en terapia renal sustitutiva sin injerto previo. Se han sugerido como indicaciones actuales de trasplantectomía el síndrome de intolerancia al injerto, la pérdida precoz de éste, la presencia de proteinuria grave, pielonefritis recurrentes o neoplasia y el síndrome de inflamación crónica. El síndrome de inflamación crónica se presenta en enfermos con elevación de los marcadores de inflamación (proteína C reactiva), anemia con resistencia al tratamiento con estimuladores de la eritropoyesis y marcadores de desnutrición en su contexto. Esta situación de inflamación está provocada por el injerto y revierte tras la trasplantectomía, como han demostrado varios estudios. Hemos revisado la literatura publicada al respecto, las indicaciones de trasplantectomía, o embolectomía, sus ventajas e inconvenientes; la incidencia del síndrome de intolerancia al injerto y la fisiopatología del síndrome de inflamación crónica, así como el algoritmo de manejo terapéutico propuesto actualmente.
Approximately 4%-10% of incident patients on dialysis have a non-functioning kidney graft, and according to series, as many as 32% require transplantectomy for a variety of reasons. Mortality in these patients is significantly higher than in those with a functioning graft or on renal replacement therapy without having received a graft. Graft intolerance syndrome, early graft loss, severe proteinuria, recurring pyelonephritis or neoplasia, and chronic inflammation syndrome have all been proposed as indications for transplantectomy. Chronic inflammation syndrome occurs in patients with high levels of inflammatory markers (C-reactive protein), anaemia resistant to treatment with erythropoiesis stimulators, and malnutrition markers. This inflammatory state is provoked by the graft, and reverts when a transplantectomy is performed, as several studies have shown. We have reviewed the medical literature published on this topic, the indications for transplantectomy and embolectomy, their advantages and disadvantages, the incidence of graft intolerance syndrome, and the pathophysiology of chronic inflammation syndrome, as well as the currently proposed therapeutic management algorithm.
INTRODUCTION
Between 4% and 10% of incident patients on dialysis have a non-functioning renal graft1; in as many as 32% of cases, according to the studies, a transplantectomy is required due to any one of a multitude of reasons. Approximately 13% of transplanted patients in 2006 had received a second graft, serving as evidence of the potential consequences of the therapeutic decisions in these patients, including the indications for transplantectomy.2 These patients may develop an immunological intolerance syndrome clinically characterised by fever without an underlying infectious disease, haematuria, pain, and increased graft size. This situation, along with early graft loss (during the first year post-transplantation), constitutes a clear indication for either transplantectomy or embolisation.3 However, chronic inflammation syndrome is an increasingly common condition in patients with late graft loss (more than 12 months post-transplantation) who remain on renal replacement therapy (RRT). This inflammatory condition, along with the closely related state of malnutrition, increases the number of episodes of infection and cardiovascular events in these patients, leading to high mortality rates.4-6 In addition, other complications inherent to decreased immunosuppressant therapy may be present. Transplantectomy is not without its own potentially severe complications, for which embolectomy should be considered as an alternative, whenever contraindications are not present.7,8
INDICATIONS FOR TRANSPLANTECTOMY
The current indications for transplantectomy are graft intolerance syndrome, early graft loss, signs of chronic inflammation, severe proteinuria, recurrent pyelonephritis or urinary infections, and cancer. In addition, nephropathy associated with polyomavirus infection is an emerging cause of loss of graft function that could constitute a new indication for transplantectomy (including ureterotomy) following graft failure that can be attributed to this cause.
Graft intolerance syndrome
Between 30% and 40% of patients that return to dialysis with a non-functioning graft develop immunological intolerance once immunosuppressant therapy is reduced. The majority of episodes occur during the first year, with described rates of cumulative risk of 28%, 38%, and 40% after 6, 12, and 24 months, respectively.9 This intolerance is manifested by the presence of fever, general discomfort, asthenia, haematuria, pain, and increased graft size, all in the absence of systemic infectious disease. In the case of isolated fever, adrenal insufficiency should also be ruled out, since these patients have generally received long-term treatment with steroids. Madore et al. described how patients with a previous history of several episodes of rejection have a greater risk for developing intolerance.10 These authors concluded that a more gradual decrease of immunosuppressant therapy, or indefinite continuation of immunosuppressant at low doses, decreases the frequency of episodes of intolerance and the need for transplantectomy. Initially, this was associated with the type of immunosuppressant previously administered; in this manner, patients treated with cyclosporine are generally at a greater risk. A retrospective study carried out at a single hospital described a 4% incidence of transplantectomy in patients treated with azathioprine and prednisone versus 21% in patients treated with cyclosporin11; other centres have reported nephrectomy rates as high as 63% in patients treated with cyclosporine + azathioprine + prednisone versus 27% of patients treated with just azathioprine + prednisone.10 Current immunosuppressant regimens include the use of an anticalcineurinic (generally tacrolimus) in the majority of cases, along with prednisone and an antimetabolite (usually mycophenolate mofetil). No conclusive relationship has been established between immunosuppression and graft intolerance syndrome. The treatment of this syndrome has typically been based on indomethacin (25-50mg/12h orally) and prednisone (5-10mg/day orally), but this condition also constitutes an established indication for transplantectomy or embolisation as long as no contraindications are present.
Early graft failure
Graft loss during the first year constitutes an established indication for transplantectomy, whether due to the risk of graft rupture due to vascular thrombosis or hyperacute/acute rejection, or due to technical complications (infection of the surgical wound, lymphocele, ureteral deinsertion, bladder rupture, etc.).
Late graft failure
After the first year, loss of graft function accompanied by one of the following conditions is an indication for transplantectomy: immunological intolerance syndrome, cancer, recurrent pyelonephritis, or extreme proteinuria after return to dialysis. Chronic inflammation syndrome due to a non-functioning graft deserves special mention as an indication for transplantectomy.12,13 Chronic inflammation syndrome is characterised by anaemia, resistance to erythropoiesis-stimulating agents, increased inflammatory markers (C-reactive protein [CRP], ferritin, erythrocyte sedimentation rate), and decreased nutritional markers (albumin).
In various studies of patients on RRT (both on haemodialysis and peritoneal dialysis), it has been observed that CRP levels in those that either perished or were affected by a cardiovascular event are significantly higher than other patients.14 In a similar manner, patients on RRT with higher levels of interleukin 6 had a significantly higher mortality rate than other patients.15
Patients with malnutrition have a greater risk of hospitalisation and mortality. Among patients on RRT, low cholesterol and/or body mass index are associated with greater mortality.16-18 It has been hypothesised that this malnutrition is mediated by the patient’s inflammatory state; certain cytokines, such as tumour necrosis factor alpha, promote catabolic processes in the body and induce anorexia.16,19 Given that low levels of albumin and/or high levels of CRP are associated with an increase in mortality rates, as well as increased risk of cardiovascular events, several authors have suggested transplantectomy in these patients, reporting a risk of mortality for various causes that is 32% lower in patients that have undergone nephrectomies (hazard ratio: 0.68; 95% confidence interval: 0.63-0.74),20 although it is true that in this study, the populations compared were not completely homogeneous (patients that underwent nephrectomy were significantly younger and healthier than those that did not), and long-term consequences, increases of infection and/or cardiovascular events, and maintenance of immunosuppression therapy were not measured in the group of patients that did not undergo nephrectomy.21
In 2004, the research group from the Hospital Clínico San Carlos de Madrid published a study involving 43 patients that returned to dialysis following graft loss; all patients had signs of chronic inflammation, and those that underwent transplantectomy improved in terms of anaemia and nutrition parameters six months after the surgery, to the point where their condition was similar to that of other patients on dialysis.12
IMMUNOSUPPRESSION MAINTENANCE
The advantages of maintaining non-functioning renal grafts in situ, such as conserving residual diuresis, erythropoietin production, and hydroxylation of calcidiol (functions that are progressively lost once dialysis is restarted), should be appropriately weighed against the risks described for the provocation of immunological intolerance or chronic inflammation states, which produce deleterious effects for these patients. A few inconclusive studies suggest that maintaining immunosuppression therapy, in an attempt to preserve residual renal function, increases the life expectancy of these patients.22 An accepted procedure for decreasing immunosuppression therapy consists of abruptly suspending the antimetabolite (azathioprine or mycophenolate), with a 25% reduction per week in the dose of anticalcineurinics or mTOR inhibitors, and a 2.5mg decrease in the steroid prescription every month, while monitoring the patient for the appearance of secondary adrenal insufficiency.9
Several studies have described the increased risk of infection and cardiovascular events present in patients on dialysis with chronic treatment of low doses of immunosuppression. Smak Gregoor et al., in a retrospective study involving 197 patients on dialysis with low doses of immunosuppression (prednisone/azathioprine/cyclosporine) as compared to patients without immunosuppression, observed infection rates of 1.7 episodes/patient/year versus 0.5 episodes/patient/year, with a 3.4 times higher risk of mortality in the group of patients that continued on immunosuppression therapy.23
The secondary side effects of chronic corticosteroid use should also be considered: increased risk of infection, diabetes, cataracts, osteoporosis, and adrenal insufficiency when the corticosteroid treatment is removed or reduced, effects that occur in as many as 30% of cases. In rare occasions, an Addisonian crisis may be produced; in addition, many patients can develop non-specific symptoms such as fatigue, myalgia, arthralgia, weight loss, mild hypercalcaemia, and eosinophilia.24,25 Neither the dose nor duration of removal from corticosteroid treatment are well established; time spans of just a few weeks to 9 months or more have been described, depending on the previous doses administered and the duration of therapy.
TRANSPLANTECTOMY VERSUS EMBOLISATION
Transplantectomy is an invasive procedure associated with potentially severe adverse side effects; however, the mortality associated with this operation decreased significantly with the advent of cyclosporine, from 73% to 38%,26 and the incidence of severe complications also decreased from 20% to 10%.27 In recent studies, the mortality rate associated with this procedure has decreased significantly: 0.7% to 5%.28-29 The most common complications are bleeding and surgical wound infection. According to Johnston et al., risks are higher in nephrectomies following early graft loss, which are associated with graft complications more than complications of the technique itself, with the risk of death or sepsis being lower in patients that have indications for late transplantectomy. These authors describe several different factors associated with transplantectomy (both early and late): age <40 years old, more than 3 HLA incompatibilities, and re-inclusion in the organ wait list during the first year following loss of graft function.31 However, Madore et al. observed that the only factor significantly correlated with early or late transplantectomy was a history of two or more episodes of acute rejection.10
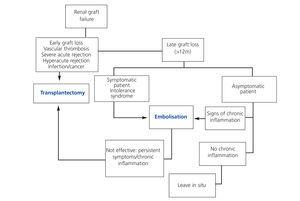
On the other hand, embolisation using ethanol or polyvinyl spheres, following insertion of metallic coils, is a less invasive procedure, associated with a shorter hospitalisation time and lower rate of complications than a nephrectomy. However, this procedure is contraindicated in cases of added infections, neo-formation of the graft, or high risk of graft rupture (surgical complications or severe rejection). The most common complication is post-embolisation syndrome, characterised by fever, local pain, haematuria, nausea, and vomiting, which, if persisting more than 72 hours, will require ruling out the presence of vascularisation in the graft and immunological intolerance. Perez Martinez et al. described an improvement in anaemia and inflammation following graft embolisation in a study involving 7 dialysis patients.8 The currently proposed treatment algorithm1 following loss of renal graft function is summarised in the Figure.
CONTROVERSIAL IMMUNOLOGICAL ASPECTS
One aspect that still produces a good deal of controversy in performing a transplantectomy or embolisation of a non-functioning graft is the formation of anti-HLA antibodies (levels of panel reactive antibodies [PRA]) following the procedure, and their implications in the survival of future transplants. It has been suggested that maintaining the non-functioning renal graft within the body would serve to trap the panel reactive antibodies as a “sponge”, which, together with the continued immunosuppression therapy, would avoid the formation of new antibodies against the graft, yielding lower levels of circulating antibodies prior to the transplantectomy.30
In two studies involving retransplanted patients, PRA levels >30% were described, with a greater frequency in patients that previously underwent a transplantectomy: 57% vs 33% and 60% vs 30%.32 In a similar manner, Gourlay et al. examined 52 retransplanted patients and observed a greater frequency of PRA>50% in patients that underwent a transplantectomy (54% vs 15%).33 In a recent comparative study of retransplanted patients with or without previous nephrectomy, patients with nephrectomy had a significant increase in PRA, which was associated with a significant increase of primary graft dysfunction and acute rejection, as well as a lower graft survival rate.34 In a sub-group of patients who received organs from elderly donors (older than 65 years) or who had received two or more previous transplants, graft survival was lower if the patient had previously undergone a transplantectomy.35 Michael Knight et al. also described a significant increase in circulating Class I and Class II antibodies following transplantectomy, which are donor-specific, and the authors also observed that the shorter the time interval between graft failure and transplantectomy (<10 months), the greater the increase in the concentrations of these molecules.36 Other authors, however, have not observed significant increases in PRA levels, or higher incidences of graft rejection or lower graft survival rates in patients that have undergone nephrectomies, and even when reporting a significant increase in these levels following transplantectomy, this was not associated with graft survival.37
To some authors, this is simply a temporary phenomenon, and the percentage of immunised patients decreases over time until matching the percentage of non-nephrectomised patients.27 Johnston et al. described two types of behaviours following nephrectomies based on the previous rate of anti-HLA antibodies; nephrectomised patients with a PRA<30% before the first transplantation have a PRA prior to retransplantation significantly higher than those who have not undergone nephrectomy, and patients with a PRA>30% before the first transplant did not have significant differences in retransplant PRA values, whether having undergone nephrectomies or not.31
There is also certain level of controversy regarding whether more intense sensitisation modifies the survival of a second graft. Sumrani et al. showed that undergoing a nephrectomy before retransplantation is associated with a greater incidence of primary dysfunction of the second graft, and lower 1-year survival rates.32 Other authors have observed that transplantectomy following early graft loss is associated with a lower risk of loss of the second graft (associated with a decrease in patient death with a functioning renal graft, more than with improved graft survival), with the inverse occurring in transplantectomies following late graft loss.31 In the study by Yagmurdur et al. involving retransplanted patients that previously underwent a nephrectomy that spent more time on dialysis, this was the factor that yielded a lower graft survival rate.38 However, Ahmad et al. found no differences in terms of graft or patient survival in patients that had received haemodialysis before retransplantation.37 In this context, it was recently described using new and more sensitive techniques for measuring donor-specific antibodies (luminex single antigen assay) that a substantial proportion of patients with negative pretransplant antibody results using CDC or ELISA are in fact positive. The greatest number of graft rejections and failures in retransplantations has been documented in patients with early transplantectomies (<6 months) and in those that receive organs from elderly donors.39
The decision to perform a transplantectomy or embolisation of a non-functioning graft following the patient’s return to dialysis must be made on an individual basis, taking into account all of the aforementioned factors. It would be advisable to monitor panel reactive antibody levels using the newest and most sensitive techniques available both before and after the nephrectomy, as well as helping to choose the appropriate donor type and immunosuppression therapy prior to the transplantation for highest risk patients.
CONCLUSIONS
Despite the scarce medical evidence available regarding decision making in patients with non-functioning grafts that return to dialysis, the indications for transplantectomy are clearly established in the form of graft intolerance, hyperacute rejection, cancer, and other conditions that imply an elevated risk of graft rupture. Chronic inflammation syndrome, with parameters of anaemia and malnutrition that cannot be explained by any other cause, is today a new indication for transplantectomy due to its implications in patient prognosis (increased mortality and morbidity rates). The maintenance of immunosuppression therapy is not advisable due to the associated increase in the rates of infection and cardiovascular events. Whenever contraindications are not present, embolectomy should be prioritised over transplantectomy, since this procedure is less invasive and is associated with lower rates of complication and hospitalisation times. In any case, the indications for transplantectomy in these patients must be evaluated on an individual basis, monitoring anti-HLA antibody levels after the procedure. More studies with larger sample sizes and control groups are needed in order to unify the criteria for managing these patients.
Conflicts of interest
The authors have no conflicts of interest to declare.
Figure 1. Treatment algorithm following loss of renal graft function